Pheromones in Crane Flies: Behaviorally Active Cuticular Compounds in Tipula autumnalis Loew (Diptera: Tipulidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Preparation of Cuticular Washes
2.3. Gas Chromatography-Electroantennogram Detection
2.4. Gas Chromatography-Mass Spectrometry
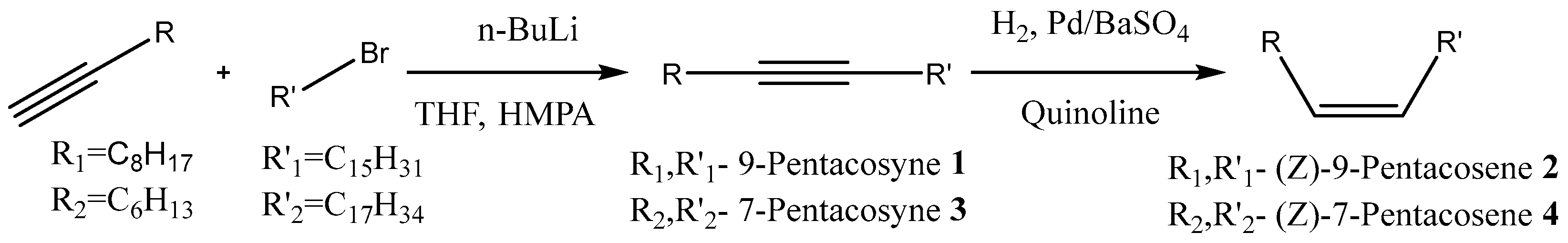
2.5. Synthesis of Chemical Compounds
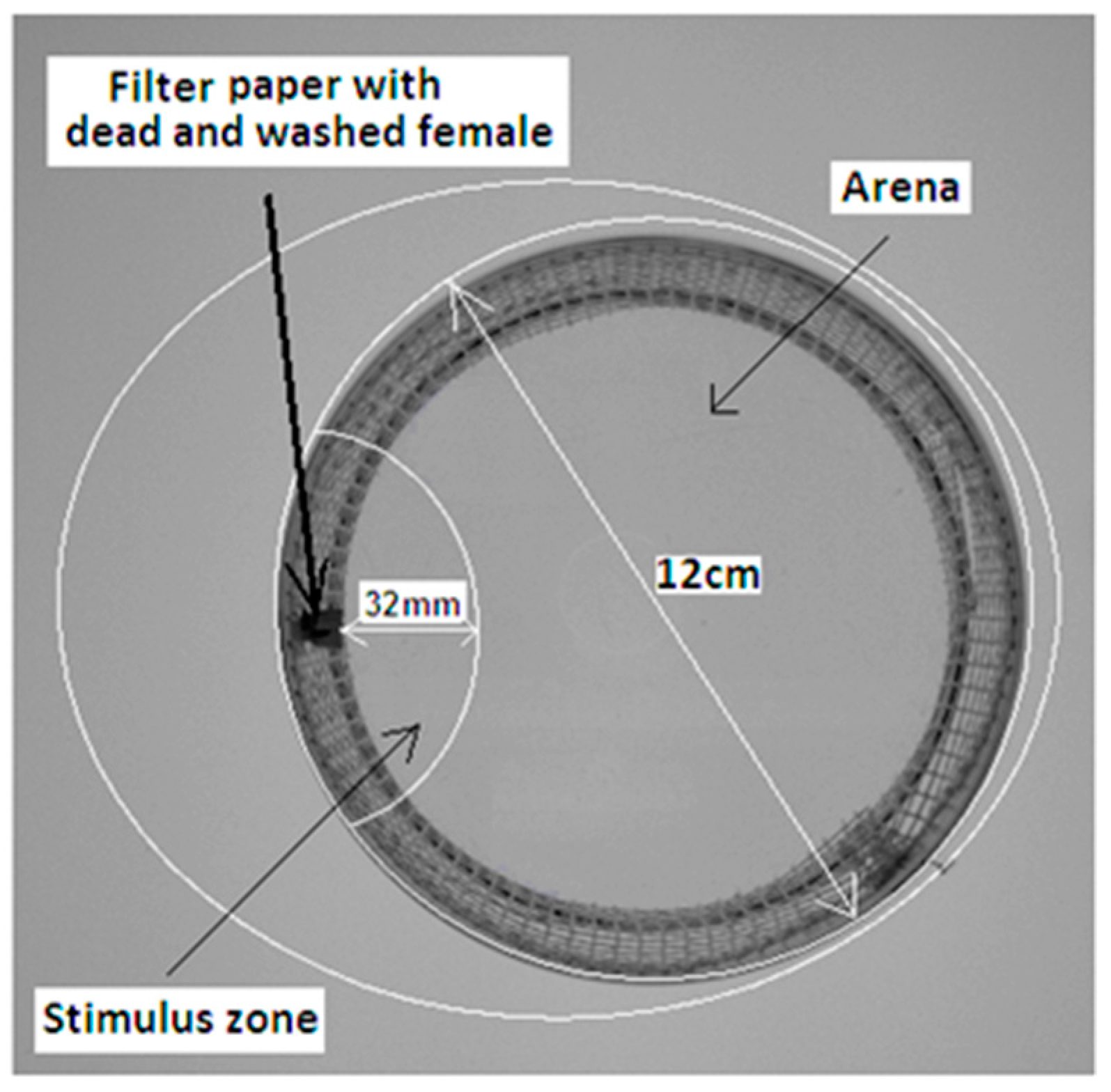
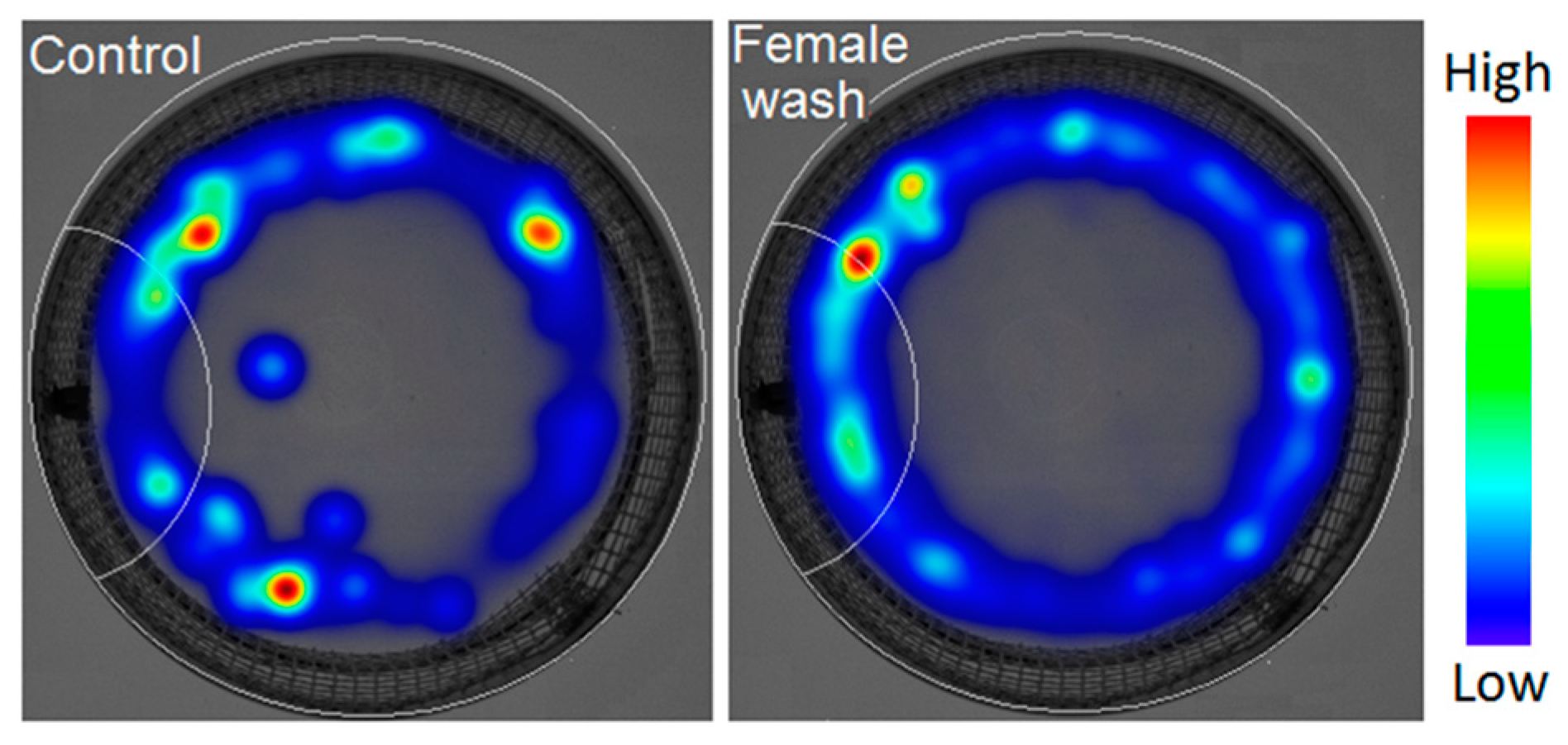
2.6. Behavioral Assay
3. Results
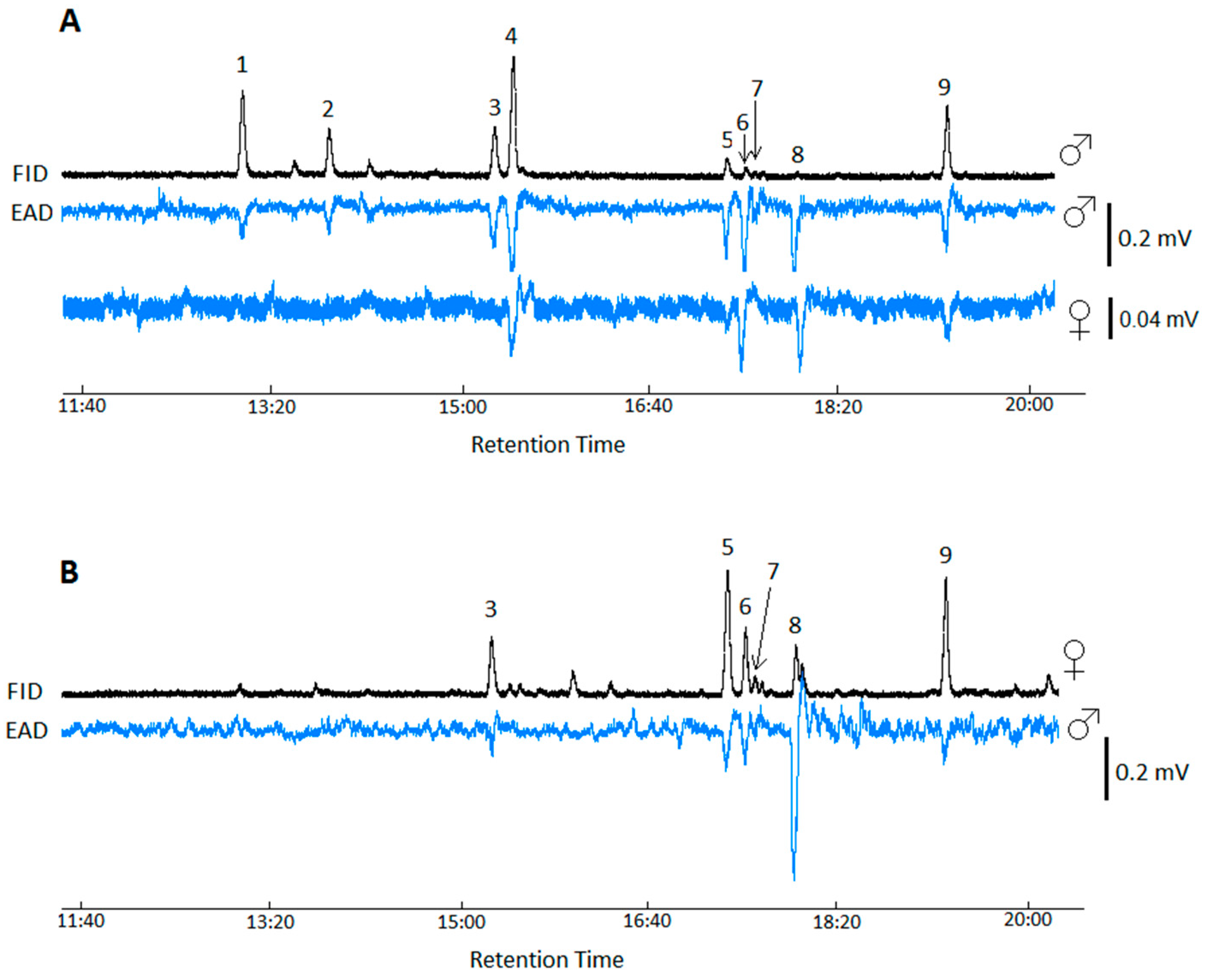
3.1. GC-EAD Analysis of Cuticular Washes
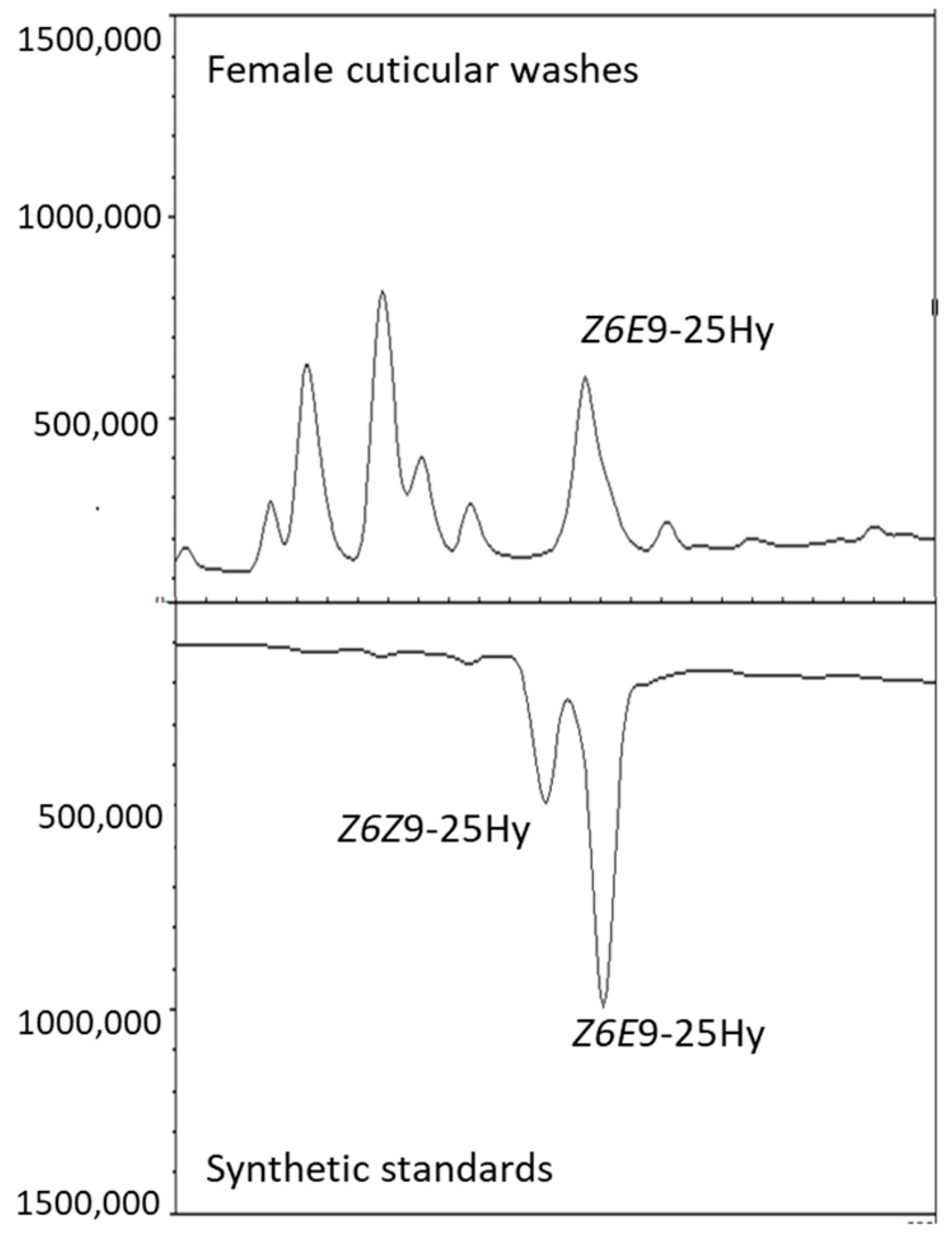
3.2. Compound Identification
3.3. Relative Abundance of the Compounds
3.4. Male Behavioral Responses to Conspecific CHCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blomquist, G.J.; Bagnères, A.G. Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology; Cambridge University Press: Cambridge, UK, 2010; pp. 1–343. [Google Scholar]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 14 October 2024).
- Curtis, S.; Sztepanacz, J.L.; White, B.E.; Dyer, K.A.; Rundle, H.D.; Mayer, P. Epicuticular compounds of Drosophila subquinaria and D. recens: Identification, quantification, and their role in female mate choice. J. Chem. Ecol. 2013, 39, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Traynier, R.M.M.; Burton, D.J. Male response to females in marsh crane fly, Tipula paludosa Mg. (Diptera: Tipulidae). J. Entomol. Soc. Br. Columbia 1970, 67, 21–22. [Google Scholar]
- de Jong, H.; Oosterbroek, P.; Gelhaus, J.; Reusch, H.; Young, C. Global diversity of craneflies (Insecta, Diptera: Tipulidea or Tipulidae sensu lato) in freshwater. In Freshwater Animal Diversity Assessment; Balian, E.V., Lévêque, C., Segers, H., Martens, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 457–467. [Google Scholar] [CrossRef]
- Blackshaw, R.P.; Coll, C. Economically important leatherjackets of grassland and cereals: Biology, impact and control. Integr. Pest Manag. Rev. 1999, 4, 143–160. [Google Scholar] [CrossRef]
- Taschereau, E.; Simard, L.; Brodeur, J.; Gelhaus, J.; Bélair, G.; Dionne, J. Seasonal ecology of the European crane fly (Tipula paludosa) and species diversity of the family Tipulidae on golf courses in Québec, Canada. Int. Turfgrass Soc. Res. J. 2009, 11, 681–693. [Google Scholar]
- Nelson, D.R.; Blomquist, G.J. Insect waxes. In Waxes: Chemistry, Molecular Biology, and Function; Hamilton, R.J., Ed.; The Oily Press: Dundee, UK, 1995; pp. 1–90. [Google Scholar]
- Carlson, D.A.; Roan, C.; Yost, R.A.; Hector, J. Dimethyl disulphide derivatives of long chain alkenes, alkadienes and alktrienes for gas chromatography/mass spectrometry. Anal. Chem. 1989, 61, 1564–1571. [Google Scholar] [CrossRef]
- Masuda, Y.; Mori, K. Synthesis of the four stereoisomers of 3,12-Dimethylheptacosane, (Z)-9-Pentacosene and (Z)-9-Heptacosene, the cuticular hydrocarbons of the Ant Diacamma sp. Biosci. Biotechnol. Biochem. 2002, 66, 1032–1038. [Google Scholar] [CrossRef]
- Fisher, I.G.; Tyman, J.H.P. The synthesis of long chain dialkylalkynes, dialkyldiynes and their hydrogenation to monoenes and dienes. Synth. Commun. 1998, 28, 1323–1338. [Google Scholar] [CrossRef]
- Vincenti, M.; Guglielmetti, G.; Cassani, G.; Tonini, C. Determination of double-bond position in diunsaturated compounds by mass spectrometry of dimethyl disulfide derivatives. Anal. Chem. 1987, 5, 694–699. [Google Scholar] [CrossRef]
- Ferveur, J.-F.; Cobb, M. Behavioural and evolutionary roles of cuticular hydrocarbons in Diptera. In Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology; Blomquist, G.J., Bagnères, A.G., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 325–343. [Google Scholar]
- Butterworth, N.J.; Wallman, J.F.; Drijfhout, F.P.; Johnston, N.P.; Keller, P.A.; Byrne, P.G. The evolution of sexually dimorphic cuticular hydrocarbons in blowflies (Diptera: Calliphoridae). J. Insect. Physiol. 2020, 33, 1468–1486. [Google Scholar] [CrossRef]
- Stich, H.F. An experimental analysis of the courtship pattern of Tipula oleracea (Diptera). Can. J. Zool. 1963, 41, 99–109. [Google Scholar] [CrossRef]
- Mozūraitis, R.; Hajkazemian, M.; Zawada, J.W.; Szymczak, J.; Pålsson, K.; Sekar, V.; Biryukova, I.; Friedländer, M.R.; Koekemoer, L.L.; Baird, J.K.; et al. Male swarming aggregation pheromones increase female attraction and mating success among multiple African malaria vector mosquito species. Nat. Ecol. Evol. 2020, 4, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Scolari, F.; Valerio, F.; Benelli, G.; Papadopoulos, N.T.; Vaníčková, L. Tephritid fruit fly semiochemicals: Current knowledge and future perspectives. Insects 2021, 12, 408. [Google Scholar] [CrossRef] [PubMed]
- Mendki, M.J.; Ganesan, K.; Shri Prakash, S.P.; Suryanarayana, M.V.S.; Malhotra, R.C.; Rao, K.M.; Vaidyanathaswamy, R. Heneicosane: An oviposition-attractant pheromone of larval origin in Aedes aegypti mosquito. Curr. Sci. 2000, 78, 1295–1296. [Google Scholar]
- Jiang, Y.; Lei, C.L.; Niu, C.Y.; Fang, Y.L.; Xiao, C.; Zhang, Z.N. Semiochemicals from ovaries of gravid females attract ovipositing female houseflies, Musca domestica. J. Insect. Physiol. 2002, 48, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, V.; Pogoda, W.; Verhoff, M.A.; Toennes, S.W.; Amendt, J. Estimating the age of the adult stages of the blow flies Lucilia sericata and Calliphora vicina (Diptera: Calliphoridae) by means of the cuticular hydrocarbon n-pentacosane. Sci. Justice 2017, 57, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Caselli, A.; Favaro, R.; Petacchi, R.; Valicenti, M.; Angeli, S. The cuticular hydrocarbons of Dasineura oleae show differences between sex, adult age and mating status. J. Chem. Ecol. 2023, 49, 369–383. [Google Scholar] [CrossRef]
- Wang, G.; Vega-Rodríguez, J.; Diabate, A.; Liu, J.; Cui, C.; Nignan, C.; Dong, L.; Li, F.; Ouedrago, C.O.; Bandaogo, A.M.; et al. Clock genes and environmental cues coordinate Anopheles pheromone synthesis, swarming, and mating. Science 2021, 371, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Li, N.; Zhang, M.; Tang, Q.; Lu, H.Z.; Zhou, Q.Y.; Niu, J.-X.; Xiao, L.; Peng, Z.-Y.; Zhang, C.; et al. The sex pheromone heptacosane enhances the mating competitiveness of sterile Aedes aegypti males. Parasit. Vectors 2023, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Mayer, M.S.; Silhacek, D.L.; James, J.D.; Beroza, M.; Bierl, B.A. Sex attractant pheromone of the house fly: Isolation, identification and synthesis. Science 1971, 174, 76–78. [Google Scholar] [CrossRef]
- Wicker-Thomas, C. Pheromonal communication involved in courtship behavior in Diptera. J. Insect Physiol. 2007, 53, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Belenioti, M.; Roditakis, E.; Sofiadis, M.; Fouskaki, M.; Apostolaki, M.; Chaniotakis, N. Effect of solvent extraction time on the hydrocarbon profile of Drosophila suzukii (Diptera: Drosophilidae) and behavioural effects of 9-pentacosene and dodecane. Eur. J. Entomol. 2022, 119, 232–241. [Google Scholar] [CrossRef]
- Carpita, A.; Canale, A.; Raffaelli, A.; Saba, A.; Benelli, G.; Raspi, A. (Z)-9-tricosene identified in rectal gland extracts of Bactrocera oleae males: First evidence of a male-produced female attractant in olive fruit fly. Naturwissenschaften 2012, 99, 77–81. [Google Scholar] [CrossRef]
- Jennings, J.H.; Etges, W.J.; Schmitt, T.; Hoikkala, A. Cuticular hydrocarbons of Drosophila montana: Geographic variation, sexual dimorphism and potential roles as pheromones. J. Insect Physiol. 2014, 61, 16–24. [Google Scholar] [CrossRef]
- Mazomenos, B.E.; Athanassiou, C.G.; Kavallieratos, N.; Milonas, P. Evaluation of the major female Eurytoma amygdali sex pheromone components, (Z, Z)-6, 9-tricosadiene and (Z, Z)-6, 9-pentacosadiene for male attraction in field tests. J. Chem. Ecol. 2004, 30, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Bello, J.E.; McElfresh, J.S.; Millar, J.G. Isolation and determination of absolute configurations of insect-produced methyl-branched hydrocarbons. Proc. Natl. Acad. Sci. USA 2015, 112, 1077–1082. [Google Scholar] [CrossRef]
- Vaníčková, L.; Svatoš, A.; Kroiss, J.; Kaltenpoth, M.; Do Nascimento, R.R.; Hoskovec, M.; Břízová, R.; Kalinová, B. Cuticular hydrocarbons of the South American fruit fly Anastrepha fraterculus: Variability with sex and age. J. Chem. Ecol. 2012, 38, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
| Peak No | RI Nonpolar | RI Polar | Compound | Abbreviation | m/z | Relative Amount 1 (Mean ± SE) (n = 4) | ||
|---|---|---|---|---|---|---|---|---|
| Untreated | Dimethyl Disulfide Derivative | Female | Male | |||||
| 1 | 2100 | 2100 | Heneicosane | 21Hy | 296 | 1.61 ± 0.02 | 21.79 ± 0.11 | |
| 2 | 2173 | 2169 | (R)-3-Methylheneicosane | R3Me-21Hy | 310, 252/253, 280/281 | 0.44 ± 0.08 | 11.55 ± 0.05 | |
| 3 | 2300 | 2300 | Tricosane | 23Hy | 324 | 13.53 ± 0.06 | 13.02 ± 0.02 | |
| 4 | 2269 | 2323 | (Z)-9-Tricosene | Z9-23Hy | 322 | 416, 173, 243 | 1.46 ± 0.01 | 30.80 ± 0.03 |
| 5 | 2500 | 2500 | Pentacosane | 25Hy | 352 | 31.45 ± 0.05 | 4.28 ± 0.04 | |
| 6 | 2471 | 2515 | (Z)-9-Pentacosene | Z9-25Hy | 350 | 444, 173, 271 | 14.17 ± 0.05 | 1.62 ± 0.08 |
| 7 | 2478 | 2523 | (Z)-7-Pentacosene | Z7-25Hy | 350 | 444, 145, 299 | 3.58 ± 0.05 | 0.49 ± 0.14 |
| 8 | 2464 | 2560 | (Z,E)-6,9-Pentacosadiene | Z6E9-25Hy | 348 | 442, 131, 155, 203, 271, 295, 343 | 9.32 ± 0.04 | 0.38 ± 0.01 |
| 9 | 2700 | 2700 | Heptacosane | 27Hy | 380 | 24.44 ± 0.19 | 16.07 ± 0.18 | |
| Behavioral Criteria | Female Wash | 25Hy 1 | Z9-25Hy 2 | Z6E9-25Hy 2 | Z9-23Hy 1 | R3Me-21Hy 1 | S3Me-21Hy 1 |
|---|---|---|---|---|---|---|---|
| Duration spent in the SZ | 159 ± 20 * | 151 ± 21 * | 221 ± 42 * | 235 ± 37 * | 112 ± 15 | 93 ± 14 | 48± 10 * |
| Latency to first visit of the SZ | 14 ± 7 * | 27 ± 18 * | 24 ± 9 | 23 ± 11 * | 7 ± 3 * | 8 ± 3 * | 26 ± 12 |
| Number of visits | 171 ± 39 | 218 ± 26 * | 176 ± 34 | 180 ± 46 | 161 ± 22 | 212 ± 32 * | 229 ± 70 |
| Distance moved | 147 ± 28 | 226 ± 26 * | 134 ± 24 | 232 ± 39 * | 199 ± 31 * | 247 ± 35 * | 226 ± 56 |
| Velocity | 158 ± 32 | 220 ± 25 * | 135 ± 24 | 230 ± 39 * | 185 ± 28 * | 246 ± 35 * | 235 ± 63 |
| Mobility | 136 ± 27 | 214 ± 24 * | 128 ± 21 | 243 ± 38 * | 215 ± 35 * | 264 ± 37 * | 201 ± 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Būda, V.; Apšegaitė, V.; Blažytė-Čereškienė, L.; Podėnas, S.; de A. Souza, J.P.; Zarbin, P.H.G.; Labanauskas, L.; Paškevičius, T.; Baužienė, V.; Radžiutė, S. Pheromones in Crane Flies: Behaviorally Active Cuticular Compounds in Tipula autumnalis Loew (Diptera: Tipulidae). Insects 2025, 16, 24. https://doi.org/10.3390/insects16010024
Būda V, Apšegaitė V, Blažytė-Čereškienė L, Podėnas S, de A. Souza JP, Zarbin PHG, Labanauskas L, Paškevičius T, Baužienė V, Radžiutė S. Pheromones in Crane Flies: Behaviorally Active Cuticular Compounds in Tipula autumnalis Loew (Diptera: Tipulidae). Insects. 2025; 16(1):24. https://doi.org/10.3390/insects16010024
Chicago/Turabian StyleBūda, Vincas, Violeta Apšegaitė, Laima Blažytė-Čereškienė, Sigitas Podėnas, João Pedro de A. Souza, Paulo H. G. Zarbin, Linas Labanauskas, Tomas Paškevičius, Vilma Baužienė, and Sandra Radžiutė. 2025. "Pheromones in Crane Flies: Behaviorally Active Cuticular Compounds in Tipula autumnalis Loew (Diptera: Tipulidae)" Insects 16, no. 1: 24. https://doi.org/10.3390/insects16010024
APA StyleBūda, V., Apšegaitė, V., Blažytė-Čereškienė, L., Podėnas, S., de A. Souza, J. P., Zarbin, P. H. G., Labanauskas, L., Paškevičius, T., Baužienė, V., & Radžiutė, S. (2025). Pheromones in Crane Flies: Behaviorally Active Cuticular Compounds in Tipula autumnalis Loew (Diptera: Tipulidae). Insects, 16(1), 24. https://doi.org/10.3390/insects16010024






