Changes in Vertical Stratification of Neotropical Nymphalid Butterflies at Forest Edges Are Not Directly Caused by Light and Temperature Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Butterfly Sampling
2.2. Temperature and Light Measurements
2.3. Statistical Analyses
2.3.1. G-Test of Decreased Canopy Probability at Edge
2.3.2. Tests of Light and Temperature Among Habitats
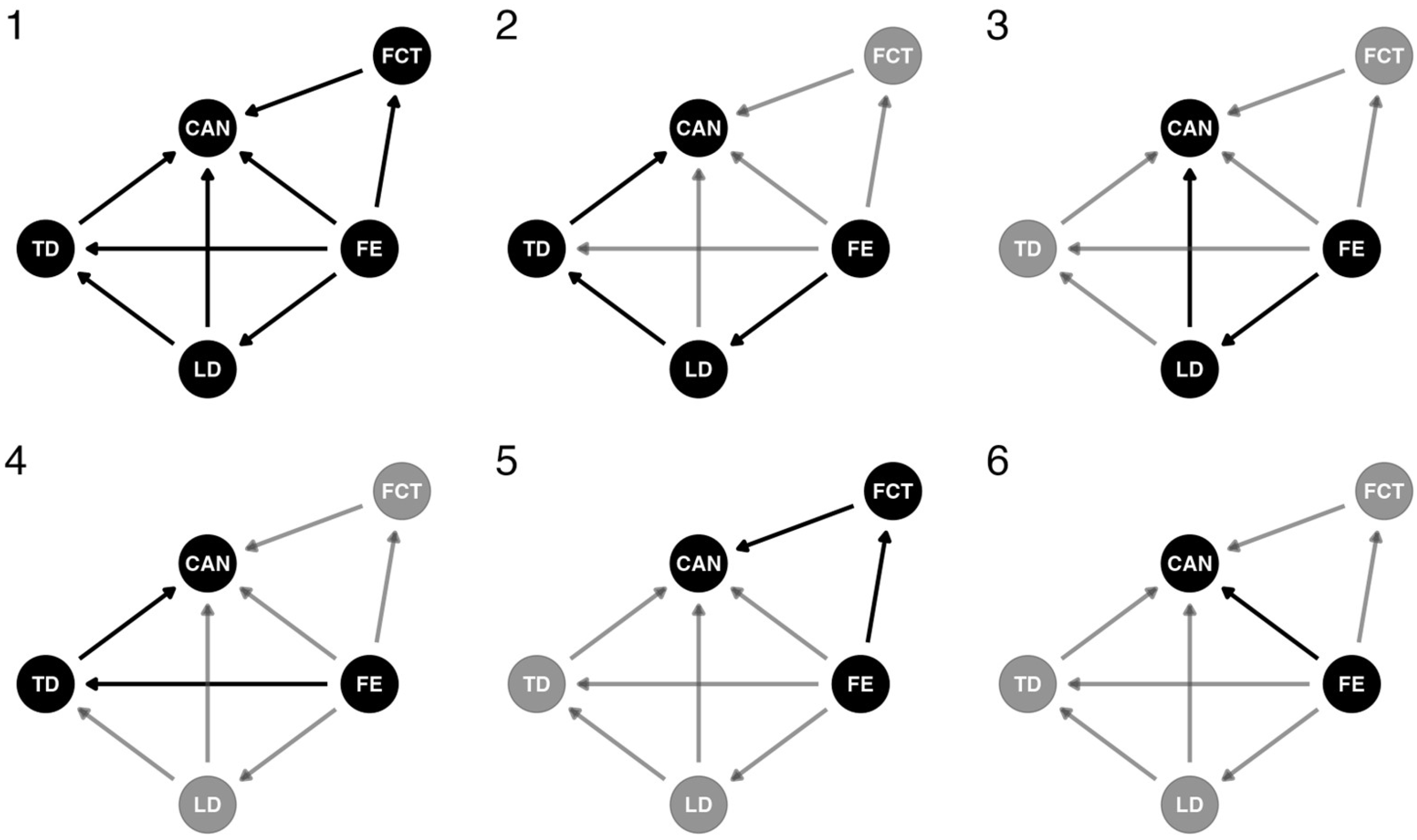
2.3.3. Bayesian Model—Definition of Causal Relationships
2.3.4. Bayesian Model—Causal Effect Definitions
2.3.5. Bayesian Model—Mediation Model Specification
2.3.6. Bayesian Model—Missing Data
2.3.7. Bayesian Model—Model Fitting
3. Results
3.1. Trapping Results, Light, and Temperature
3.1.1. Butterfly Observations
3.1.2. Temperature and Light Differences
3.2. Species Canopy Probability Changes
3.2.1. Changes in Species Canopy Probability at Forest Edges (G-Tests)
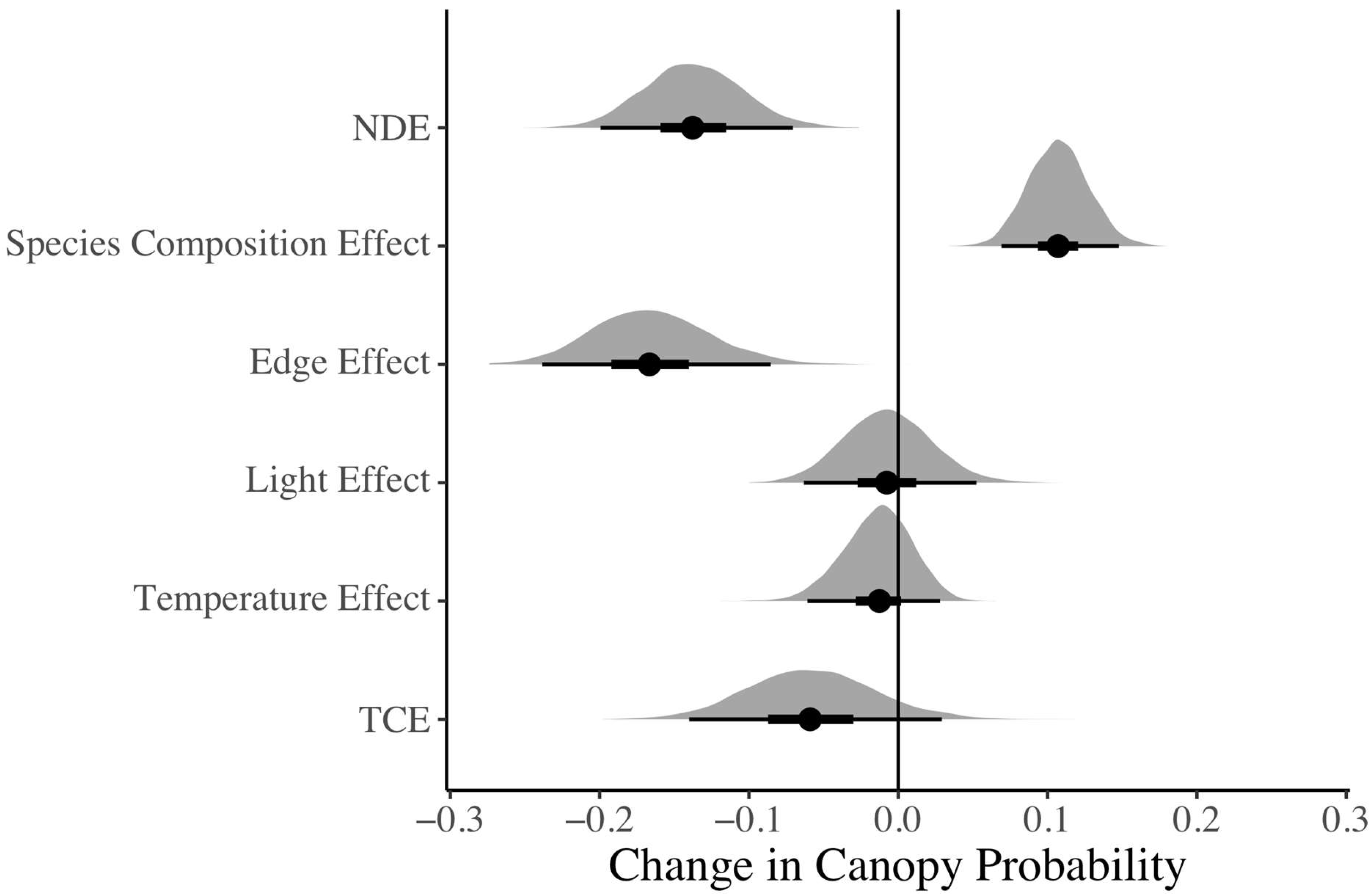
3.2.2. Forest Edge Causal Effects on Canopy Probability (Mediation Model)
4. Discussion
4.1. Edge Effect on Canopy Probabilty and Abiotic Variables
4.2. Edge Effect on Canopy Probability Is Not Explained by Light and Temperature Variation
4.3. Conservation Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pielke, R.A., Sr.; Pitman, A.; Niyogi, D.; Mahmood, R.; McAlpine, C.; Hossain, F.; Goldewijk, K.K.; Nair, U.; Betts, R.; Fall, S.; et al. Land use/land cover changes and climate: Modeling analysis and observational evidence. WIREs Clim. Change 2011, 2, 828–850. [Google Scholar] [CrossRef]
- Fagan, M.; Defries, R.; Sesnie, S.; Arroyo, P.; Walker, W.; Soto-Castro, C.; Chazdon, R.; Sanchun, A. Land cover dynamics following a deforestation ban in northern Costa Rica. Environ. Res. Lett. 2013, 8, 034017. [Google Scholar] [CrossRef]
- Bach, O. Agricultura e implicaciones ambientales con énfasis en algunas cuencas hidrográficas principales. Decimotercer Inf. Eatado de la Nación En Desarro. Hum. Sosten. 2007; 1–22. [Google Scholar]
- Martinelli, L.A.; Filoso, S. Expansion of sugarcane ethanol production in Brazil: Environmental and social challenges. Ecol. Appl. 2008, 18, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, H.K.; Ruesch, A.S.; Achard, F.; Clayton, M.K.; Holmgren, P.; Ramankutty, N.; Foley, J.A. Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc. Natl. Acad. Sci. USA 2010, 107, 16732–16737. [Google Scholar] [CrossRef]
- Bebbington, A.J.; Humphreys Bebbington, D.; Sauls, L.A.; Rogan, J.; Agrawal, S.; Gamboa, C.; Imhof, A.; Johnson, K.; Rosa, H.; Royo, A.; et al. Resource extraction and infrastructure threaten forest cover and community rights. Proc. Natl. Acad. Sci. USA 2018, 115, 13164–13173. [Google Scholar] [CrossRef]
- Wilson, M.; Chen, X.-Y.; Corlett, R.; Didham, R.; Ding, P.; Holt, R.; Holyoak, M.; Jiang, L.; Hu, G.; Hughes, A.; et al. Habitat fragmentation and biodiversity conservation: Key findings and future challenges. Landsc. Ecol. 2016, 31, 219–227. [Google Scholar] [CrossRef]
- Haddad, N.; Brudvig, L.; Clobert, J.; Davies, K.; Gonzalez, A.; Holt, R.; Lovejoy, T.; Sexton, J.; Austin, M.; Collins, C.; et al. Habitat fragmentation and its lasting impact on Earth ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Filgueiras, B.K.C.; Melo, D.H.A.; Leal, I.R.; Tabarelli, M.; Freitas, A.V.L.; Iannuzzi, L. Fruit-feeding butterflies in edge-dominated habitats: Community structure, species persistence and cascade effect. J. Insect Conserv. 2016, 20, 539–548. [Google Scholar] [CrossRef]
- Feer, F. Responses of dung beetle assemblages to characteristics of rain forest edges. Ecotropica 2008, 14, 49–62. [Google Scholar]
- Schneider-Maunoury, L.; Lefebvre, V.; Ewers, R.M.; Medina-Rangel, G.F.; Peres, C.A.; Somarriba, E.; Urbina-Cardona, N.; Pfeifer, M. Abundance signals of amphibians and reptiles indicate strong edge effects in Neotropical fragmented forest landscapes. Biol. Conserv. 2016, 200, 207–215. [Google Scholar] [CrossRef]
- Bolt, L.M.; Schreier, A.L.; Voss, K.A.; Sheehan, E.A.; Barrickman, N.L. Down by the riverside: Riparian edge effects on three monkey species in a fragmented Costa Rican forest. Biotropica 2020, 52, 541–553. [Google Scholar] [CrossRef]
- Murcia, C. Edge effects in fragmented forests: Implications for conservation. Trends Ecol. Evol. 1995, 10, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Horner-Devine, M.C.; Daily, G.C.; Ehrlich, P.R.; Boggs, C.L. Countryside biogeography of tropical butterflies. Conserv. Biol. 2003, 17, 168–177. [Google Scholar] [CrossRef]
- Wirth, R.; Meyer, S.T.; Leal, I.R.; Tabarelli, M. Plant Herbivore Interactions at the Forest Edge. In Progress in Botany; Lüttge, U., Beyschlag, W., Murata, J., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2008; pp. 423–448. [Google Scholar]
- DeVries, P.J. Stratification of fruit-feeding nymphalid butterflies in a Costa Rican rainforest. J. Res. Lepid. 1988, 26, 98–108. [Google Scholar]
- DeVries, P.J.; Walla, T.R.; Greeney, H. Species diversity in spatial and temporal dimensions of fruit-feeding butterflies from two Ecuadorian rainforests. Biol. J. Linn. Soc. 1999, 68, 333–353. [Google Scholar] [CrossRef]
- DeVries, P.J.; Walla, T.R. Species diversity and community structure in neotropical fruit-feeding butterflies. Biol. J. Linn. Soc. 2001, 74, 1–15. [Google Scholar] [CrossRef]
- Basset, Y.; Hammond, P.; Barrios, H.; Holloway, J.; Miller, S. Vertical stratification of arthropod assemblages. In Arthropods of Tropical Forests: Spatio-Temporal Dynamics and Resource Use in the Canopy; Cambridge Unversity Press: Cambridge, UK, 2003; pp. 17–27. [Google Scholar]
- Walla, T.R.; Engen, S.; DeVries, P.J.; Lande, R. Modeling vertical beta-diversity in tropical butterfly communities. Oikos 2004, 107, 610–618. [Google Scholar] [CrossRef]
- Fermon, H.; Waltert, M.; Vane-Wright, D.; Mühlenberg, M. Forest use and vertical stratification in fruit-feeding butterflies of Sulawesi, Indonesia: Impacts for conservation. Biodivers. Conserv. 2005, 14, 333–350. [Google Scholar] [CrossRef]
- Molleman, F.; Kop, A.; Brakefield, P.M.; DeVries, P.J.; Zwaan, B.J. Vertical and temporal patterns of biodiversity of fruit-feeding butterflies in a tropical forest in Uganda. Biodivers. Conserv. 2006, 15, 107–121. [Google Scholar] [CrossRef]
- Nice, C.C.; Fordyce, J.A.; Bell, K.L.; Forister, L.M.; Gompert, Z.; DeVries, P.J. Vertical differentiation in tropical forest butterflies: A novel mechanism generating insect diversity? Biol. Lett. 2019, 15, 20180723. [Google Scholar] [CrossRef]
- Fordyce, J.A.; DeVries, P.J. A tale of two communities: Neotropical butterfly assemblages show higher beta diversity in the canopy compared to the understory. Oecologia 2016, 181, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.B.; Pequeno, P.A.C.L.; Franklin, E.; Souza, J.L.P.; Morais, J.W. Taxonomic, functional, and phylogenetic perspectives on butterfly spatial assembly in northern Amazonia. Ecol. Entomol. 2017, 42, 816–826. [Google Scholar] [CrossRef]
- DeVries, P.J.; Alexander, L.G.; Chacon, I.A.; Fordyce, J.A. Similarity and difference among rainforest fruit-feeding butterfly communities in Central and South America. J. Anim. Ecol. 2012, 81, 472–482. [Google Scholar] [CrossRef] [PubMed]
- DeVries, P.J.; Murray, D.; Lande, R. Species diversity in vertical, horizontal, and temporal dimensions of a fruit-feeding butterfly community in an Ecuadorian rainforest. Biol. J. Linn. Soc. 1997, 62, 343–364. [Google Scholar] [CrossRef]
- Freitas, A.V.L.; Iserhard, C.A.; Santos, J.P.; CarreiraI, J.Y.O.; Ribeiro, D.B.; Melo, D.H.A.; Rosa, A.H.B.; Marini-filho, O.J.; Mattos Accacio, G.; Uehara-Prado, M. Studies with butterfly bait traps: An overview. Rev. Colomb. De Entomol. 2014, 40, 203–212. [Google Scholar]
- Austin, G.T.; Riley, T.J. Portable bait traps for the study of butterflies. Trop. Lepid. Res. 1995, 6, 5–9. [Google Scholar]
- Checa, M.F.; Rodriguez, J.; Willmott, K.R.; Liger, B. Microclimate variability significantly affects the composition, abundance and phenology of butterfly communities in a highly threatened neotropical dry forest. Fla. Entomol. 2014, 97, 1–13. [Google Scholar] [CrossRef]
- Checa, M.F.; Donoso, D.A.; Rodriguez, J.; Levy, E.; Warren, A.; Willmott, K.R. Combining sampling techniques aids monitoring of tropical butterflies. Insect Conserv. Divers. 2019, 12, 362–372. [Google Scholar] [CrossRef]
- Downes, J.A. Lepidoptera feeding at puddle-margins, dung, and carrion. J. Lepid. Soc. 1973, 27, 89–99. [Google Scholar]
- DeVries, P.J. The Butterflies of Costa Rica and Their Natural History. Vol I: Papilionidae, Pieridae, Nymphalidae; Princeton University Press: Princeton, NJ, USA, 1987; Volume I. [Google Scholar]
- Glassberg, J. A Swift Guide to the Butterflies of Mexico and Central America, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2007. [Google Scholar]
- Warren, A.D.; Davis, K.J.; Stangeland, E.M.; Pelham, J.P.; Willmott, K.R.; Grishin, N.V. Illustrated Lists of American Butterflies. Butterflies Am. Available online: https://www.butterfliesofamerica.com/ (accessed on 5 January 2025).
- Lamas, G.; Callaghan, C.; Casagrande, M.; Mielke, O.; Pyrcz, T.; Robbins, R.; Viloria, A. Checklist: Part 4A. Hesperioidea–Papilionoidea; Scientific Publishers: Gainesville, FL, USA, 2004; Volume 5. [Google Scholar]
- Gelman, A.; Carlin, J.; Stern, H.; Dunson, D.; Vehtari, A.; Rubin, D. Bayesian Data Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Clark, D.A.; Demb, J.B. Parallel computations in insect and mammalian visual motion processing. Curr. Biol. 2016, 26, R1062–R1072. [Google Scholar] [CrossRef]
- Laughlin, S.B. The role of sensory adaptation in the retina. J. Exp. Biol. 1989, 146, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.R.; Cole, W.H. The response of Popillia japonica to light and the Weber-Fechner Law. J. Gen. Physiol. 1921, 3, 331–335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing, R version 4.4.2; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wickham, H. ggplot2: Elegant graphics for data analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2016, 174, 245–246. [Google Scholar]
- Burchett, W.W.; Ellis, A.R.; Harrar, S.W.; Bathke, A.C. Nonparametric inference for multivariate data: The R Package npmv. J. Stat. Softw. 2017, 76, 1–18. [Google Scholar] [CrossRef]
- Liu, C.; Bathke, A.C.; Harrar, S.W. A nonparametric version of Wilks’ lambda—Asymptotic results and small sample approximations. Stat. Probab. Lett. 2011, 81, 1502–1506. [Google Scholar] [CrossRef]
- Richiardi, L.; Bellocco, R.; Zugna, D. Mediation analysis in epidemiology: Methods, interpretation and bias. Int. J. Epidemiol. 2013, 42, 1511–1519. [Google Scholar] [CrossRef]
- Pearl, J. An introduction to causal inference. Int. J. Biostat. 2010, 6, 7. [Google Scholar] [CrossRef]
- Pearl, J. Causal diagrams for empirical research. Biometrika 1995, 82, 669–688. [Google Scholar] [CrossRef]
- Pearl, J. The mediation formula: A guide to the assessment of causal pathways in nonlinear models. In Causality: Statistical Perspectives and Applications; Willy: Hoboken, NJ, USA, 2012; pp. 151–179. [Google Scholar]
- Imai, K.; Keele, L.; Tingley, D. A general approach to causal mediation analysis. Psychol. Methods 2010, 15, 309. [Google Scholar] [CrossRef]
- Daniel, R.M.; De Stavola, B.L.; Cousens, S.N.; Vansteelandt, S. Causal mediation analysis with multiple mediators. Biometrics 2015, 71, 1–14. [Google Scholar] [CrossRef]
- Belgorodski, N.; Greiner, M.; Tolksdorf, K.; Schueller, K. Rriskdistributions: Fitting Distributions to Given Data or Known Quantiles, R Package version 2.1.2; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Gabry, J.; Simpson, D.; Vehtari, A.; Betancourt, M.; Gelman, A. Visualization in Bayesian workflow. J. R. Stat. Soc. Ser. A (Stat. Soc.) 2019, 182, 389–402. [Google Scholar] [CrossRef]
- Kuhn, M. Caret: Classification and Regression Training, R Package version 6.0-86; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- van Buuren, S. Flexible Imputation of Missing Data; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Little, R.J.A.; Rubin, D.B. Statistical Analysis with Missing Data, 2nd ed.; John Wiley & Sons, Inc.: NewYork, NY, USA, 2002. [Google Scholar]
- Erler, N.S.; Rizopoulos, D.; Rosmalen, J.v.; Jaddoe, V.W.V.; Franco, O.H.; Lesaffre, E.M.E.H. Dealing with missing covariates in epidemiologic studies: A comparison between multiple imputation and a full Bayesian approach. Stat. Med. 2016, 35, 2955–2974. [Google Scholar] [CrossRef] [PubMed]
- Little, R.J.A. Regression with missing X’s: A review. J. Am. Stat. Assoc. 1992, 87, 1227–1237. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Donders, R.A.R.T.; Stijnen, T.; Harrell, F.E. Using the outcome for imputation of missing predictor values was preferred. J. Clin. Epidemiol. 2006, 59, 1092–1101. [Google Scholar] [CrossRef]
- Shumway, R.H.; Stoffer, D.S. Time Series Analysis and Its Applications: With R Examples; Springer Nature: London, UK, 2017; p. 558. [Google Scholar]
- McDade, L.; Bawa, K.; Hespenheide, H.; Hartshorn, G. La Selva: Ecology and Natural History of a Neotropical Rain Forest; Unversity of Chicago Press: Chicago, IL, USA, 1995; Volume 122. [Google Scholar]
- Dominoni, D.; Quetting, M.; Partecke, J. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123017. [Google Scholar] [CrossRef]
- Stan Development Team. The Stan Core Library, version 2.34.0; Stan Development Team: Los Angeles, CA, USA, 2024. [CrossRef]
- Stan Development Team. CmdStan: The Command-Line Interface to Stan, version 2.34.0; Stan Development Team: Los Angeles, CA, USA, 2024. [CrossRef]
- Nguyen, C.; Carlin, J.; Lee, K. Model checking in multiple imputation: An overview and case study. Emerg. Themes Epidemiol. 2017, 14, 1–12. [Google Scholar] [CrossRef]
- Cespedes, A.; Penz, C.M.; DeVries, P.J. Cruising the rain forest floor: Butterfly wing shape evolution and gliding in ground effect. J. Anim. Ecol. 2015, 84, 808–816. [Google Scholar] [CrossRef]
- Gueratto, P.E.; Carreira, J.Y.; Santos, J.P.; Tacioli, A.; Freitas, A.V. Effects of forest trails on the community structure of tropical butterflies. J. Insect Conserv. 2020, 24, 309–319. [Google Scholar] [CrossRef]
- Fetcher, N.; Oberbauer, S.; Strain, B. Vegetation effects on microclimate in lowland forest in Costa Rica. Int. J. Biometeorol. 1985, 29, 145–155. [Google Scholar] [CrossRef]
- Briscoe, A.D.; Chittka, L. The evolution of color vision in insects. Annu. Rev. Entomol. 2001, 46, 471–510. [Google Scholar] [CrossRef] [PubMed]
- Endler, J.A. The color of light in forests and its implications. Ecol. Monogr. 1993, 63, 1–27. [Google Scholar] [CrossRef]
- Chai, P.; Srygley, R.B. Predation and the flight, morphology, and temperature of Neotropical rain-forest butterflies. Am. Nat. 1990, 135, 748–765. [Google Scholar] [CrossRef]
- Peixoto, P.E.C.; Benson, W.W. Daily activity patterns of two co-occurring tropical satyrine butterflies. J. Insect Sci. 2009, 9, 54. [Google Scholar] [CrossRef]
- Pinheiro, C.; Cintra, R. Butterfly Predators in the Neotropics: Which Birds are Involved? J. Lepid. Soc. 2017, 71, 109–114. [Google Scholar] [CrossRef]
- Graça, M.B.; Pequeno, P.A.C.L.; Franklin, E.; Morais, J.W. Coevolution between flight morphology, vertical stratification and sexual dimorphism: What can we learn from tropical butterflies? J. Evol. Biol. 2017, 30, 1862–1871. [Google Scholar] [CrossRef]
- Beck, J.; Mühlenberg, E.; Fiedler, K. Mud-puddling behavior in tropical butterflies: In search of proteins or minerals? Oecologia 1999, 119, 140–148. [Google Scholar] [CrossRef]
- Boggs, C.L.; Dau, B. Resource specialization in puddling Lepidoptera. Environ. Entomol. 2004, 33, 1020–1024. [Google Scholar] [CrossRef]
- Kaspari, M.; Yanoviak, S.P.; Dudley, R. On the biogeography of salt limitation: A study of ant communities. Proc. Natl. Acad. Sci. USA 2008, 105, 17848–17851. [Google Scholar] [CrossRef]
- Daily, G.C.; Ceballos, G.; Pacheco, J.; Suzán, G.; Sánchez-Azofeifa, A. Countryside biogeography of neotropical mammals: Conservation opportunities in agricultural landscapes of Costa Rica. Conserv. Biol. 2003, 17, 1814–1826. [Google Scholar] [CrossRef]
- Melo, D.H.; Freitas, A.V.; Tabarelli, M.; Filgueiras, B.K.; Leal, I.R. Aridity and chronic anthropogenic disturbance as organizing forces of fruit-feeding butterfly assemblages in a Caatinga dry forest. Biotropica 2023, 55, 173–184. [Google Scholar] [CrossRef]
- Daily, G.C.; Ehrlich, P.R. Preservation of biodiversity in small rainforest patches: Rapid evaluations using butterfly trapping. Biodivers. Conserv. 1995, 4, 35–55. [Google Scholar] [CrossRef]
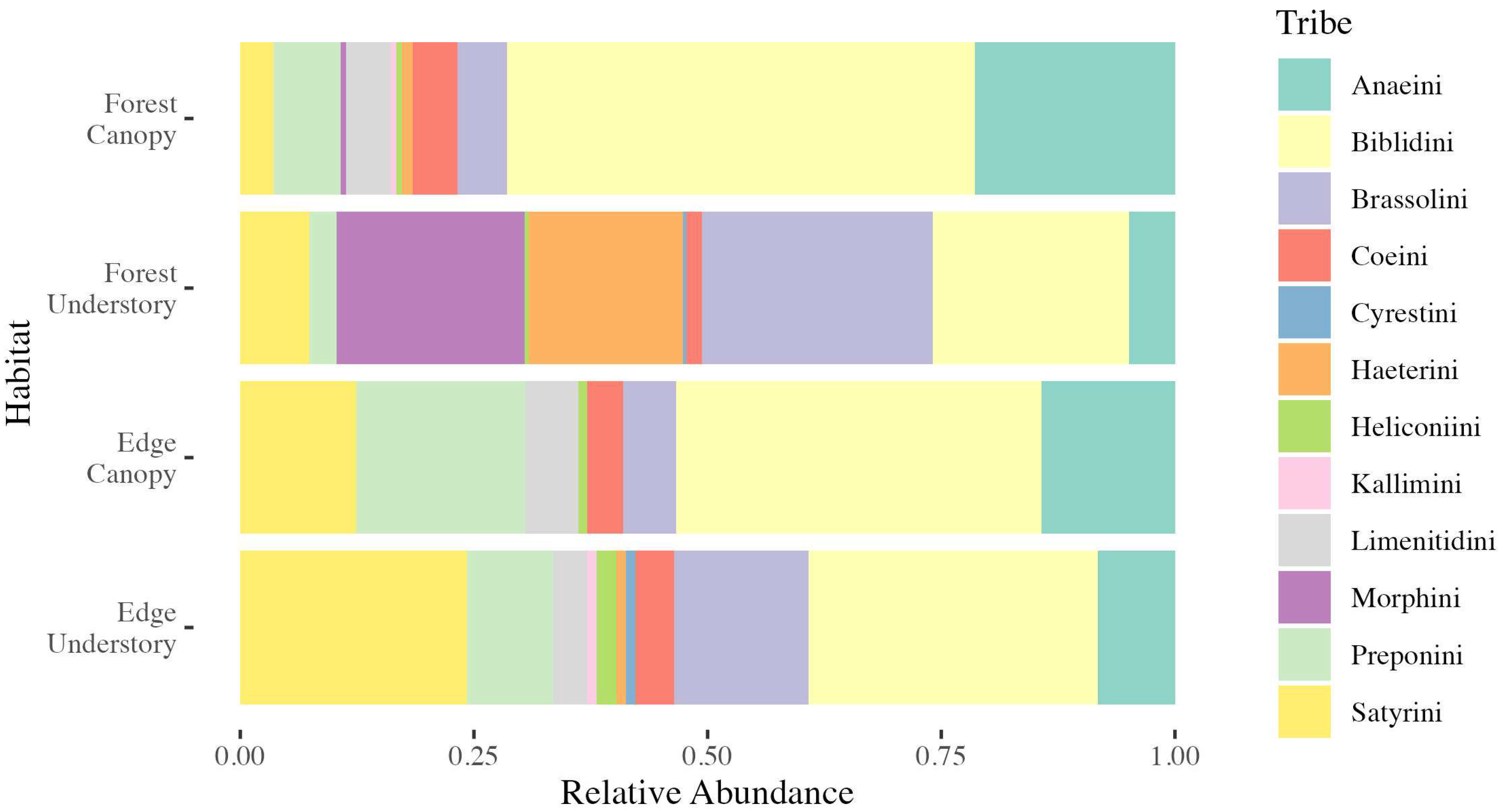

| FC | FU | EC | EU | |
|---|---|---|---|---|
| FC | - | |||
| FU | * | - | ||
| EC | NS | * | - | |
| EU | NS | * | NS | - |
| Species | EC | EU | FC | FU | Edge Canopy Probability | Forest Canopy Probability | Delta Edge |
|---|---|---|---|---|---|---|---|
| Adelpha iphiclus | 5 | 6 | 7 | 0 | 0.4545 | 1.0000 | 0.5455 |
| Adelpha naxia | 1 | 0 | 1 | 0 | 1.0000 | 1.0000 | 0.0000 |
| Archaeoprepona demophon | 2 | 14 | 1 | 3 | 0.1250 | 0.2500 | 0.1250 |
| Archaeoprepona demophoon | 1 | 0 | 2 | 0 | 1.0000 | 1.0000 | 0.0000 |
| Archaeoprepona meander | 0 | 2 | 0 | 1 | 0.0000 | 0.0000 | 0.0000 |
| Caligo atreus | 0 | 4 | 0 | 20 | 0.0000 | 0.0000 | 0.0000 |
| Caligo brasiliensis | 0 | 9 | 0 | 12 | 0.0000 | 0.0000 | 0.0000 |
| Catoblepia orgetorix | 0 | 2 | 1 | 24 | 0.0000 | 0.0400 | 0.0400 |
| Catonephele numilia | 4 | 17 | 4 | 2 | 0.1905 | 0.6667 | 0.4762 |
| Catonephele orites | 1 | 13 | 14 | 12 | 0.0714 | 0.5385 | 0.4670 |
| Cissia confusa | 1 | 1 | 3 | 2 | 0.5000 | 0.6000 | 0.1000 |
| Colobura annulata | 1 | 1 | 5 | 2 | 0.5000 | 0.7143 | 0.2143 |
| Dryas iulia | 0 | 4 | 1 | 0 | 0.0000 | 1.0000 | 1.0000 |
| Dulcedo polita | 0 | 2 | 1 | 26 | 0.0000 | 0.0370 | 0.0370 |
| Epiphile adrasta | 0 | 1 | 1 | 0 | 0.0000 | 1.0000 | 1.0000 |
| Eryphanis lycomedon | 0 | 7 | 0 | 2 | 0.0000 | 0.0000 | 0.0000 |
| Fountainea eurypyle | 0 | 1 | 1 | 0 | 0.0000 | 1.0000 | 1.0000 |
| Hamadryas amphinome | 4 | 0 | 4 | 0 | 1.0000 | 1.0000 | 0.0000 |
| Hamadryas arinome | 1 | 1 | 10 | 3 | 0.5000 | 0.7692 | 0.2692 |
| Hamadryas laodamia | 9 | 1 | 12 | 0 | 0.9000 | 1.0000 | 0.1000 |
| Historis odius | 2 | 2 | 2 | 0 | 0.5000 | 1.0000 | 0.5000 |
| Magneuptychia gomezi | 1 | 1 | 2 | 1 | 0.5000 | 0.6667 | 0.1667 |
| Megeuptychia antonoe | 1 | 0 | 0 | 1 | 1.0000 | 0.0000 | −1.0000 |
| Memphis artacaena | 1 | 1 | 2 | 0 | 0.5000 | 1.0000 | 0.5000 |
| Memphis cleomestra | 1 | 0 | 2 | 1 | 1.0000 | 0.6667 | −0.3333 |
| Memphis mora | 0 | 1 | 1 | 0 | 0.0000 | 1.0000 | 1.0000 |
| Memphis moruus | 11 | 3 | 3 | 0 | 0.7857 | 1.0000 | 0.2143 |
| Myscelia cyaniris | 3 | 14 | 0 | 1 | 0.1765 | 0.0000 | −0.1765 |
| Myscelia leucocyana | 6 | 2 | 5 | 0 | 0.7500 | 1.0000 | 0.2500 |
| Nessaea aglaura | 0 | 5 | 7 | 29 | 0.0000 | 0.1944 | 0.1944 |
| Nica flavilla | 1 | 0 | 1 | 0 | 1.0000 | 1.0000 | 0.0000 |
| Opsiphanes cassina | 6 | 0 | 4 | 1 | 1.0000 | 0.8000 | −0.2000 |
| Pareuptychia metaleuca | 0 | 5 | 1 | 3 | 0.0000 | 0.2500 | 0.2500 |
| Prepona laertes | 16 | 2 | 7 | 0 | 0.8889 | 1.0000 | 0.1111 |
| Pyrrhogyra neaerea | 0 | 1 | 5 | 1 | 0.0000 | 0.8333 | 0.8333 |
| Pyrrhogyra otolais | 1 | 1 | 2 | 0 | 0.5000 | 1.0000 | 0.5000 |
| Taygetis thamyra | 0 | 15 | 0 | 5 | 0.0000 | 0.0000 | 0.0000 |
| Temenis laothoe | 3 | 1 | 7 | 2 | 0.7500 | 0.7778 | 0.0278 |
| Tigridia acesta | 0 | 2 | 1 | 2 | 0.0000 | 0.3333 | 0.3333 |
| Zaretis isidora | 0 | 2 | 5 | 3 | 0.0000 | 0.6250 | 0.6250 |
| Zaretis itys | 0 | 3 | 7 | 7 | 0.0000 | 0.5000 | 0.5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oye, B.K.; Hill, R.I. Changes in Vertical Stratification of Neotropical Nymphalid Butterflies at Forest Edges Are Not Directly Caused by Light and Temperature Conditions. Insects 2025, 16, 64. https://doi.org/10.3390/insects16010064
Oye BK, Hill RI. Changes in Vertical Stratification of Neotropical Nymphalid Butterflies at Forest Edges Are Not Directly Caused by Light and Temperature Conditions. Insects. 2025; 16(1):64. https://doi.org/10.3390/insects16010064
Chicago/Turabian StyleOye, Brian K., and Ryan I. Hill. 2025. "Changes in Vertical Stratification of Neotropical Nymphalid Butterflies at Forest Edges Are Not Directly Caused by Light and Temperature Conditions" Insects 16, no. 1: 64. https://doi.org/10.3390/insects16010064
APA StyleOye, B. K., & Hill, R. I. (2025). Changes in Vertical Stratification of Neotropical Nymphalid Butterflies at Forest Edges Are Not Directly Caused by Light and Temperature Conditions. Insects, 16(1), 64. https://doi.org/10.3390/insects16010064






