Bioactive Potential of Some Bacillus thuringiensis Strains from Macapá, Amazon, Brazil, Against the Housefly Musca domestica (Diptera: Muscidae) Under Laboratory Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. M. domestica Colonies
2.2. Bt Strains and Growth Conditions
2.3. Selective Bioassays on M. domestica
2.4. Data Analysis
2.5. DNA Extraction and Molecular Characterization
2.6. Scanning Electron Microscopy
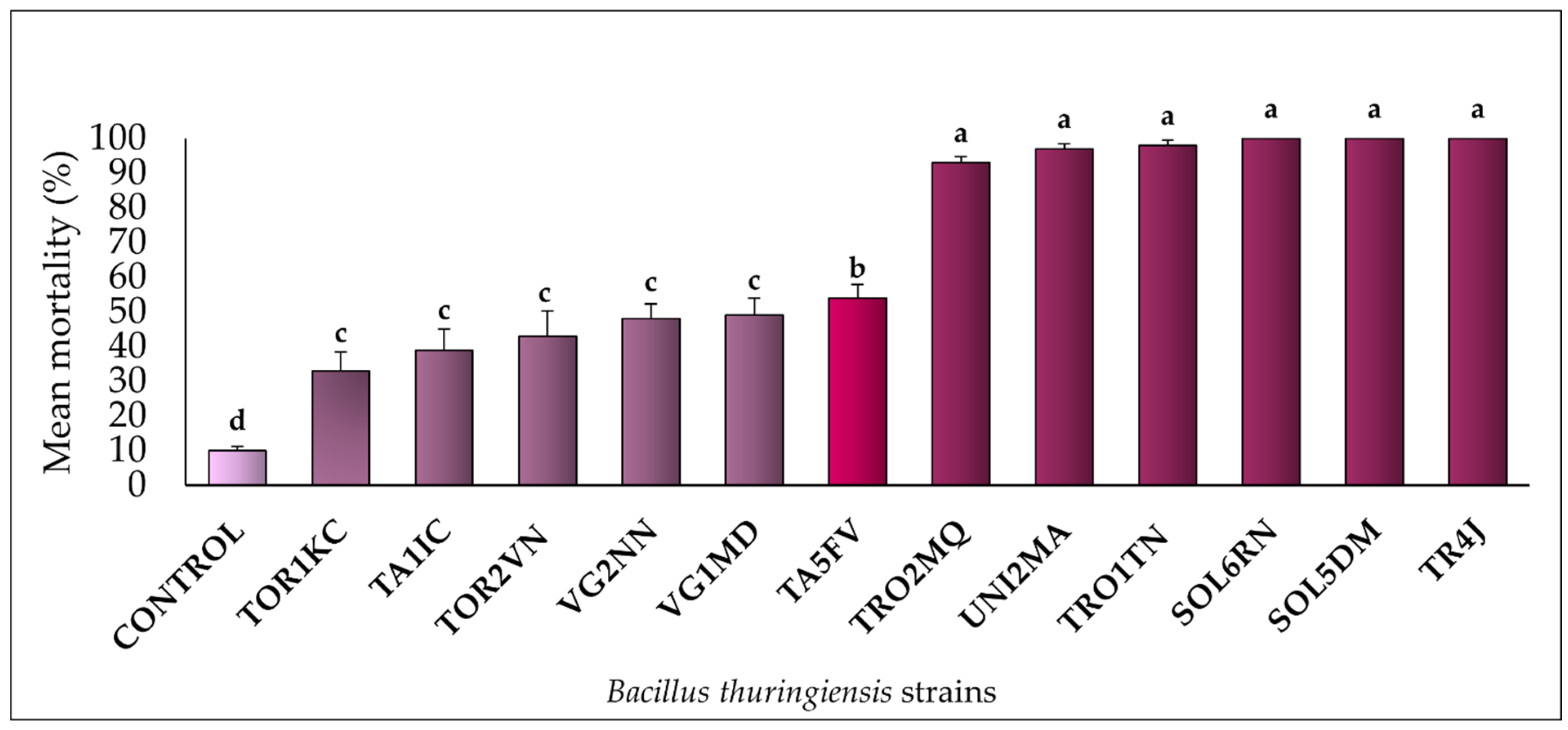
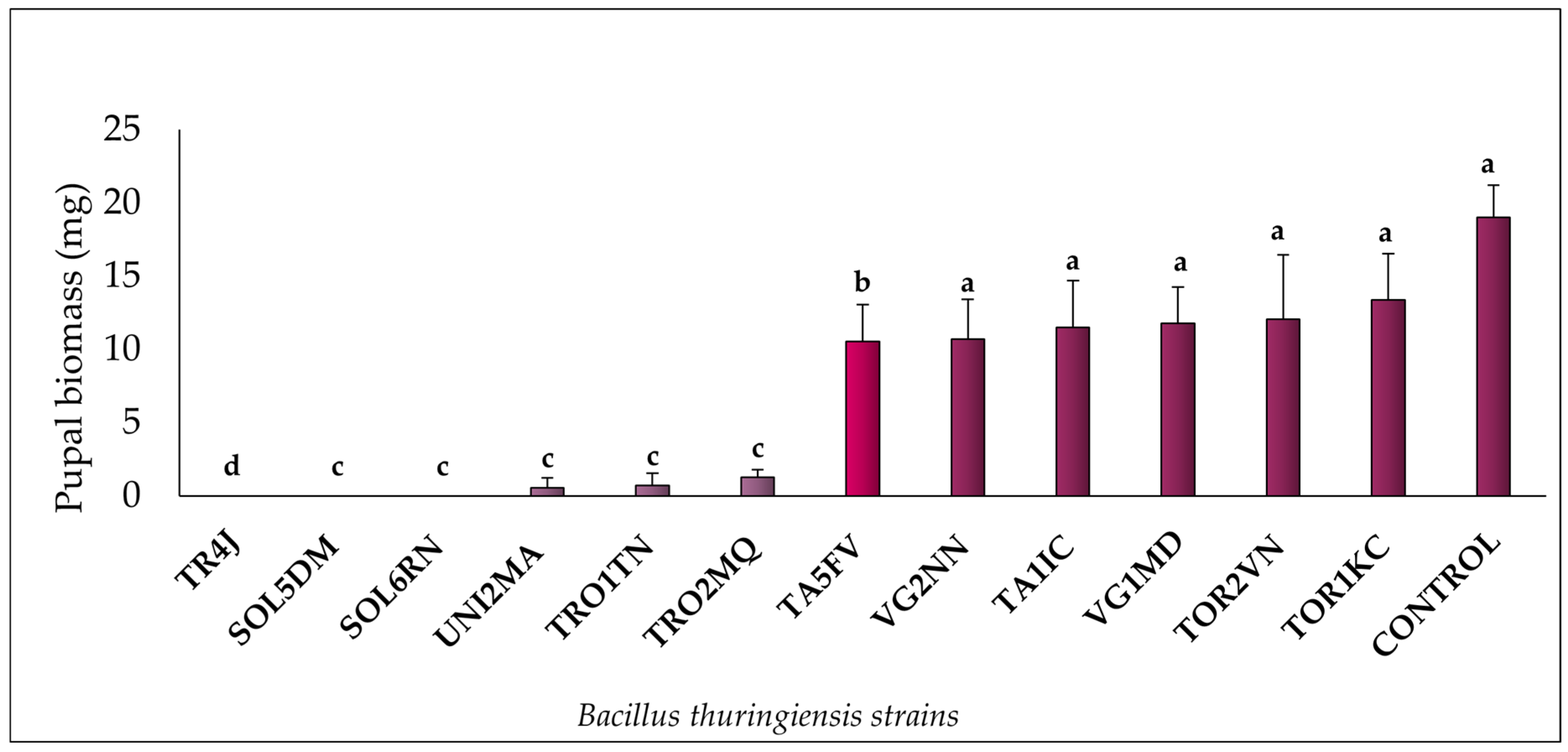
3. Results
3.1. Bt Virulence
3.2. Detection of cry, cyt, and vip Genes
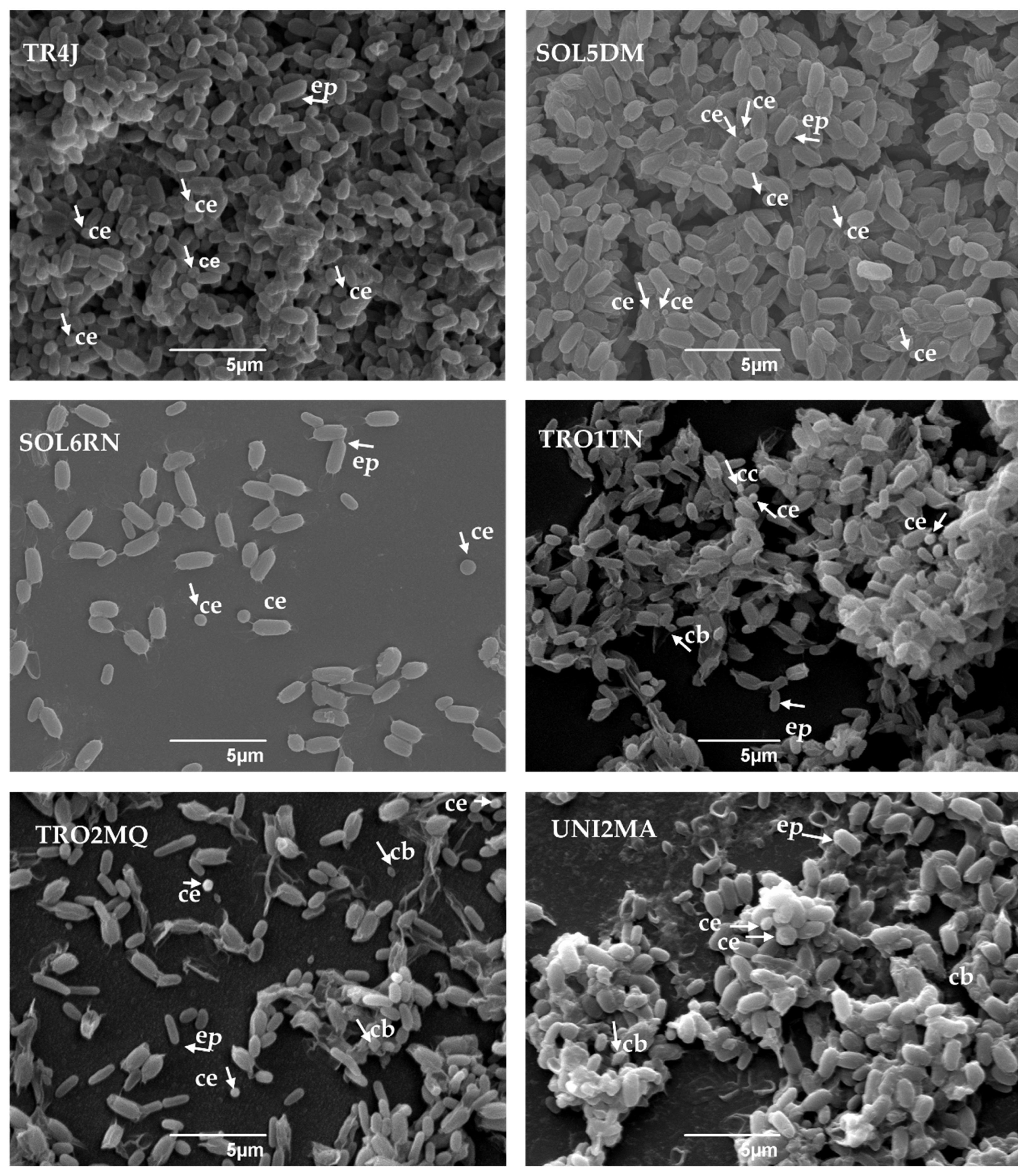
3.3. SEM Analysis of Crystalline Inclusions of Bt Strains
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Pitkin, A.; Deen, J.; Otake, S.; Moon, R.; Dee, S. Further assessment of houseflies Musca domestica as vectors for the mechanical transport and transmission of porcine reproductive and respiratory syndrome virus under field conditions. Can. J. Vet. Res. 2009, 73, 91–96. [Google Scholar] [PubMed]
- Baker, S.Z.; Atiyae, Q.M.; Khairallah, M.S. Isolation and Identification of some Species of Bacterial Pathogens from Musca Domestica and Test their Susceptibility Againts Antibiotics. Tikrit J. Pure Sci. 2018, 23, 20–27. [Google Scholar] [CrossRef]
- Dogra, S.S.; Mahajan, V.K. Oral myiasis caused by Musca domestica larvae in a child. Int. J. Pediatr. Otorhinolaryngol. 2010, 5, 105–107. [Google Scholar] [CrossRef]
- Salem, H.M.; Attia, M.M. Accidental intestinal myiasis caused by Musca domestica L. (Diptera: Muscidae) larvae in broiler chickens: A field study. Int. J. Trop. Insect Sci. 2021, 41, 2549–2554. [Google Scholar] [CrossRef]
- Abellán, M.; Ruano, F.; Rojo, S.; Martínez-Sánchez, A. Effect of two larval diets on the reproductive parameters of the housefly, Musca domestica L. (Diptera, Muscidae). J. Insects Food Feed 2023, 1, 1–14. [Google Scholar] [CrossRef]
- Couto, L.C.M.; Queiroz, M.M.C. Characterization of intrapuparial development of Musca domestica (Diptera: Muscidae), under different temperatures in laboratory conditions. J. Med. Entomol. 2024, 61, 64–73. [Google Scholar] [CrossRef]
- Greenberg, B. Flies and Disease: II. Biology and Disease Transmission; Princeton University Press: Princeton, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Moon, R.D. Muscid flies (muscidae). In Medical and Veterinary Entomology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–368. [Google Scholar] [CrossRef]
- Sasaki, T.; Kobayashi, M.; Agui, N. Epidemiological potential of excretion and regurgitation by Musca domestica (Diptera: Muscidae) in the dissemination of Escherichia coli O157: H7 to food. J. Med. Entomol. 2020, 37, 945–949. [Google Scholar] [CrossRef]
- Brito, L.G.; Oliveira, M.D.S.; Giglioti, R.; Barbieri, F.D.S.; Silva Neto, F.G.; Chagas, A.D.S.; Celestino, O.D.O. Manual de Identificação, Importância e Manutenção de Colônias Estoque de Dípteras de Interesse Veterinário em Laboratório; Documentos/Embrapa Rondonia; Embrapa Rondônia: Porto Velho, Brazil, 2008; 25p, ISSN 0103-9865. [Google Scholar]
- Nayduch, D.; Neupane, S.; Pickens, V.; Purvis, T.; Olds, C. House flies are underappreciated yet important reservoirs and vectors of microbial threats to animal and human health. Microorganisms 2023, 11, 583. [Google Scholar] [CrossRef]
- Carramaschi, I.N.; Castro, E.A.R.D.; Leite, J.A.; Queiroz, M.M.D.C.; Boas, M.H.S.V.; Rangel, K.; Zahner, V. First report of Raoultella ornithinolytica carrying blaKPC-2 isolated from a dipteran muscoid collected in a garbage from a public hospital in Rio de Janeiro, Brazil. Ver. Inst. Med. Trop. São Paulo 2019, 61, e32. [Google Scholar] [CrossRef]
- Carramaschi, I.N.; Lopes, J.C.O.; Leite, J.A.; Carneiro, M.T.; Barbosa, R.R.; Boas, M.H.V.; Zahner, V. Surveillance of antimicrobial resistant bacteria in flies (Diptera) in Rio de Janeiro city. Acta Trop. 2021, 220, 105962. [Google Scholar] [CrossRef]
- Abbas, M.N.; Sajeel, M.; Kausar, S. House fly (Musca domestica), a challenging pest; biology, management and control strategies. Elixir. Entomol. 2013, 64, 19333–19338. [Google Scholar]
- Young, B.W. The need for a greater understanding in the application of pesticides. Outlook Agric. 1996, 15, 80–87. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Sharma, A.; Dewali, S.; Kumari, R.; Mohapatra, A.; Cunill, J.M. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef] [PubMed]
- Achari, T.S.; Barik, T.K.; Acharya, U.R. Toxins of Bacillus thuringiensis: A Novel Microbial Insecticide for Mosquito Vector Control. In Molecular Identification of Mosquito Vectors and Their Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 89–116. [Google Scholar] [CrossRef]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberón, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef]
- Pinos, D.; Andrés-Garrido, A.; Ferré, J.; Hernández-Martínez, P. Response mechanisms of invertebrates to Bacillus thuringiensis and its pesticidal proteins. Microbiol. Mol. Biol. 2021, 85, 10-1128. [Google Scholar] [CrossRef]
- Estruch, J.J.; Warren, G.W.; Mullins, M.A.; Nye, G.J.; Craig, J.A.; Koziel, M.G. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc. Natl. Acad. Sci. USA 1996, 93, 5389–5394. [Google Scholar] [CrossRef]
- Chakroun, M.; Banyuls, N.; Bel, Y.; Escriche, B.; Ferré, J. Bacterial vegetative insecticidal proteins (Vip) from entomopathogenic bacteria. Microbiol. Mol. Biol. 2016, 80, 329–350. [Google Scholar] [CrossRef]
- Wang, C.; Li, M.; Chen, X.; Fan, S.; Lan, J. Binding Analysis of Sf-SR-C MAM Domain and Sf-FGFR Ectodomain to Vip3Aa. Insects 2024, 15, 428. [Google Scholar] [CrossRef]
- Pacheco, S.; Gómez, I.; Peláez-Aguilar, A.E.; Verduzco-Rosas, L.A.; García-Suárez, R.; do Nascimento, N.A.; Rivera-Nájera, L.Y.; Cantón, P.E.; Soberón, M.; Bravo, A. Structural changes upon membrane insertion of the insecticidal pore-forming toxins produced by Bacillus thuringiensis. Front. Insect Sci. 2023, 3, 1188891. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Mengist, H.M.; Shi, C.; Zhang, C.; Wang, B.; Li, T.; Huang, Y.; Xu, Y.; Jin, T. Structural basis of the pore-forming toxin/membrane interaction. Toxins 2021, 13, 128. [Google Scholar] [CrossRef]
- Chilcott, C.N.; Ellar, D.J. Comparative toxicity of Bacillus thuringiensis var. israelensis crystal proteins in vivo and in vitro. Microbiology 1988, 134, 2551–2558. [Google Scholar] [CrossRef] [PubMed]
- Guerchicoff, A.; Delécluse, A.; Rubinstein, C.P. The Bacillus thuringiensis cyt genes for hemolytic endotoxins constitute a gene family. Appl. Environ. Microbiol. 2001, 67, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, S.; Jin, M.; Wu, C.; Chakraborty, P.; Xiao, Y. Bacillus thuringiensis vegetative insecticidal protein family Vip3A and mode of action against pest Lepidoptera. Pest Manag. Sci. 2020, 76, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Şahin, B.; Gomis-Cebolla, J.; Güneş, H.; Ferré, J. Characterization of Bacillus thuringiensis isolates by their insecticidal activity and their production of Cry and Vip3 proteins. PLoS ONE 2018, 13, e0206813. [Google Scholar] [CrossRef]
- Höfte, H.; Whiteley, H.R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 1989, 53, 242–255. [Google Scholar] [CrossRef]
- Golding, N.; Wilson, A.L.; Moyes, C.L.; Cano, J.; Pigott, D.M.; Velayudhan, R.; Brooker, S.J.; Smith, D.L.; Hay, S.I.; Lindsay, S.W. Integrating vector control across diseases. BMC Med. 2015, 13, 249. [Google Scholar] [CrossRef]
- Rajagopal, G.; Ilango, S. Native Bacillus strains from infected insects: A potent bacterial agent for controlling mosquito vectors Aedes aegypti and Culex quinquefasciatus. Int. J. Mosq. Res. 2020, 7, 51–52. [Google Scholar]
- Valicente, F.H. Bacillus thuringiensis. In Natural Enemies of Insect Pests in Neotropical Agroecosystems; Souza, B., Vázquez, L., Marucci, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Queiroz, P.R.; Posso, M.C.; Martins, É.S.; Grynberg, P.; Togawa, R.; Monnerat, R.G. Identification of cry genes in Bacillus thuringiensis by multiplex real-time PCR. J. Microbiol. Methods 2023, 205, 106665. [Google Scholar] [CrossRef]
- Van Den Berg, H.; Takken, W. Viewpoint: A framework for decision-making in integrated vector management to prevent disease. Trop. Med. Int. Health 2007, 12, 1230–1238. [Google Scholar] [CrossRef]
- Li, K.; Chen, M.; Shi, J.; Mao, T. An overview of the production and use of Bacillus thuringiensis toxin. Open Life Sci. 2024, 19, 20220902. [Google Scholar] [CrossRef]
- Merdan, B.A. Bacillus thuringiensis as a feed additive to control Musca domestica associated with poultry houses. J. Basic Appl. Zool. 2012, 65, 83–87. [Google Scholar] [CrossRef]
- Nascimento, T.A.; Santos, M.J.P.; Valicente, F.H.; Dutok-Sánchez, C.M.; Queiroz, M.M.d.C. Desarrollo postembrionario de Musca domestica (Diptera: Muscidae) criada en diferentes dietas a base de harinas, en condiciones de laboratorio. Contrib. Cienc. Soc. 2024, 17, e8447. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência Agrotecnologia 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Ceron, J.; Ortíz, A.; Quintero, R.; Güereca, L.; Bravo, A. Specific PCR primers directed to identify cryI and cryIII genes within a Bacillus thuringiensis strain collection. Appl. Environ. Microbiol. 1995, 61, 3826–3831. [Google Scholar] [CrossRef]
- Valicente, F.H.; de Toledo Picoli, E.A.; de Vasconcelos, M.J.V.; Carneiro, N.P.; Carneiro, A.A.; Guimarães, C.T.; Lana, U.G. Molecular characterization and distribution of Bacillus thuringiensis cry1 genes from Brazilian strains effective against the fall armyworm, Spodoptera frugiperda. Biol. Control 2010, 53, 360–366. [Google Scholar] [CrossRef]
- Ceron, J.; Covarrubias, L.; Quintero, R.; Ortiz, A.; Ortiz, M.; Aranda, E.; Bravo, A. PCR Analysis of the cryI insecticidal crystal family genes from Bacillus thuringiensis. Appl. Environ. Microbiol. 1994, 60, 353–356. [Google Scholar] [CrossRef]
- Fagundes, R.B.S. Laboratório de Controle Biológico, Embrapa Milho e Sorgo, Sete Lagoas, Minas Gerais, Brazil. 2019; unpublished work. [Google Scholar]
- Valicente, F.H. Laboratório de Controle Biológico, Embrapa Milho e Sorgo, Sete Lagoas, Minas Gerais, Brazil. 2010; unpublished work. [Google Scholar]
- Ibarra, J.E.; del Rincón, M.C.; Ordúz, S.; Noriega, D.; Benintende, G.; Monnerat, R.; Bravo, A. Diversity of Bacillus thuringiensis strains from Latin America with insecticidal activity against different mosquito species. Appl. Environ. Microbiol. 2003, 69, 5269–5274. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, C.S.; Boets, A.; Van Rie, J.; Ferré, J. Screening and identification of vip genes in Bacillus thuringiensis strains. Appl. Environ. Microbiol. 2009, 107, 219–225. [Google Scholar] [CrossRef]
- Valicente, F.H.; Souza, I.R.P. Cultivo e preparo de Bacillus thuringiensis para macroscopia eletrônica de varredura. In Congresso Nacional de Milho e Sorgo, 25, Proceedings of the Simpósio Brasileiro Sobre a Lagarta-do-Cartucho, Spodoptera frugiperda; Embrapa Milho e Sorgo; Brazilian Association of Corn and Sorghum (ABMS): Sete Lagoas, Brazil; Empaer: Cuiabá, Brazil, 2004. [Google Scholar]
- Lambert, B.; Peferoen, M. Insecticidal promise of Bacillus thuringiensis. BioScience 1992, 42, 112–122. [Google Scholar] [CrossRef]
- Habib, M.E.M.; Andrade, C.F.S. Bactérias entomopatogênicas. Controle Microbiano Insetos 1998, 2, 383–446. [Google Scholar]
- Valicente, F.H.; Barreto, M.R. Bacillus thuringiensis survey in Brazil: Geographical distribution and insecticidal activity against Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2003, 32, 639–644. [Google Scholar] [CrossRef]
- Djenane, Z.; Nateche, F.; Amziane, M.; Gomis-Cebolla, J.; El-Aichar, F.; Khorf, H.; Ferré, J. Assessment of the Antimicrobial Activity and the Entomocidal Potential of Bacillus thuringiensis Isolates from Algeria. Toxins 2017, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Travers, R.S.; Martin, P.A.; Reichelderfer, C.F. Selective process for efficient isolation of soil Bacillus spp. Appl. Environ. Microbiol. 1987, 53, 1263–1266. [Google Scholar] [CrossRef]
- Uribe, D.; Martinez, W.; Ceron, J. Distribution and diversity of cry genes in native strains of Bacillus thuringiensis obtained from different ecosystems from Colombia. J. Invert. Pathol. 2003, 82, 119–127. [Google Scholar] [CrossRef]
- Mukhija, B.; Khanna, V. Isolation, characterization and crystal morphology study of Bacillus thuringiensis isolates from soils of Punjab. J. Pure Appl. Microbiol. 2018, 12, 189–193. [Google Scholar] [CrossRef]
- da Costa Fernandes, G.; de Prado Costa, D.K.; de Oliveira, N.S.; de Sousa, E.C.P.; Machado, D.H.B.; Polanczyk, R.A.; de Siqueira, H.Á.A.; da Silva, M.C. Genetic diversity of Brazilian Bacillus thuringiensis isolates with toxicity against Aedes aegypti (Diptera: Culicidae). Sci. Rep. 2022, 12, 14408. [Google Scholar] [CrossRef]
- Seifinejad, A.; Jouzani, G.S.; Hosseinzadeh, A.; Abdmishani, C. Characterization of Lepidoptera-active cry and vip genes in Iranian Bacillus thuringiensis strain collection. Biol. Control 2008, 44, 216–226. [Google Scholar] [CrossRef]
- Espinasse, S.; Chaufaux, J.; Buisson, C.; Perchat, S.; Gohar, M.; Bourguet, D.; Sanchis, V. Occurrence and Linkage Between Secreted Insecticidal Toxins in Natural Isolates of Bacillus thuringiensis. Curr. Microbiol. 2003, 47, 501–507. [Google Scholar] [CrossRef]
- Nascimento, T.A.; de Carvalho, K.S.; Martins, D.C.; de Oliveira, C.R.; de Carvalho Queiroz, M.M.; Valicente, F.H. Bacillus thuringiensis strains to control Noctuidae pests. J. Appl. Entomol. 2024, 148, 371–381. [Google Scholar] [CrossRef]
- Ben-Dov, E. Bacillus thuringiensis subsp. israelensis and Its Dipteran-Specific. Toxins 2014, 6, 1222–1243. [Google Scholar] [CrossRef]
- Hemthanon, T.; Promdonkoy, B.; Boonserm, P. Screening and characterization of Bacillus thuringiensis isolates for high production of Vip3A and Cry proteins and high thermostability to control Spodoptera spp. J. Invertebr. Pathol. 2023, 201, 108020. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, L.; Zhou, Z.; Pacheco, S.; Gomez, I.; Song, F.; Soberon, M.; Zhang, J.; Bravo, A. Specific binding between Bacillus thuringiensis Cry9Aa and Vip3Aa toxins synergizes their toxicity against Asiatic rice borer (Chilo suppressalis). J. Biol. Chem. 2018, 293, 11447–11458. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; He, J.; Wu, J.; Sun, J.; Wang, R.; Zhang, W. Vip3Aa from Bacillus thuringiensis subsp. kurstaki HD1 is toxic to Aedes aegypti (Diptera: Culicidae). J. Invertebr. Pathol. 2020, 171, 107342. [Google Scholar] [CrossRef] [PubMed]
- Boukedi, H.; Khedher, S.B.; Abdelkefi-Mesrati, L.; Van Rie, J.; Tounsi, S. Comparative analysis of the susceptibility/tolerance of Spodoptera littoralis to Vip3Aa, Vip3Ae, Vip3Ad and Vip3Af toxins of Bacillus thuringiensis. J. Invertebr. Pathol. 2018, 152, 30–34. [Google Scholar] [CrossRef]
- Bernardi, O.; Bernardi, D.; Horikoshi, R.J.; Okuma, D.M.; Miraldo, L.L.; Fatoretto, J.; Medeiros, F.C.; Burd, T.; Omoto, C. Selection and characterization of resistance to the Vip3Aa20 protein from Bacillus thuringiensis in Spodoptera frugiperda. Pest Manag. Sci. 2016, 72, 1794–1802. [Google Scholar] [CrossRef]
- Chen, D.; Moar, W.J.; Jerga, A.; Gowda, A.; Milligan, J.S.; Bretsynder, E.C.; Rydel, T.J.; Baum, J.A.; Semeao, A.; Fu, X.; et al. Bacillus thuringiensis chimeric proteins Cry1A.2 and Cry1B.2 to control soybean lepidopteran pests: New domain combinations enhance insecticidal spectrum of activity and novel receptor contributions. PLoS ONE 2021, 16, e0249150. [Google Scholar] [CrossRef]
- Federici, B.A.; Park, H.-W.; Sakano, Y. Insecticidal protein crystals of Bacillus thuringiensis. In Inclusions in Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2006; pp. 195–236. [Google Scholar] [CrossRef]
- Johnson, C.; Bishop, A.H.; Turner, C.L. Isolation and Activity of Strains of Bacillus thuringiensis Toxic to Larvae of the Housefly (Diptera: Muscidae) and Tropical Blowflies (Diptera: Calliphoridae). J. Invertebr. Pathol. 1998, 71, 138–144. [Google Scholar] [CrossRef]
- Abdul-Rauf, M.; Ellar, D.J. Toxicity and Receptor Binding Properties of aBacillus thuringiensis CryIC Toxin Active against Both Lepidoptera and Diptera. J. Invertebr. Pathol. 1999, 73, 52–58. [Google Scholar] [CrossRef]
- Valtierra-de-Luis, D.; Villanueva, M.; Berry, C.; Caballero, P. Potential for Bacillus thuringiensis and other bacterial toxins as biological control agents to combat dipteran pests of medical and agronomic importance. Toxins 2020, 12, 773. [Google Scholar] [CrossRef]
- Mendoza, G.; Portillo, A. New combinations of cry genes from Bacillus thuringiensis strains isolated from northwestern Mexico. Int. Microbiol. 2012, 15, 209–216. [Google Scholar] [CrossRef]
- Van Frankenhuyzen, K. Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 2009, 101, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, F.A.; Crickmore, N. N-terminal proteolysis determines the differential activity of Bacillus thuringiensis Cry2A toxins towards Aedes aegypti. J. Invertebr. Pathol. 2024, 204, 108100. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.-Z.; Xu, L.; Liu, B.; Chen, Q.-X.; Zhu, Y.-J. Key residues of Bacillus thuringiensis Cry2Ab for oligomerization and pore-formation activity. AMB Express 2011, 11, 112. [Google Scholar] [CrossRef]
- Polanczyk, R.A.; van Frankenhuyzen, K.; Pauli, G. The American Bacillus thuringiensis based biopesticides market. In Bacillus thuringiensis and Lysinibacillus sphaericus: Characterization and Use in the Field of Biocontrole; Fiuza, L., Polanczyk, R., Crickmore, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 173–184. [Google Scholar] [CrossRef]
- Raymond, B.; Johnston, P.R.; Nielsen-LeRoux, C.; Lereclus, D.; Crickmore, N. Bacillus thuringiensis: An impotent pathogen? Trends Microbiol. 2010, 18, 189–194. [Google Scholar] [CrossRef]
- Costa, M.L.; Lana, U.G.; Barros, E.C.; Paiva, L.V.; Valicente, F.H. Molecular characterization of Bacillus thuringiensis cyt genes efficient against fall armyworm, Spodoptera frugiperda. J. Agric. Sci. 2014, 6, 1–10. [Google Scholar] [CrossRef][Green Version]
- Vilas-Bôas, G.T.; Lemos, M.V.F. Diversity of cry genes and genetic characterization of Bacillus thuringiensis isolated from Brazil. Can. J. Microbiol. 2004, 50, 605–613. [Google Scholar] [CrossRef]
- Porcar, M.; Juárez-Pérez, V. PCR-based identification of Bacillus thuringiensis pesticidal crystal genes. FEMS Microbiol. Rev. 2003, 26, 419–432. [Google Scholar] [CrossRef]
- Agaisse, H.; Lereclus, D. How does Bacillus thuringiensis produce so much insecticidal crystal protein? J. Bacteriol. 1995, 177, 6027–6032. [Google Scholar] [CrossRef]
- Nair, K.; Al-Thani, R.; Ahmed, T.; Jaoua, S. Diversity of Bacillus thuringiensis strains from Qatar as shown by crystal morphology, δ-endotoxins and cry gene content. Front. Microbiol. 2018, 9, 339313. [Google Scholar] [CrossRef]
- Djenane, Z.; Lázaro-Berenguer, M.; Nateche, F.; Ferré, J. Evaluation of the toxicity of supernatant cultures and spore–crystal mixtures of Bacillus thuringiensis strains isolated from Algeria. Curr. Microbiol. 2020, 77, 2904–2914. [Google Scholar] [CrossRef]
- Yamamoto, T.; McLaughlin, R.E. Isolation of a protein from the parasporal crystal of Bacillus thuringiensis var kurstaki toxic to the mosquito larva, Aedes taeniorhynchus. Biochem. Biophys. Res. Commun. 1981, 103, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Sarabia, S.; Lopez, L.; Ontiveros, H.; Abarca, C.; Ortiz, A.; Ortiz, M.; Lina, L.; Villalobos, F.J.; Peña, G.; et al. Characterization of cry genes in a Mexican Bacillus thuringiensis strain collection. Appl. Environ. Microbiol. 1998, 64, 4965–4972. [Google Scholar] [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef]
- Loutfi, H.; Fayad, N.; Pellen, F.; Le Jeune, B.; Chakroun, M.; Benfarhat, D.; Lteif, R.; Kallassy, M.; Le Brun, G.; Abboud, M. Morphological study of Bacillus thuringiensis crystals and spores. Appl. Sci. 2020, 11, 155. [Google Scholar] [CrossRef]
- Gitahy, P.D.M.; Souza, M.T.D.; Monnerat, R.G.; Arrigoni, E.D.B.; Baldani, J.I. A Brazilian Bacillus thuringiensis strain highly active to sugarcane borer Diatraea saccharalis (Lepidoptera: Crambidae). Braz. J. Microbiol. 2007, 38, 531–537. [Google Scholar] [CrossRef]
- Arsov, A.; Gerginova, M.; Paunova-Krasteva, T.; Petrov, K.; Petrova, P. Multiple cry genes in Bacillus thuringiensis strain BTG suggest a broad-spectrum insecticidal activity. Int. J. Mol. Sci. 2023, 24, 11137. [Google Scholar] [CrossRef]
- Scott, J.G.; Warren, W.C.; Beukeboom, L.W.; Bopp, D.; Clark, A.G.; Giers, S.D.; Hediger, M.; Jones, A.K.; Kasai, S.; Leichter, C.A.; et al. Genome of the house fly, Musca domestica L., a global vector of diseases with adaptations to a septic environment. Genome Biol. 2014, 15, 466. [Google Scholar] [CrossRef]
- Geden, C.J.; Nayduch, D.; Scott, J.G.; Burgess, E.R., IV; Gerry, A.C.; Kaufman, P.E.; Machtinger, E.T. House fly (Diptera: Muscidae): Biology, pest status, current management prospects, and research needs. J. Integr. Pest Manag. 2021, 12, 39. [Google Scholar] [CrossRef]
- Soares-da-Silva, J.; Pinheiro, V.C.S.; Litaiff-Abreu, E.; Polanczyk, R.A.; Tadei, W.P. Isolation of Bacillus thuringiensis from the state of Amazonas, in Brazil, and screening against Aedes aegypti (Diptera, Culicidae). Rev. Bras. Entomol. 2015, 59, 1–6. [Google Scholar] [CrossRef]
- Šebesta, K.; Horska, K.; Vaňková, J. Inhibition of de novo RNA synthesis by the insecticidal exotoxin of Bacillus thuringiensis var. gelechiae. Collect. Czechoslov. Chem. Commun. 1969, 34, 1786–1791. [Google Scholar] [CrossRef]
- Gowelo, S.; Chirombo, J.; Spitzen, J.; Koenraadt, C.J.; Mzilahowa, T.; Van Den Berg, H.; Takken, W.; McCann, R. Effects of larval exposure to sublethal doses of Bacillus thuringiensis var. israelensis on body size, oviposition and survival of adult Anopheles coluzzii mosquitoes. Parasites Vectors 2020, 13, 259. [Google Scholar] [CrossRef]
- Iqbal, H.; Fatima, A.; Khan, H.A.A. ZnO nanoparticles produced in the culture supernatant of Bacillus thuringiensis ser. israelensis affect the demographic parameters of Musca domestica using the age-stage, two-sex life table. Pest Manag. 2022, 78, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Hanski, I. Carrion fly community dynamics: Patchiness, seasonality and coexistence. Ecol. Entomol. 1987, 12, 257–266. [Google Scholar] [CrossRef]
- Davidowitz, G.; D’Amico, L.J.; Nijhout, H.F. Critical weight in the development of insect body size. Evol. Dev. 2003, 5, 188–197. [Google Scholar] [CrossRef]
- Chapman, R.F.; Simpson, S.J.; Douglas, A.E. The Insects: Structure and Function, 5th ed.; Cambridge University Press: New York, NY, USA, 2013; ISBN 978-0-521-11389-2. [Google Scholar]
- Ullyett, G. Pupation habits of sheep blowflies in relation to parasitism by Mormoniella vitripennis, Wlk.(Hym., Pteromalid.). Bull. Entomol. Res. 1950, 40, 533–537. [Google Scholar] [CrossRef]
- Goodbrod, J.R.; Goff, M.L. Effects of larval population density on rates of development and interactions between two species of Chrysomya (Diptera: Calliphoridae) in laboratory culture. J. Med. Entomol. 1990, 27, 338–343. [Google Scholar] [CrossRef]
- Panizzi, A.R.; Parra, J.R.P.P. Insect Bioecology and nutrition for integrated pest management. Fla. Entomol. 2013, 96, 298–299. [Google Scholar]
- Miranda, C.D.; Cammack, J.A.; Tomberlin, J.K. Large-scale production of house fly, Musca domestica (Diptera: Muscidae), larvae fed 3 manure types. J. Econ. Entomol. 2023, 116, 1102–1109. [Google Scholar] [CrossRef]
- Fisher, R. The evolution of dominance in certain polymorphic species. Am. Nat. 1930, 64, 385–406. [Google Scholar] [CrossRef]
- Norris, K. The bionomics of blow flies. Annu. Rev. Entomol. 1965, 10, 47–68. [Google Scholar] [CrossRef]
- Oliveira, E.M.D.; Sales, V.H.G.; Andrade, M.S.; Zilli, J.É.; Borges, W.L.; Souza, T.M.D. Isolation and Characterization of Biosurfactant-Producing Bacteria from Amapaense Amazon Soils. Intern. J. Microbiol. 2021, 2021, 9959550. [Google Scholar] [CrossRef]


| Strain | Substrate | Region | Coordinates |
|---|---|---|---|
| TRO1TN | Dead tree trunk | Comunidade Areal do Matapí—AP | 0°15′17″ N; 51°09′57″ W |
| TRO2MQ | Dead tree trunk | Comunidade Areal do Matapí—AP | 0°15′17″ N; 51°09′57″ W |
| TA5FV | Spider web inside a tree trunk | Comunidade Areal do Matapí—AP | 0°15′18″ N; 51°09′54″ W |
| TA1IC | Spider web inside a tree trunk | Comunidade Areal do Matapí—AP | 0°15′18″ N; 51°09′54″ W |
| VG1MD | Organic matter (leaves) | Comunidade Areal do Matapí—AP | 0°15′18″ N; 51°09′57″ W |
| VG2NN | Organic matter (leaves) | Comunidade Areal do Matapí—AP | 0°15′18″ N; 51°09′57″ W |
| TOR1KC | Roadside soil | Comunidade Torrão do Matapí—AP | 0°14′09″ N; 51°11′03″ W |
| TOR2VN | Roadside soil | Comunidade Torrão do Matapí—AP | 0°14′09″ N; 51°11′03″ W |
| UNI2MA | Forest remnant | Universidade Federal do Amapá—AP | 0°00′26″ S; 51°05′02″ W |
| TR4J | Spider web near the Matapí River | Comunidade Areal do Matapí—AP | 0°15′18″ N; 51°09′56″ W |
| SOL5DM | Soil close to the Matapí River | Comunidade Areal do Matapí—AP | 0°15′29″ N; 51°10′00″ W |
| SOL6RN | Soil close to the Matapí River | Comunidade Areal do Matapí—AP | 0°15′18″ N; 51°09′56″ W |
| Target Genes | Primers Sequences (5′-3′) | Tm (°C) | Fragment Size (bp) | Reference |
|---|---|---|---|---|
| cry1Aa | TGTAGAAGAGGAAGTCTATCCA | 53 | 272 | Cerón et al. [39] |
| TATCGGTTTCTGGGAAGTA | ||||
| cry1Ab | CGCCACAGGACCTCTTAT | 55 | 232 | Valicente et al. [40] |
| TGCACAACCACCTGACCCA | ||||
| cry1B | CTTCATCACGATGGAGTAA | 55 | 367 | Cerón et al. [41] |
| CATAATTTGGTCGTTCTGTT | ||||
| cry1C | AAAGATCTGGAACACCTTT | 58 | 130 | Cerón et al. [41] |
| CAAACTCTAAATCCTTTCAC | ||||
| cry1D | CTGCAGCAAGCTATCCAA | 55 | 290 | Cerón et al. [39] |
| ATTTGAATTGTCAAGGCCTG | ||||
| cry1Ea | GGAACCAAGACGAACTATTGC | 56 | 147 | Cerón et al. [39] |
| GGTTGAATGAACCCTACTCCC | ||||
| cry1G | ATATGGAGTGAATAGGGCG | 55 | 235 | Cerón et al. [39] |
| TGAACGGCGATTACATGC | ||||
| cry9A | CATAATAGGCGATGCAGCAA | 53 | 395 | Fagundes [42] * |
| CTAACGAGCCACCATTCGTT | ||||
| cry9B | TCATTGGTATAAGAGTTGGTGATAGAC | 60 | 402 | Valicente [43] * |
| CCGCTTCCAATAACATCTTTT | ||||
| cry2Aa | GGGGCGACTAATCTCAATCA | 53 | 318 | Fagundes [42] * |
| AGGTGTTCCCGAAGGACTTT | ||||
| cry2Ac | ACAGCAGTCGCTAGCCTTGT | 55 | 475 | Fagundes [42] * |
| CAAATTGTGGATTGCCGTTA | ||||
| cry2Ad | ACGATATCGCCACCTTTGTC | 53 | 282 | Fagundes [42] * |
| AGGTGTTCCTGAAGGGCTTT | ||||
| cyt1 | CCTCAATCAACAGCAAGGGTTATT | 52 | 477 | Ibarra et al. [44] |
| TGCAAACAGGACATTGTATGTGTAATT | ||||
| cyt2 | ATTACAAATTGCAAATGGTATTCC | 50 | 356 | Ibarra et al. [44] |
| TTTCAACATCCACAGTAATTTCAAATGC | ||||
| vip1 | TTATTAGATAAACAACAACAAGAATATCAATCTATTMGNTGGATHGG | 48 | 585 | Hernández-Rodríguez et al. [45] |
| GATCTATATCTCTAGCTGCTTTTTCATAATCTSARTANGGRTC | ||||
| vip2 | GATAAAGAAAAAGCAAAAGAATGGGRNAARRA | 48 | 845 | Hernández-Rodríguez et al. [45] |
| CCACACCATCTATATACAGTAATATTTTCTGGDATNGG | ||||
| vip3 | TGCCACTGGTATCAARGA | 48 | 1621 | Hernández-Rodríguez et al. [45] |
| TCCTCCTGTATGATCTACATATGCATTYTTRTTRTT |
| Strains 4 mL Bacillus thuringiensis 80 g Diet | Larval Stage (Days) X ± SD | Pupal Stage (Days) X ± DP | Newly Hatched Larvae to Adults (Days) X ± SD | Deformity (%) X ± DP | Sex Ratio X ± DP |
|---|---|---|---|---|---|
| SOL5DM | 0.0 ± 0.0 c | 0.0 ± 0.0 b | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.00 ± 0.0 b |
| SOL6RN | 0.0 ± 0.0 c | 0.0 ± 0.0 b | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.00 ± 0.0 b |
| TR4J | 0.0 ± 0.0 c | 0.0 ± 0.0 b | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.00 ± 0.0 b |
| TRO1TN | 3.0 ± 0.0 b | 4.0 ± 0.0 b | 7.0 ± 0.0 c | 0.0 ± 0.0 c | 0.33 ± 0.4 a |
| UNI2MA | 3.0 ± 0.0 b | 5.5 ± 4.6 a | 8.5 ± 7.0 b | 0.0 ± 0.0 c | 0.41 ± 0.5 a |
| TA5FV | 6.0 ± 0.0 a | 7.0 ± 0.0 a | 13.0 ± 0.0 a | 0.0 ± 0.0 c | 0.55 ± 0.1 a |
| VG2NN | 6.0 ± 0.0 a | 7.0 ± 0.0 a | 13.0 ± 0.0 a | 0.0 ± 0.0 c | 0.48 ± 0.1 a |
| VG1MD | 6.0 ± 0.0 a | 7.0 ± 0.0 a | 13.0 ± 0.0 a | 0.0 ± 0.0 c | 0.36 ± 0.0 a |
| TRO2MQ | 6.0 ± 0.0 a | 10.0 ± 0.0 a | 16.0 ± 0.0 a | 0.0 ± 0.0 c | 0.64 ± 0.2 a |
| CONTROL | 6.0 ± 0.0 a | 7.0 ± 0.0 a | 13.0 ± 0.0 a | 0.0 ± 0.0 c | 0.47 ± 0.0 a |
| TA1IC | 6.0 ± 0.0 a | 8.2 ± 5.5 a | 14.2 ± 5.5 a | 1.0 ± 0.5 b | 0.52 ± 0.0 a |
| TOR1KC | 7.0 ± 0.0 a | 8.0 ± 0.0 a | 15.0 ± 0.0 a | 0.0 ± 0.0 c | 0.47 ± 0.1 a |
| TOR2VN | 7.0 ± 0.0 a | 8.0 ± 0.0 a | 15.0 ± 0.0 a | 3.0 ± 0.8 a | 0.46 ± 0.0 a |
| Bt Strains | Genes | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cry | cyt | vip | ||||||||||||||
| cry1Aa | cry1Ab | cry1B | cry1C | cry1G | cry1Ea | cry9A | cry9B | cry2Aa | cry2Ac | cry2Ad | cyt1 | cyt2 | vip1 | vip2 | vip3 | |
| TRO1TN | − | − | + | − | − | − | − | − | − | − | + | − | − | + | − | + |
| TRO2MQ | − | − | + | − | − | − | − | − | − | + | − | − | − | + | − | + |
| UNI2MA | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + |
| TR4J | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | + |
| SOL5DM | − | − | − | − | − | − | − | − | + | − | + | − | − | − | − | − |
| SOL6RN | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, T.A.; Paes, M.J.; Valicente, F.H.; Queiroz, M.M.d.C. Bioactive Potential of Some Bacillus thuringiensis Strains from Macapá, Amazon, Brazil, Against the Housefly Musca domestica (Diptera: Muscidae) Under Laboratory Conditions. Insects 2025, 16, 27. https://doi.org/10.3390/insects16010027
Nascimento TA, Paes MJ, Valicente FH, Queiroz MMdC. Bioactive Potential of Some Bacillus thuringiensis Strains from Macapá, Amazon, Brazil, Against the Housefly Musca domestica (Diptera: Muscidae) Under Laboratory Conditions. Insects. 2025; 16(1):27. https://doi.org/10.3390/insects16010027
Chicago/Turabian StyleNascimento, Tatiane Aparecida, Maria José Paes, Fernando Hercos Valicente, and Margareth Maria de Carvalho Queiroz. 2025. "Bioactive Potential of Some Bacillus thuringiensis Strains from Macapá, Amazon, Brazil, Against the Housefly Musca domestica (Diptera: Muscidae) Under Laboratory Conditions" Insects 16, no. 1: 27. https://doi.org/10.3390/insects16010027
APA StyleNascimento, T. A., Paes, M. J., Valicente, F. H., & Queiroz, M. M. d. C. (2025). Bioactive Potential of Some Bacillus thuringiensis Strains from Macapá, Amazon, Brazil, Against the Housefly Musca domestica (Diptera: Muscidae) Under Laboratory Conditions. Insects, 16(1), 27. https://doi.org/10.3390/insects16010027







