Fat Body Metabolome Revealed Glutamine Metabolism Pathway Involved in Prepupal Apis mellifera Responding to Cold Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honeybee Capped Brood and Cold Treatment
2.2. Fat Body Metabolite Identification
2.3. Metabolite Data Process and Analysis
2.4. Measurement of Gene Expression Levels
2.5. Biochemical Assay
2.6. Data Analysis
3. Results
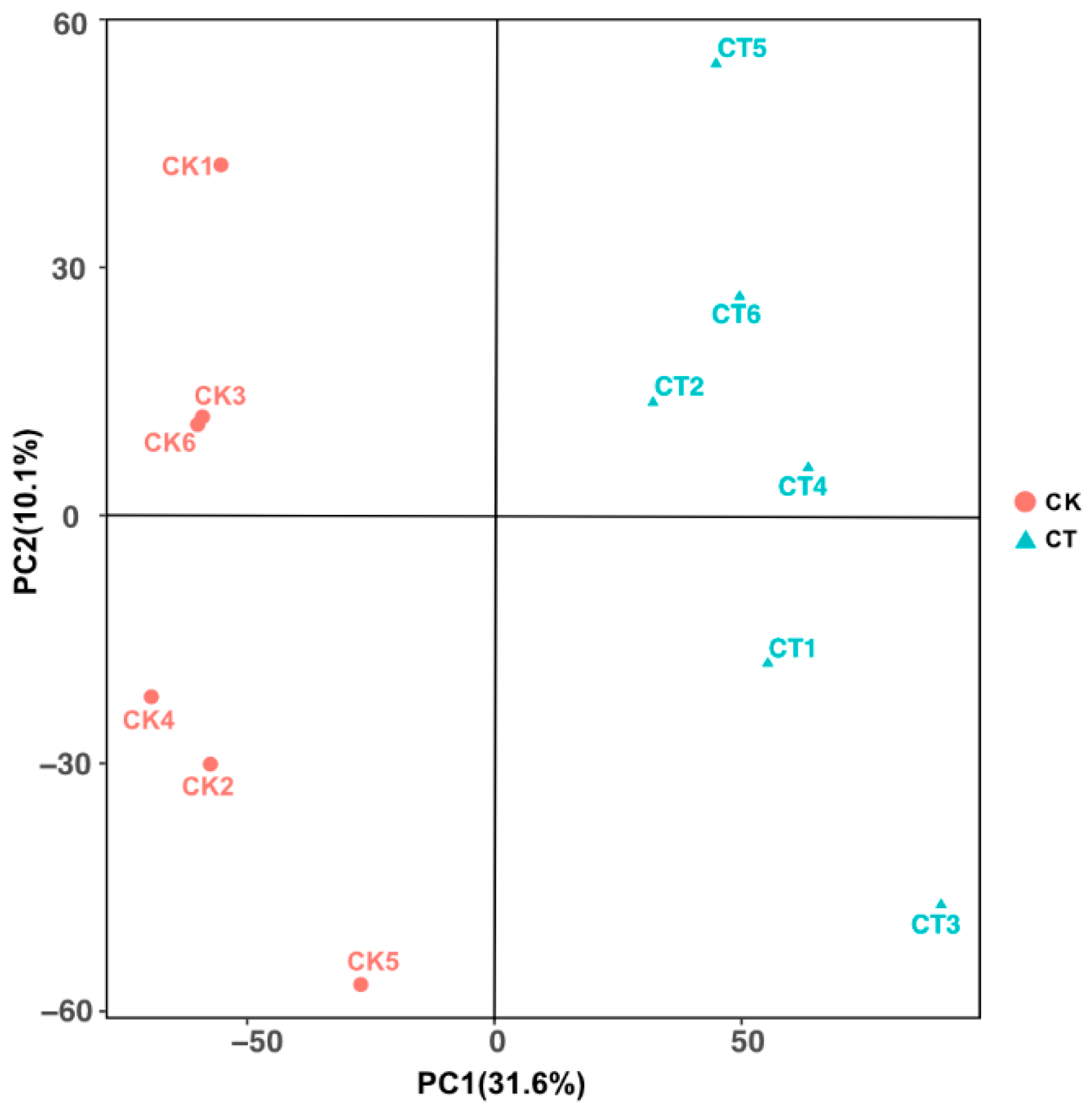
3.1. Data Assessment and Analysis of Significantly Differential Metabolites
3.2. Enrichment Analysis of Metabolites
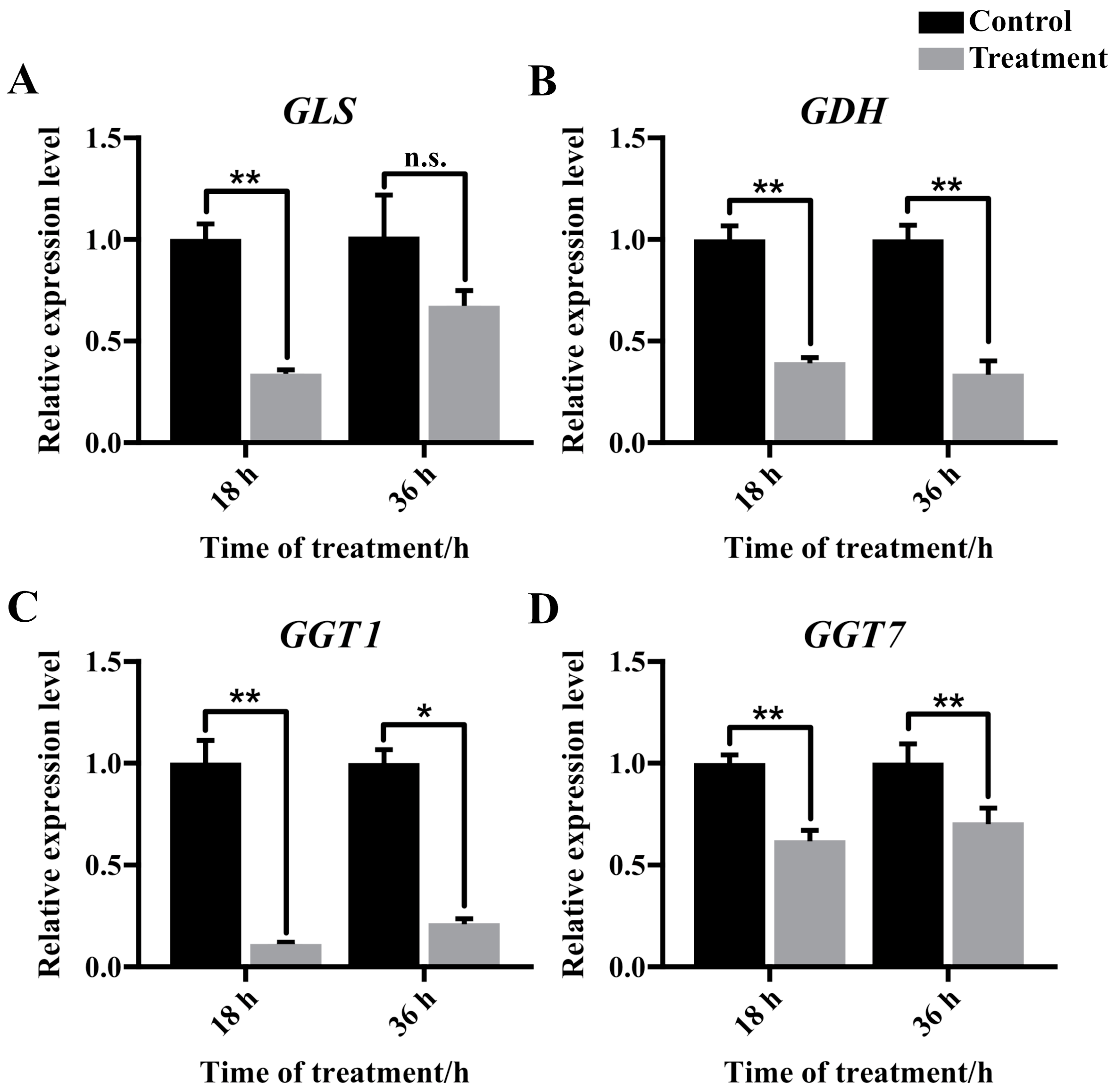
3.3. Gene Expression Quantification
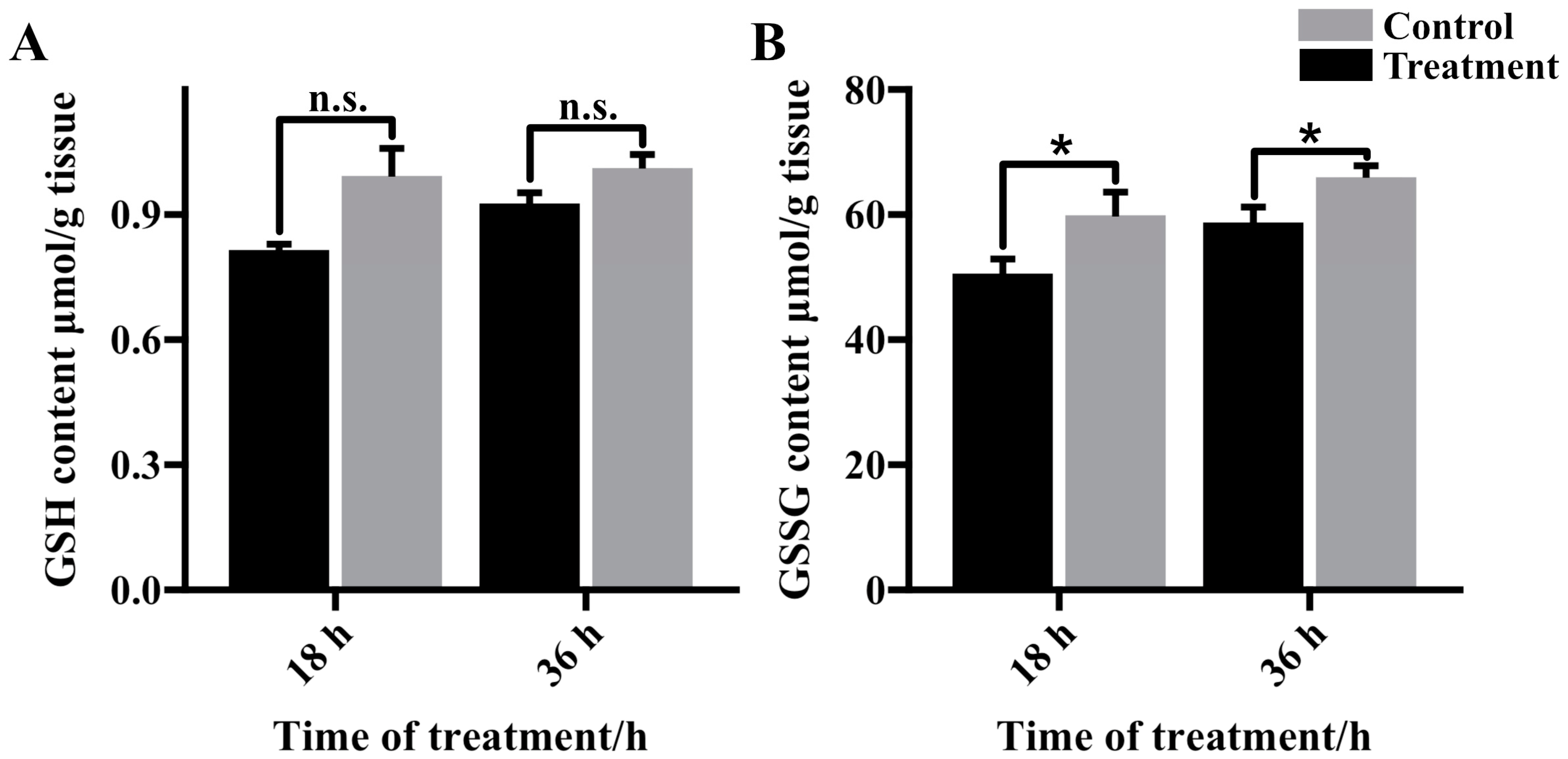
3.4. Glutathione Content in Cold Stressed Fat Body
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, K.; Ma, C.; Zhao, F.; Han, J. Effects of Large Temperature Fluctuations on Hatching and Subsequent Development of the Diamondback Moth (Lepidoptera: Plutellidae). Fla. Entomol. 2015, 98, 651–659. [Google Scholar] [CrossRef]
- Zimmerman, L.C.; Tracy, C.R. Interactions between the Environment and Ectothermy and Herbivory in Reptiles. Physiol. Zool. 1989, 62, 374–409. [Google Scholar] [CrossRef]
- Kleinhenz, M.; Bujok, B.; Fuchs, S.; Tautz, J. Hot Bees in Empty Broodnest Cells: Heating from Within. J. Exp. Biol. 2003, 206, 4217–4231. [Google Scholar] [CrossRef]
- Kovac, H.; Stabentheiner, A.; Brodschneider, R. Contribution of Honeybee Drones of Different Age to Colonial Thermoregulation. Apidologie 2009, 40, 82–95. [Google Scholar] [CrossRef]
- Seeley, T.D.; Visscher, P.K. Survival of Honeybees in Cold Climates: The Critical Timing of Colony Growth and Reproduction. Ecol. Entomol. 1985, 10, 81–88. [Google Scholar] [CrossRef]
- Stabentheiner, A.; Kovac, H.; Brodschneider, R. Honeybee Colony Thermoregulation—Regulatory Mechanisms and Contribution of Individuals in Dependence on Age, Location and Thermal Stress. PLoS ONE 2010, 5, e8967. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, H.; Xu, X.; Zhou, S.; Zhou, B.; Li, X.; Xu, H.; Tian, Y.; Wang, Y.; Chu, Y.; et al. The Mushroom Body Development and Learning Ability of Adult Honeybees Are Influenced by Cold Exposure during Their Early Pupal Stage. Front. Physiol. 2023, 14, 1173808. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, X.; Zhu, X.; Chen, L.; Zhou, S.; Huang, Z.Y.; Zhou, B. Low-Temperature Stress during Capped Brood Stage Increases Pupal Mortality, Misorientation and Adult Mortality in Honey Bees. PLoS ONE 2016, 11, e0154547. [Google Scholar] [CrossRef]
- Becher, M.A.; Scharpenberg, H.; Moritz, R.F.A. Pupal Developmental Temperature and Behavioral Specialization of Honeybee Workers (Apis mellifera L.). J. Comp. Physiol. A 2009, 195, 673–679. [Google Scholar] [CrossRef]
- Ramirez, L.; Negri, P.; Sturla, L.; Guida, L.; Vigliarolo, T.; Maggi, M.; Eguaras, M.; Zocchi, E.; Lamattina, L. Abscisic Acid Enhances Cold Tolerance in Honeybee Larvae. Proc. R. Soc. Biol. Sci. 2017, 284, 20162140. [Google Scholar] [CrossRef] [PubMed]
- Medrzycki, P.; Sgolastra, F.; Bortolotti, L.; Bogo, G.; Tosi, S.; Padovani, E.; Porrini, C.; Sabatini, A.G. Influence of Brood Rearing Temperature on Honey Bee Development and Susceptibility to Poisoning by Pesticides. J. Apic. Res. 2010, 49, 52–59. [Google Scholar] [CrossRef]
- Tautz, J.; Maier, S.; Groh, C.; Rössler, W.; Brockmann, A. Behavioral Performance in Adult Honey Bees Is Influenced by the Temperature Experienced during Their Pupal Development. Proc. Natl. Acad. Sci. USA 2003, 100, 7343–7347. [Google Scholar] [CrossRef]
- Groh, C.; Tautz, J.; Rössler, W. Synaptic Organization in the Adult Honey Bee Brain Is Influenced by Brood-Temperature Control during Pupal Development. Proc. Natl. Acad. Sci. USA 2004, 101, 4268–4273. [Google Scholar] [CrossRef]
- Jones, J.C.; Helliwell, P.; Beekman, M.; Maleszka, R.; Oldroyd, B.P. The Effects of Rearing Temperature on Developmental Stability and Learning and Memory in the Honey Bee, Apis mellifera. J. Comp. Physiol. A 2005, 191, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Nelliot, A.; Bond, N.; Hoshizaki, D.K. Fat-Body Remodeling in Drosophila Melanogaster. Genesis 2006, 44, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. Proliferation and Programmed Cell Death in the Fat Body in Workers of the Italian Honeybee (Apis mellifera ligustica) during Postembryonic Development. Acta Entomol. Sin. 2013, 56, 1252–1257. [Google Scholar] [CrossRef]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. Fat Body—Multifunctional Insect Tissue. Insects 2021, 12, 547. [Google Scholar] [CrossRef]
- Des Marteaux, L.E.; Štětina, T.; Koštál, V. Insect Fat Body Cell Morphology and Response to Cold Stress Is Modulated by Acclimation. J. Exp. Biol. 2018, 221, jeb189647. [Google Scholar] [CrossRef] [PubMed]
- Chowanski, S.; Lubawy, J.; Spochacz, M.; Ewelina, P.; Grzegorz, S.; Rosinski, G.; Slocinska, M. Cold Induced Changes in Lipid, Protein and Carbohydrate Levels in the Tropical Insect Gromphadorhina coquereliana. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 183, 57–63. [Google Scholar] [CrossRef]
- Chowański, S.; Lubawy, J.; Paluch-Lubawa, E.; Spochacz, M.; Rosiński, G.; Słocińska, M. The Physiological Role of Fat Body and Muscle Tissues in Response to Cold Stress in the Tropical Cockroach Gromphadorhina coquereliana. PLoS ONE 2017, 12, e0173100. [Google Scholar] [CrossRef]
- Wang, Y.; Brent, C.S.; Fennern, E.; Amdam, G.V. Gustatory Perception and Fat Body Energy Metabolism Are Jointly Affected by Vitellogenin and Juvenile Hormone in Honey Bees. PLoS Genet. 2012, 8, e1002779. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, S.; He, L.; Yao, K. The Antioxidative Function of Alpha-Ketoglutarate and Its Applications. BioMed Res. Int. 2018, 2018, e3408467. [Google Scholar] [CrossRef]
- Xiao, D.; Zeng, L.; Yao, K.; Kong, X.; Wu, G.; Yin, Y. The Glutamine-Alpha-Ketoglutarate (AKG) Metabolism and Its Nutritional Implications. Amino Acids 2016, 48, 2067–2080. [Google Scholar] [CrossRef]
- Hu, H.; Chen, L.; Dai, S.; Li, J.; Bai, X. Effect of Glutamine on Antioxidant Capacity and Lipid Peroxidation in the Breast Muscle of Heat-Stressed Broilers via Antioxidant Genes and HSP70 Pathway. Animals 2020, 10, 404. [Google Scholar] [CrossRef]
- Curthoys, N.P.; Watford, M. Regulation of Glutaminase Activity and Glutamine Metabolism. Annu. Rev. Nutr. 1995, 15, 133–159. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Pochini, L.; Galluccio, M.; Indiveri, C. Glutamine Transport. From Energy Supply to Sensing and Beyond. Biochim. Biophys. Acta (BBA) Bioenerg. 2016, 1857, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Jungreis, A.M.; Omilianowski, D.R. γ-Amino Butyric Acid and Glutamic Acid in Manduca Sexta: Proposed Roles in Insect Development. Comp. Biochem. Physiol. C Comp. Pharmacol. 1980, 67, 173–185. [Google Scholar] [CrossRef]
- Wang, X.-P.; Sun, S.-P.; Li, Y.-X.; Wang, L.; Dong, D.-J.; Wang, J.-X.; Zhao, X.-F. 20-Hydroxyecdysone Reprograms Amino Acid Metabolism to Support the Metamorphic Development of Helicoverpa Armigera. Cell Rep. 2023, 42, 112644. [Google Scholar] [CrossRef] [PubMed]
- Mackei, M.; Sebők, C.; Vöröházi, J.; Tráj, P.; Mackei, F.; Oláh, B.; Fébel, H.; Neogrády, Z.; Mátis, G. Detrimental Consequences of Tebuconazole on Redox Homeostasis and Fatty Acid Profile of Honeybee Brain. Insect Biochem. Mol. Biol. 2023, 159, 103990. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Loscalzo, J. Metabolic Responses to Reductive Stress. Antioxid. Redox Signal. 2020, 32, 1330–1347. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of Glutathione Synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Engelen, M.P.K.J.; Schols, A.M.W.J.; Does, J.D.; Deutz, N.E.P.; Wouters, E.F.M. Altered Glutamate Metabolism Is Associated with Reduced Muscle Glutathione Levels in Patients with Emphysema. Am. J. Respir. Crit. Care Med. 2000, 161, 98–103. [Google Scholar] [CrossRef]
- Mitrić, A.; Castellano, I. Targeting Gamma-Glutamyl Transpeptidase: A Pleiotropic Enzyme Involved in Glutathione Metabolism and in the Control of Redox Homeostasis. Free Radic. Biol. Med. 2023, 208, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, A.K.; Kaur, A. Glutathione Degradation. Antioxid. Redox Signal. 2017, 27, 1200–1216. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Gene ID | Forward Primer 5′-3′ | Reverse Primer 5′-3′ |
|---|---|---|---|
| β-actin | XM_623378 | TGCCAACACTGTCCTTTCTG | AGAATTGACCCACCAATCCA |
| GLS | LOC408991 | CGCCACTCGAGTCTATCCAC | ATTCATTCGCTTCGCGTTCG |
| GDH | LOC409253 | GAGCAGTCGGCAGTGACAAG | GTCATCCTGGAACGTTTGCC |
| GGT-1 | LOC551194 | GCGAGAAATGCGCAAACTCA | CTAGCATACGCGGAACTGGT |
| GGT-7 | LOC551194 | TGCTGAAGGAGGTCCACAGA | CCACCACCGAGACCAGTTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Cao, M.; Zhu, C.; Mo, L.; Huang, H.; Xie, J.; Zhou, B.; Zhou, S.; Zhu, X. Fat Body Metabolome Revealed Glutamine Metabolism Pathway Involved in Prepupal Apis mellifera Responding to Cold Stress. Insects 2025, 16, 37. https://doi.org/10.3390/insects16010037
Xu X, Cao M, Zhu C, Mo L, Huang H, Xie J, Zhou B, Zhou S, Zhu X. Fat Body Metabolome Revealed Glutamine Metabolism Pathway Involved in Prepupal Apis mellifera Responding to Cold Stress. Insects. 2025; 16(1):37. https://doi.org/10.3390/insects16010037
Chicago/Turabian StyleXu, Xinjian, Mingjie Cao, Chenyu Zhu, Lingqing Mo, Huajiao Huang, Jiaying Xie, Bingfeng Zhou, Shujing Zhou, and Xiangjie Zhu. 2025. "Fat Body Metabolome Revealed Glutamine Metabolism Pathway Involved in Prepupal Apis mellifera Responding to Cold Stress" Insects 16, no. 1: 37. https://doi.org/10.3390/insects16010037
APA StyleXu, X., Cao, M., Zhu, C., Mo, L., Huang, H., Xie, J., Zhou, B., Zhou, S., & Zhu, X. (2025). Fat Body Metabolome Revealed Glutamine Metabolism Pathway Involved in Prepupal Apis mellifera Responding to Cold Stress. Insects, 16(1), 37. https://doi.org/10.3390/insects16010037






