Simple Summary
In the southeastern United States of America, shifts in the environment such as climate change and host availability are pushing tick populations to spread into new areas. It is hypothesized that, as they migrate, tick populations have developed a behavior known as incomplete feeding. With this, ticks feed on more than one host at each life stage, increasing the chance of pathogen transmission. In South Carolina, we found evidence of ticks displaying this behavior. We collected engorged female ticks from stray dogs at animal shelters across the state in 2022. Testing showed that about a third of these ticks had fed on humans. The patterns varied depending on the tick species, where they were found, and the time of collection. This pilot study reflects the growing trend of tick-borne diseases in the southeastern USA. It is crucial to dig deeper into how factors like the season, location, and species are linked to incomplete feeding behavior in South Carolina’s tick populations.
Abstract
Dynamic environmental conditions, such as climate change and host availability, have greatly influenced the expansion of medically relevant tick vectors into new regions throughout the southeastern United States of America. As tick populations migrate into new areas, it has been suggested they can exhibit a phenomenon known as incomplete feeding. With this phenomenon, tick vectors feed on more than one host at each life stage, thus increasing the likelihood of pathogen transmission. Although this behavior is not well understood, it presents an important threat to human health. Here we present evidence of incomplete feeding behaviors in multiple tick species in South Carolina. Engorged, blood-fed female ticks were collected from feral dogs at animal shelters across South Carolina in 2022. All ticks were tested for human blood meals using rapid stain identification blood tests. Approximately one third (33.78%) of all ticks tested positive for a human blood meal, with various patterns seen across species, geographic location, and collection month. The results of this pilot study follow the current national trend of increasing rates of tick-borne disease incidence in the southeastern United States of America and warrant further investigation into the relationship between seasonality, geographic distribution, species, and incomplete feeding among tick populations in South Carolina.
1. Introduction
The epidemiology of tick-borne diseases (TBDs) in the United States of America (USA) has dramatically changed over the last two decades. Human TBD incidence has doubled, and seven novel tick-transmitted pathogens have been identified nationally [1,2]. Moreover, according to the Centers for Disease Control and Prevention (CDC), upwards of five hundred thousand people are diagnosed and treated for some form of TBD every year, and this number is expected to rise in prospective years [3]. The increased incidence of federally reported TBDs can be attributed to the geographic range expansion of tick species into new regions [4]. Currently, there are nine tick species of medical and veterinary importance in the USA [5]. The most common among these have greatly expanded outside of their historic ranges, solidifying range expansion as a serious public health threat. For instance, Amblyomma americanum, one of the most abundant and common tick species in the USA, has gradually disseminated outside its historic range both north and westwards [6]. In the past two decades, Ixodes scapularis has significantly expanded into several Midwest, northeast, and mid-Atlantic states, while remaining an established species in the southeastern USA [7]. Additionally, Amblyomma maculatum, a tick species most found along the southern Atlantic coast, has steadily expanded its range into the mid-Atlantic and is projected to continue this expansion northwards [8].
Historically, tick vectors had well-defined geographic ranges due to local abiotic environmental factors and host–vector associations [6]. Therefore, the geographic expansion of ticks in the USA is believed to be heavily impacted by climate change, which transforms the natural environment, ultimately impacting these ecologic communities and relationships [3]. Different vector adaptation processes allow various tick species to establish themselves in these new domains [6]. One adaptation of importance is incomplete feeding, also referred to as interrupted or partial feeding. Ticks have four distinct life stages: egg, larva, nymph, and adult [9]. Once the eggs hatch, larvae must take a blood meal to molt to the nymphal stage; nymphs must take a blood meal to molt into an adult; and adults must take a blood meal to propagate [9]. Most ticks of public health importance in the USA, including A. americanum, A. maculatum, I. scapularis, Ixodes keiransi, Dermacentor variabilis, and Rhipicephalus sanguineus, are considered three-host ticks [9,10]. Three-host ticks hunt, attach, and feed on a different animal reservoir at every life stage [9,10]. With this behavior, incomplete feeding can occur if a tick detaches from its host before completion of a blood meal [11].
This behavior could be a sign of new ticks into a new area they have not previously inhabited. Both host type and atmospheric conditions are key factors that have been shown to influence how many hosts a tick will utilize throughout their life cycle [12]. It has been shown that adult female I. ricinus, D. reticulatus, and Hyalomma asiaticum ticks are able to detach from a host mid-blood meal and reattach to a different host, so long as they have not reached the critical mass needed to reproduce [12]. However, this phenomenon remains poorly studied, especially in ticks native to the USA. Therefore, the current study aims to explore the potential factors related to the increased number of tick populations across South Carolina (SC) by (1) testing the hypothesis that engorged female tick species are exhibiting incomplete feeding and (2) analyzing potential risk factors associated with these ticks. To address these aims, blood meal analysis was performed on host-attached ticks collected from feral dogs presenting at animal shelters throughout the state.
2. Materials and Methods
2.1. Tick Collection and Processing
From January 2022 to July 2022, ticks were collected through an ongoing statewide tick surveillance project that included a partnership with animal shelters throughout SC. Participating animal shelters were located in all four regions of SC: the Upstate, located in the upper half of the state; the Midlands, located in the middle of the state; the Lowcountry, located on the lower coastline bordering Georgia (GA); and PeeDee, located on the upper coastline and bordering North Carolina (NC). At the start of each month, participating animal shelters were given a new ‘tick kit’ containing a mason jar filled with 75% ethanol, a data log, and a prepaid mailer. The shelters were instructed to place any ticks removed from a feral dog into the jar and fill out the data log upon doing so. The data log collected data on date, host species, and the number of ticks removed. Once the jar was received, the ticks were identified morphologically using published pictorial keys [13,14,15]. Each individual tick was then stored in a new 15 mL centrifuge tube and labeled with the collecting animal shelter, date, species, sex, life stage, and engorgement status. These ticks were stored at room temperature in 75% ethanol on the lab bench away from sunlight until further processing. Each individual tick was then bisected vertically using a scalpel sterilized in a Micro Bead Sterilizer at 300 °C. Half of each individual tick was placed in a new 15 mL centrifuge tube and stored in a −80° freezer to be used for pathogen testing. The other half was used for blood meal analysis.
Only engorged adult female ticks were included in this study. Although all life stages and both sexes blood-feed, only adult female ticks become fully engorged, increasing their body weight by almost 100 times their unfed weight [16,17]. Therefore, engorged adult female ticks exhibit the highest likelihood of completing a substantial blood meal.
2.2. Blood Meal Analysis
As the ticks used for this study were removed directly from a canine host, canine blood meal testing was not conducted. Instead, it was inferred all ticks were positive for a canine blood meal. However, rapid stain identification (RSID) blood test kits were used to test for the presence of human blood according to the manufacturer’s instructions (Independent Forensics, Lombard, IL, USA). Individual tick halves were scored across the inside of its body cavity using a disinfected and sterilized scalpel to remove a sample of coagulated blood. Each coagulated blood sample was then added to the buffer solution provided by the test kit in its own well. The samples were covered and left undistributed at room temperature for one hour to allow the coagulated blood to soften and dissolve. Once mostly dissolved, thin swabs were used to crush up and mix in any remaining congealed pieces. Next, 100 μL of each well mixture was transferred to the sample well of the test cassette using a sterile micropipette tip. The tests were allowed to sit at room temperature, undisturbed for 10 min before the results were read. The results were interpreted per the test kit’s instructions: visible red lines at both the control (C) and test (T) position indicated a positive test. All other results were considered negative.
2.3. Pathogen Testing
Tick DNA was extracted using a Qiagen DNEasy kit (Qiagen Sciences Inc., Germantown, MD, USA), and dsDNA was collected and purified using the QIAcube HT (Qiagen Sciences Inc., Germantown, MD, USA) as previously described by Dye-Braumuller, 2023 [18]. Finally, ticks were screened for various pathogens using a QuantStudio 5 Real-Time PCR System (Appendix A). Amblyomma and Dermacentor tick species were tested for spotted fever group Rickettsioses (SFGR, R. parkeri, R. amblyommatis, R. rickettsii, and SFGR general ompA) and Ehrlichia species (E. ewingii, E. chaffeensis, and Panola Mountain Ehrlichia), while Ixodes spp. and Haemaphyalis longicornis ticks were tested for Borrelia burgdorferi, Anaplasma phagocytophilum and Theileria orientalis.
2.4. Statistical Analysis
Descriptive statistics were performed to describe the ecological differences between human blood meal positives vs. negatives. Univariate logistic regression was performed to statistically assess the variance between feeding groups. Six multinomial logistic regressions were run for each category to accommodate for potential dilution effects of low counts within individual category variables. Variables with a p-value < 0.05 were considered statistically significant. Statistical analysis was conducted using both SAS OnDemand for Academics v3.81 (SAS Institute Inc., Cary, NC, USA), and Stata SE v15.1 (State Corporation, College Station, TX, USA). Finally, a geospatial map of human blood meal-positive ticks was created using ArcGIS Pro (Esri Corp, Redlands, CA, USA).
3. Results
A total of 225 eligible animal shelter ticks were received from 8 different veterinary practices across SC between January 2022 and July 2022. Only fully fed adult female ticks were included in the final analysis. Of these, 48% (n = 108) were Prostriata (Ixodes spp. only), and 52% (n = 117) were Metastriate (Amblyomma, Dermacentor, and Haemaphyalis spp.). Furthermore, six different tick species were obtained: 39.6% (n = 89) were I. scapularis, 26.7% (n = 60) were D. variabilis, 17.8% (n = 40) were A. americanum, 8.4% (n = 19) were I. keiransi, 5.3% (n = 12) were H. longicornis and 2.2% (n = 5) were A. maculatum.
Overall, 33.8% (n = 76) tested positive for a human blood meal (RSID positive). Of these, 34.2% (n = 26) were D. variabilis, 32.9% (n = 25) were I. scapularis, 14.5% (n = 11) were A. americanum, 10.5% (n = 8) were I. keiransi, and 7.9% (n = 6) were H. longicornis. No A. maculatum ticks were found to be positive for a mixed blood meal.
Few variables were found to be statistically associated with human blood meal-positive ticks in the univariate analysis (Table 1). Despite the diversity among the study population, genus and species were not likely risk factors associated with incomplete feeding. Ticks received in the month of March, however, had 2.26 times greater odds (p-value = 0.027; CI: 1.10 to 4.65) of testing positive for a mixed blood meal compared to those collected in all other months.

Table 1.
Variables associated with mixed blood meal analysis among 225 engorged adult female ticks.
When assessing general location, 40.8% (n = 31) of positive ticks came from animal shelters in the Upstate, while only 2.6% (n = 2) came from animal shelters in PeeDee. However, no statistically significant association was observed between mixed blood meal-positive ticks and region. When evaluating location, county as a collective variable (p-value = 0.012), and Horry County (p-value = 0.027; OR: 2.26; CI: 1.10 to 4.65) were significant. In addition, Charleston (p-value = 0.068) and Greenville (p-value = 0.13) both neared significances. Taken together, this indicates that while region does not appear to be a risk factor, the county a tick was collected from potentially plays a big role in predicting if that tick was human blood meal-positive or not. Further, when looking at the rate of RSID positive ticks by county, 100% of ticks collected from animal shelters in York County, 67% from Aiken, 50% from Horry and 50% from Pickens were human blood meal-positive (Figure 1).
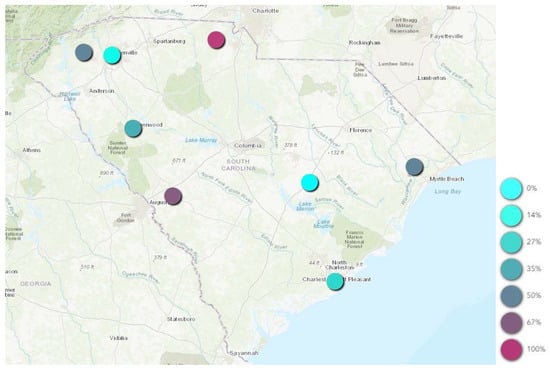
Figure 1.
Geospatial distribution of percent mixed blood meal-positive adult ticks by county.
Overall, 48.7% (n = 37) of human blood meal-positive ticks, and 45.6% (n = 68) of human blood meal-negative ticks, tested positive for one or more pathogens. Although the infection rate for both mixed blood meal-positive ticks, and mixed blood meal-negative ticks were similar, pathogen testing was not statistically significant. This indicates being infected with a pathogen does not affect if a tick exhibits incomplete feeding or not.
4. Discussion
The current study aimed to (1) test the hypothesis that engorged female tick populations in SC are exhibiting incomplete feeding, and (2) analyze the risk factors associated with mixed blood meal-positive ticks. This investigation revealed calendar month and collection county may be important risk factors of incomplete feeding behavior. Moreover, the highest rate of human bloodmeal-positive ticks were from counties bordering both NC and GA, suggesting vector control differences between states may impact tick blood meal selection. Although tick genus and species were not significant, the results show I. scapularis and D. variabilis were most likely to demonstrate incomplete feeding. Moreover, while pathogen testing was not a significant risk factor associated with incomplete feeding in this pilot study, previous literature suggests pathogen-induced behavior changes may play a role in this. Taken together, the current study shows several preliminary results that can be built upon.
Although incomplete feeding among tick populations in SC is poorly studied, existing literature on other tick species and vectors can still give insight into potential risk factors that might influence this behavior. A study conducted in Switzerland found 19.5% of nymphs and 18.9% of adult I. ricinus ticks contained DNA from more than one vertebrate host [19]. Similar observations were recorded in St. Louis, Missouri where 16.2% of A. americanum nymphs tested positive for a mixed blood meal [20]. Another study in Pennsylvania suggested previously fed adult and nymphal H. longicornis ticks continued to quest for a new host despite their engorgement status [21]. In addition to ticks, this behavior has been widely described in other common vectors such as mosquitoes, sandflies, and triatomines. For instance, multiple studies have shown various mosquito species including: Culex tritaeniorhynchus, Cx. quinquefasciatus, Anopheles coustani, An. pharonesis, An. funestus, and An. sacharovi, feed on more than one host within the same gonotrophic cycle [22,23,24,25,26,27]. Furthermore, a study conducted on Aedes aegypti and A. albopictus mosquitoes found multiple blood meals were more likely to meet the insect’s nutritional needs [28]. A study conducted in the Republic of Tunisia discovered mixed blood meals in Phlebotomus perniciosus [29]. Similarly, mixed blood meals were detected in both P. perniciosus and P. ariasi sandflies in Spain [30]. A study from Maleki-Ravasan et al. found the presence of a mixed blood meal in field-captured sandflies [31]. In closing, incomplete feeding is not a phenomenon restrictive to our tick population, further validating the study’s findings.
A tick’s life cycle and feeding behavior are greatly influenced by their surrounding environment, particularly seasonality and ecology [6]. Although just under half of all ticks were collected in the month of May, only ticks collected in March were statistically significantly associated with a higher odds of a mixed blood meal, and thus incomplete feeding. Half (n = 9) of all dual blood meal-positive ticks collected in March were I. scapularis, while 27.8% (n = 5) were I. keiransi, and 22.2% (n = 4) were D. variabilis. In the southeastern USA, adult I. scapularis ticks commonly emerge in October but remain active throughout the winter, with females typically laying eggs in the spring [32]. Unlike I. scapularis, however, adult I. keiransi overwinter and begin questing in March [33]. Furthermore, I. keiransi females most commonly lay eggs in the late summer or early fall months [33]. Finally, adult D. variabilis ticks are most active between March and August, but typically peak in May and June [34,35]. Although there are minor differences in the seasonality of these species, all three are active in the month of March. Given these overlapping biotic factors combined with March representing a transitional climate month, the mix of species competition and abiotic uncertainly could be a driver for incomplete feeding behaviors.
Location was determined to be an important factor for mixed blood meal ticks. First, county as a collective variable was significant, suggesting location may be an important predictor for incomplete feeding in SC. Horry County was the only individual county with a statistically significant relationship with incomplete feeding. Most interestingly, the highest rate of mixed blood meal-positive ticks were collected from counties boarding surrounding states. For example, Horry, Pickens, and York counties, all which border NC, had mixed blood meal rates of 50%, 50%, and 100%, respectively. Similarly, Aiken, which borders GA, had a positive infection rate of 66.7%. Although every state has their own policies regarding vector control, SC is heavily underfunded. Surrounding states receive up to 2.5 times more financial assistance for vector and tick-borne disease-related programs [36]. As a result, tick surveillance and intervention programs are less common, making it easier for tick populations to expand and establish in new areas.
Previous studies have shown ticks originating from the north are genetically distinct from those originating from the south in the USA, although the current paper did not explore this [37,38]. This distinction is useful in not only pinpointing where tick populations originate from, but also if they have expanded outside their historic range. For example, Monzon et al. (2016) showed A. americanum ticks from New York and Oklahoma were distinct not just from each other, but also from historic range populations in North and South Carolina [37]. Similarly, a study from Xu et al. (2020) found northern (North Carolina, Virginia, Delaware, New Jersy, Rhode Island, Massachusetts, and New Hampshire) and southern (Florida, Georgia, and South Carolina) I. scapularis ticks had different 16S haplotype distributions [38]. The current study provides the groundwork for future, more in-depth, studies about these ticks’ distinct linages and migration patterns. Understanding these distinct lineages will allow for a more detailed comprehension of how geospatial and migratory patterns influence incomplete feeding.
Ixodes scapularis and D. variabilis were also the two most prevalent species to test positive for a mixed blood meal. Although A. americanum is regarded as the most abundant and aggressive human biting tick in the USA, two separate studies from Felz et al. (1996, 1999) demonstrated D. variabilis and I. scapularis are the second and third most common human-attached ticks [39,40]. In addition, several studies have directly demonstrated incomplete feeding among I. scapularis ticks in both controlled and natural conditions. Piesman et al. (1991) demonstrated that 38% of semi-engorged I. scapularis larvae, who previously detached from a dead host, reattached to a new host, and feed to completion [41]. A similar study observed semi-engorged I. scapularis nymphs voluntarily reattached to a new host and fed to repletion after spending up to 48 h on their first host [42]. However, fewer reports of incomplete feeding among D. variabilis ticks have been reported. Although one study from Reese et al. (2010) reported incomplete feeding in nymphal D. variabilis ticks, the authors did not consider or report reattachment rate [43]. Another laboratory study forcibly removed partially fed adult female D. variabilis ticks from their hosts and infected them with either a growth solution or a solution containing pathogenic material [44]. These ticks were then allowed to reattach and feed to completion [44]. Although D. variabilis incomplete feeding studies are limited, studies on other species belonging to the Dermacentor genus have been conducted. For example, it has been demonstrated that 47% of male D. reticulatus ticks can attach to a new canine host after spending up to 88 hours on the first [45]. In addition, both D. reticulatus and D. andersoni ticks have been shown to exhibit intrastadial pathogen transmission, which requires an attached tick to detach and reattach to a new host under natural condition [46,47]. Overall, existing literature, though limited, shows that incomplete feeding has been documented among these, and other closely related species.
Just under half of all mixed blood meal-positive ticks tested positive for at least one pathogen. Despite this, pathogen infection was not a statistically significant risk factor for incomplete feeding. Although this may be due to the overall small sample size, it is important to consider other explanations as well, such as pathogen-induced behavioral changes. Like incomplete feeding, this phenomenon is well studied in other arthropod vectors such as mosquitoes, sandflies, kissing bugs, and fleas [48,49,50,51,52]. While tick studies are more limited, research has shown a tick’s locomotion, questing heights, vertical and horizontal walks, tendency to overcome obstacles, and host-seeking ability are associated with pathogen infection [53]. While there is currently no evidence for this relationship, it is plausible pathogen infection may also influence incomplete feeding among specific tick populations. In addition, it has been suggested that partially fed ticks have a significantly shorter transmission time, allowing them to spread disease more readily [11,54]. Therefore, it is crucial to analyze other tick pathogen types to better understand the mechanisms behind interrupted feeding and pathogenic transmission.
While this study presented several notable preliminary results, it is important to address the potential limitations. First, the tick samples utilized were not representative of the entire state’s tick populations. Only seven months of sampling was conducted between January 2022 and July 2022. During this time, half of all ticks were collected in May, while no ticks were received in April or June, a potential sampling bias. Furthermore, although ticks were collected from each region of SC, only 8 out of 46 total counties were represented in the analysis. Finally, only fully engorged adult female ticks were included. While female ticks take a larger blood meal and become engorged, Ixodidae male ticks are naturally intermittent feeders, suggesting they commonly exhibit incomplete feeding as well [11]. In addition, both larvae and nymphs have demonstrated incomplete feeding behavior [19,20,21,41,42,43]. Therefore, it is likely the results of this study were bound by the small, uniform sample size.
Future studies should utilize a larger sample encompassing all life stages, sex, and engorgement statuses. Another potential limitation arises from the RSID test kits used to detect human blood. Although RSID testing is fast and cost-effective and has previously been used to ascertain human blood in vectors, such as triatomines, the exact sensitivity of the test is not known [55]. Furthermore, more validated vector blood meal testing, such as qPCR and DNA sequencing is available. Future studies should compare these methodologies to find the most reliable test. It is also a possibility that the human blood detected was simply a remanent left over from prior life stages. Therefore, inclusion of all blood feeding life stages is crucial in future studies. Finally, only human blood was directly tested for; however, due to it is preliminary nature, this was beyond the scope of the current study. In an attempt at qPCR blood meal analysis, several primer/probe sequences were screened. These sequences mainly targeted mitochondrial DNA (mtDNA) of humans, dogs, cattle, and deer. These methods and targets have been explored recently in the literature for blood meal identification of arthropods [56]. However, all sequences screened showed cross-reactivity between the previously mentioned animal species using pure species-specific genomic DNA (gDNA) controls purchased from Zyagen, Inc. (San Diego, CA, USA). Although these primer/probe sequences were designed in silico to be species-specific, there is increasing evidence of in vitro cross-reactivity in qPCR methods [25,57,58]. Therefore, further optimization of this protocol or other methods such as next-generation sequencing should be explored. As the tick samples were removed directly from feral dogs, it was assumed they all consumed some amount of canine blood. Future studies should also complete a full comprehensive blood meal analysis, including other common host animals such as cattle, deer, and goats [59]. This will not only greatly expand the scope of the study but will also provide a greater understanding of how many hosts a tick potentially feeds on at each life stage.
5. Conclusions
This pilot investigation provided new evidence for incomplete feeding among tick populations in SC. Currently, it is believed ticks feed on a set number of hosts throughout their life [9]. However, this study’s findings suggest otherwise. Seasonality and location are two important risk factors for mixed blood meal-positive ticks. Given that most mixed blood meal-positive ticks were from counties bordering both NC and GA, future surveillance efforts in these areas should be considered. Additionally, it must be considered that pathogen infected, partially fed ticks may be more likely to seek a host, take larger blood meals, and transmit the bacteria at a faster rate [11,54,60,61,62,63,64]. With this, future initiatives to minimize human tick exposure should be implemented, especially in the previously mentioned high risk areas. As global climate change continues to intensify, tick populations will continue to expand their geographic ranges. This range expansion will not only have lasting consequences on tick behavior, but also on human and animal hosts. Incomplete feeding is one adaptation that will allow these ticks to persist in new environments. Therefore, the state of SC must prioritize proactive vector surveillance and control to limit the threat these tick species pose concerning the transmission of tick-borne diseases to human populations. Overall, this investigation has laid the groundwork for future research studies, and surveillance programs to help improve the understanding of this emerging phenomenon.
Author Contributions
Conceptualization, M.S.N. and K.C.D.-B.; methodology, K.Z. and M.M.M.; formal analysis, K.E.B., L.E.W. and M.S.N.; investigation, L.E.W., K.Z. and M.M.M.; resources, M.S.N.; data curation, K.E.B. and L.E.W.; writing—original draft preparation, K.E.B., L.E.W. and K.C.D.-B.; writing—review and editing, K.E.B., L.E.W., K.Z., M.M.M., M.S.N. and K.C.D.-B.; supervision, M.S.N. and K.C.D.-B.; project administration, M.S.N.; funding acquisition, M.S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by the Office of the Vice-President for Research at the University of South Carolina. This work was partially supported by the Centers for Disease Control and Prevention Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases 5 NU50CK000542. The CDC did not have a role in the study’s design, the collection, analysis, or interpretation of data, nor in writing the manuscript.
Data Availability Statement
The data in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank our animal shelter partners for their collaboration and tick sample contributions.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the study’s design, the collection, analyses, or interpretation of data, the writing of the manuscript, or the decision to publish the results.
Appendix A

Table A1.
Primers and Probes for identification of pathogens in ticks by RT-qPCR.
Table A1.
Primers and Probes for identification of pathogens in ticks by RT-qPCR.
| Target Species | Gene | Sequence | Reference |
|---|---|---|---|
| Pathogen | |||
| Panola Mountain Ehrlichia | gltA | Forward TGTCATTTCCACAGCATTCTCATC | [65] |
| Reverse ATTAGCGCAATCATACTTGCAA | |||
| Probe TGCCTTAGCTGCACATTATTGTGAT | |||
| Ehrlichia ewingii | 16S | Forward GCGGCAAGCCTAACACATG | [65] |
| Reverse CCCGTCTGCCACTAACAACTATC | |||
| Probe TCGAACGAACAATTCCTAAATAGTCTCTGACTATT | |||
| Ehrlichia chaffeensis | 16S | Forward GCGGCAAGCCTAACACATG | [65] |
| Reverse CCCGTCTGCCACTAACAATTATT | |||
| Probe AGTCGAACGGACAATTGCTTATAACCTTTTGGT | |||
| Rickettsia species | 23S rRNA | Forward AGCTTGCTTTTGGATCATTTGG | [66] |
| Reverse TTCCTTGCCTTTTCATACATCTAGT | |||
| Probe CCTGCTTCTATTTGTCTTGCAGTAACACGCCA | |||
| Rickettsia parkeri | ompB | Forward CAAATGTTGCAGTTCCTCTAAA | [67] |
| Reverse AAA ACA AAC CGT TAA AAC TAC CG | |||
| Probe CGCGAAATTAATACCCTTATGAGCAGCAGTCGCG | |||
| Rickettsia rickettsii | Hypothetical Protein (HP) | Forward AAATCAACGGAAGAGCAAAAC | [66] |
| Reverse CCCTCCACTACCTGCATCAT | |||
| Probe TCCTCTCCAATCAGCGATTC | |||
| Rickettsia amblyommatis | ompB | Forward GGTGCTGCGGCTTCTACATTAG | [65] |
| Reverse CTGAAACTTGAATAAATCCATTAGTAACAT | |||
| Probe TCCTCTTACACTTGGACAGAATGCT | |||
| Borrelia burgdorferi | fliD | Forward TGG TGA CAG AGT GTA TGA TAA TGG AA | [68] |
| Reverse ACT CCT CCG GAA GCC ACA A | |||
| Probe TGC TAA AAT GCT AGG AGA TTG TCT GTC GCC | |||
| Anaplasma phagocytophilum | P44 | Forward ATG GAA GGT AGT GTT GGT TAT GGT ATT | [69] |
| Reverse TTG GTC TTG AAG CGC TCG TA | |||
| Probe TGG TGC CAG GGT TGA GCT TGA GAT TG | |||
| Theileria orientalis | MPSP | Forward GCA AAC AAG GAT TTG CAC GC | [70] |
| Reverse TGT GAG ACT CAA TGC GCC TAG A | |||
| Probe TCG ACA AGT TCT CAC CAC | |||
References
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Vital Signs: Trends in reported vectorborne disease cases—United States and territories, 2004–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Prevention, C.f.D.C.a. A National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans; Centers for Disease Control and Prevention and Prevention National Center for Emerging and Zoonotic Infectious Disease: Atlanta, Georgia, 2020. [Google Scholar]
- Prevention, C.f.D.C.a. Understanding Lyme and Other Tickborne Diseases; 2022. Available online: https://www.cdc.gov/ticks/communication-resources/press-kit.html (accessed on 12 February 2024).
- Bouchard, C.; Dibernardo, A.; Koffi, J.; Wood, H.; Leighton, P.; Lindsay, L. Climate change and infectious diseases: The challenges: N increased risk of tick-borne diseases with climate and environmental changes. Can. Commun. Dis. Rep. 2019, 45, 83. [Google Scholar] [CrossRef] [PubMed]
- PPrevention, C.f.D.C.a. Tick ID. Tickborne Diseases of the United States. 2022. Available online: https://www.cdc.gov/ticks/tickbornediseases/TickborneDiseases-P.pdf (accessed on 13 February 2024).
- Sonenshine, D.E. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Eisen, L. The Blacklegged Tick, Ixodes scapularis: An Increasing Public Health Concern. Trends Parasitol. 2018, 34, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Phillips, V.C.; Zieman, E.A.; Kim, C.H.; Stone, C.M.; Tuten, H.C.; Jiménez, F.A. Documentation of the Expansion of the Gulf Coast Tick (Amblyomma maculatum) and Rickettsia parkeri: First Report in Illinois. J. Parasitol. 2020, 106, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Prevention, C.f.D.C.a. How Ticks Spread Disease; 2020. Available online: https://www.cdc.gov/ticks/about/tick-lifecycles.html?CDC_AAref_Val=https://www.cdc.gov/ticks/life_cycle_and_hosts.html (accessed on 12 February 2024).
- Concern, C.f.D.C.a.P.D.-L.I.o.P.o.P.H. Ticks; 2017. Available online: https://www.cdc.gov/dpdx/ticks/index.html (accessed on 13 February 2024).
- Tahir, D.; Meyer, L.; Fourie, J.; Jongejan, F.; Mather, T.; Choumet, V.; Blagburn, B.; Straubinger, R.K.; Varloud, M. Interrupted Blood Feeding in Ticks: Causes and Consequences. Microorganisms 2020, 8, 910. [Google Scholar] [CrossRef] [PubMed]
- Apanaskevich, D.A.; Oliver, J.H., Jr. Biology of Ticks, 2nd ed.; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: New York, NY, USA, 2014; Volume 1. [Google Scholar]
- Keirans, J.E.; Litwak, T.R. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi River. J. Med. Entomol. 1989, 26, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Nadolny, R.M.; Toliver, M.; Gaff, H.D.; Snodgrass, J.G.; Robbins, R.G. Focus Stacking Images of Morphological Character States for Differentiating the Adults of Ixodes affinis and Ixodes scapularis (Acari: Ixodidae) in Areas of Sympatry. J. Med. Entomol. 2021, 58, 1941–1947. [Google Scholar] [CrossRef]
- Egizi, A.M.; Robbins, R.G.; Beati, L.; Nava, S.; Vans, C.R.; Occi, J.L.; Fonseca, D.M. A pictorial key to differentiate the recently detected exotic Haemaphysalislongicornis Neumann, 1901 (Acari, Ixodidae) from native congeners in North America. Zookeys 2019, 818, 117–128. [Google Scholar] [CrossRef]
- Flynn, P.C.; Kaufman, W.R. Female ixodid ticks grow endocuticle during the rapid phase of engorgement. Exp. Appl. Acarol. 2011, 53, 167–178. [Google Scholar] [CrossRef]
- Island, T.U.o.R. American Dog Tick. TickEncounter 2022. Available online: https://web.uri.edu/tickencounter/species/dog-tick/#:~:text=Male%20ticks%20blood%20feed%20briefly,or%20more%20to%20completely%20engorge (accessed on 13 February 2024).
- Dye-Braumuller, K.C.; Gual-Gonzalez, L.; Abiodun, T.; Rustin, L.P.; Evans, C.L.; Meyer, M.M.; Zellars, K.; Neault, M.J.; Nolan, M.S. Invasive Haemaphysalis longicornis (Acari: Ixodidae) investigation in South Carolina: New records of establishment, pathogen prevalence, and blood meal analyses. J. Med. Entomol. 2023, 60, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Morán Cadenas, F.; Rais, O.; Humair, P.F.; Douet, V.; Moret, J.; Gern, L. Identification of host bloodmeal source and Borrelia burgdorferi sensu lato in field-collected Ixodes ricinus ticks in Chaumont (Switzerland). J. Med. Entomol. 2007, 44, 1109–1117. [Google Scholar] [CrossRef][Green Version]
- Allan, B.F.; Goessling, L.S.; Storch, G.A.; Thach, R.E. Blood Meal Analysis to Identify Reservoir Hosts for Amblyomma americanum Ticks. Emerg. Infect. Dis. 2010, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Price, K.J.; Witmier, B.J.; Eckert, R.A.; Boyer, C.N. Recovery of Partially Engorged Haemaphysalis longicornis (Acari: Ixodidae) Ticks from Active Surveillance. J. Med. Entomol. 2022, 59, 1842–1846. [Google Scholar] [CrossRef] [PubMed]
- Muriu, S.M.; Muturi, E.J.; Shililu, J.I.; Mbogo, C.M.; Mwangangi, J.M.; Jacob, B.G.; Irungu, L.W.; Mukabana, R.W.; Githure, J.I.; Novak, R.J. Host choice and multiple blood feeding behaviour of malaria vectors and other anophelines in Mwea rice scheme, Kenya. Malar. J. 2008, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Muriu, S.; Shililu, J.; Mwangangi, J.M.; Jacob, B.G.; Mbogo, C.; Githure, J.; Novak, R.J. Blood-feeding patterns of Culex quinquefasciatus and other culicines and implications for disease transmission in Mwea rice scheme, Kenya. Parasitol. Res. 2008, 102, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, N.; Samuel, P.P.; Hiriyan, J.; Rajendran, R.; Dash, A. Observations on the multiple feeding behavior of Culex tritaeniorhynchus (Diptera: Culicidae), the vector of Japanese encephalitis in Kerala in southern India. Am. J. Trop. Med. Hyg. 2005, 72, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Keven, J.B.; Artzberger, G.; Gillies, M.L.; Mbewe, R.B.; Walker, E.D. Probe-based multiplex qPCR identifies blood-meal hosts in Anopheles mosquitoes from Papua New Guinea. Parasites Vectors 2020, 13, 111. [Google Scholar] [CrossRef]
- Lifson, A.R. Mosquitoes, models, and dengue. Lancet 1996, 347, 1201–1202. [Google Scholar] [CrossRef]
- Boreham, P.F.; Garrett-Jones, C. Prevalence of mixed blood meals and double feeding in a malaria vector (Anopheles sacharovi Favre). Bull. World Health Organ. 1973, 48, 605–614. [Google Scholar]
- Ponlawat, A.; Harrington, L.C. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J. Med. Entomol. 2005, 42, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Remadi, L.; Chargui, N.; Jiménez, M.; Molina, R.; Haouas, N.; González, E.; Chaabane-Banaouas, R.; Ben Salah, E.; Haddaji, M.; Chaabouni, Y.; et al. Molecular detection and identification of Leishmania DNA and blood meal analysis in Phlebotomus (Larroussius) species. PLoS Negl. Trop. Dis. 2020, 14, e0008077. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Gállego, M.; Molina, R.; Abras, A.; Alcover, M.M.; Ballart, C.; Fernández, A.; Jiménez, M. Identification of blood meals in field captured sand flies by a PCR-RFLP approach based on cytochrome b gene. Acta Trop. 2015, 152, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Maleki-Ravasan, N.; Oshaghi, M.; Javadian, E.; Rassi, Y.; Sadraei, J.; Mohtarami, F. Blood Meal Identification in Field-Captured Sand flies: Comparison of PCR-RFLP and ELISA Assays. Iran. J. Arthropod Borne Dis. 2009, 3, 8–18. [Google Scholar] [PubMed]
- Patnaude, M.R.; Mather, T.N. Blacklegged Tick or Deer Tick. Featured Creatures 2000 April 2021. Available online: https://entnemdept.ufl.edu/creatures/urban/medical/deer_tick.htm#syn (accessed on 11 February 2024).
- Clark, K.L.; Oliver, J.H., Jr.; McKechnie, D.B.; Williams, D.C. Distribution, abundance, and seasonal activities of ticks collected from rodents and vegetation in South Carolina. J. Vector Ecol. 1998, 23, 89–105. [Google Scholar] [PubMed]
- Newhouse, V.F. Variations in Population Density, Movement, and Rickettsial Infection Rates in a Local Population of Dermacentor variabilis (Acarina: Ixodidae) Ticks in the Piedmont of Georgia 1. Environ. Entomol. 1983, 12, 1737–1746. [Google Scholar] [CrossRef]
- Burg, J.G. Seasonal activity and spatial distribution of host-seeking adults of the tick Dermacentor variabilis. Med. Vet. Entomol. 2002, 15, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Spending, U. Advanced Search. Available online: https://www.usaspending.gov/ (accessed on 13 February 2024).
- Xu, G.; Wielstra, B.; Rich, S.M. Northern and southern blacklegged (deer) ticks are genetically distinct with different histories and Lyme spirochete infection rates. Sci. Rep. 2020, 10, 10289. [Google Scholar] [CrossRef]
- Monzón, J.D.; Atkinson, E.G.; Henn, B.M.; Benach, J.L. Population and evolutionary genomics of Amblyomma americanum, an expanding arthropod disease vector. Genome Biol. Evol. 2016, 8, 1351–1360. [Google Scholar] [CrossRef]
- Felz, M.W.; Durden, L.A. Attachment Sites of Four Tick Species (Acari: Ixodidae) Parasitizing Humans in Georgia and South Carolina. J. Med. Entomol. 1999, 36, 361–364. [Google Scholar] [CrossRef]
- Felz, M.W.; Durden, L.A.; Oliver, J.H., Jr. Ticks parasitizing humans in Georgia and South Carolina. J. Parasitol. 1996, 82, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Piesman, J. Experimental acquisition of the Lyme disease spirochete, Borrelia burgdorferi, by larval Ixodes dammini (Acari: Ixodidae) during partial blood meals. J. Med. Entomol. 1991, 28, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.M.; Spielman, A. Accelerated transmission of Lyme disease spirochetes by partially fed vector ticks. J. Clin. Microbiol. 1993, 31, 2878–2881. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.M.; Dietrich, G.; Dolan, M.C.; Sheldon, S.W.; Piesman, J.; Petersen, J.M.; Eisen, R.J. Transmission dynamics of Francisella tularensis subspecies and clades by nymphal Dermacentor variabilis (Acari: Ixodidae). Am. J. Trop. Med. Hyg. 2010, 83, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, K.R.; Sonenshine, D.E.; Ceraul, S.M.; Azad, A.F. Infection and transovarial transmission of rickettsiae in Dermacentor variabilis ticks acquired by artificial feeding. Vector Borne Zoonotic Dis. 2001, 1, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Varloud, M.; Liebenberg, J.; Fourie, J. Early Babesia canis transmission in dogs within 24 h and 8 h of infestation with infected pre-activated male Dermacentor reticulatus ticks. Parasites Vectors 2018, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Lysyk, T.J. Movement of Male Dermacentor andersoni (Acari: Ixodidae) Among Cattle. J. Med. Entomol. 2013, 50, 977–985. [Google Scholar] [CrossRef]
- Buczek, A.; Zając, Z.; Woźniak, A.; Kulina, D.; Bartosik, K. Locomotor activity of adult Dermacentor reticulatus ticks (Ixodida: Ixodidae) in natural conditions. Ann. Agric. Enviorn. Med. 2017, 24, 271–275. [Google Scholar] [CrossRef]
- Platt, K.B.; Linthicum, K.J.; Myint, K.; Innis, B.L.; Lerdthusnee, K.; Vaughn, D.W. Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am. J. Trop. Med. Hyg. 1997, 57, 119–125. [Google Scholar] [CrossRef]
- Killick-Kendrick, R.; Leaney, A.; Ready, P.; Molyneux, D. Leishmania in phlebotomid sandflies-IV. The transmission of Leishmania mexicana amazonensis to hamsters by the bite of experimentally infected Lutzomyia longipalpis. Proc. R. Soc. London. Ser. B. Biol. Sci. 1977, 196, 105–115. [Google Scholar]
- Botto-Mahan, C.; Cattan, P.E.; Medel, R. Chagas disease parasite induces behavioural changes in the kissing bug Mepraia spinolai. Acta Trop. 2006, 98, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, P.R.; Ross, Q.E.; Craig, G.B., Jr. Aedes Triseriatus (Diptera: Culicidae) and La Crosse Virus: II. Modification of mosquito feeding behavior by virus infection. J. Med. Entomol. 1980, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bacot, A.W.; Martin, C.J. LXVII. Observations on the mechanism of the transmission of plague by fleas. J. Hyg. 1914, 13, 423. [Google Scholar] [PubMed]
- Javed, N.; Bhatti, A.; Paradkar, P.N. Advances in Understanding Vector Behavioural Traits after Infection. Pathogens 2021, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.R. Interrupted feeding of blood-sucking insects: Causes and effects. Parasitol. Today 1990, 6, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Beatty, N.L.; Behrens-Bradley, N.; Love, M.; McCants, F.; Smith, S.; Schmidt, J.O.; Hamer, S.A.; Dorn, P.L.; Ahmad, N.; Klotz, S.A. Rapid detection of human blood in triatomines (kissing bugs) utilizing a lateral flow immunochromatographic assay—A pilot study. Mem. Inst. Oswaldo Cruz 2019, 114, e190047. [Google Scholar] [CrossRef] [PubMed]
- Borland, E.M.; Kading, R.C. Modernizing the Toolkit for Arthropod Bloodmeal Identification. Insects 2021, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.G.d.S.; Miranda, D.E.d.O.; Paiva, M.H.S.; Figueredo, L.A.; Otranto, D.; Dantas-Torres, F. Fast multiplex real-time PCR assay for simultaneous detection of dog and human blood and Leishmania parasites in sand flies. Parasites Vectors 2020, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- MR, V.D.S.; Gu, X.; Ward, M.P.; Kirkland, P.D. Development and evaluation of real-time PCR assays for bloodmeal identification in Culicoides midges. Med. Vet. Entomol. 2016, 30, 155–165. [Google Scholar] [CrossRef]
- Koch, H.G. Suitability of white-tailed deer, cattle, and goats as hosts for the lone star tick, Amblyomma americanum (Acari: Ixodidae). J. Kans. Entomol. Soc. 1988, 61, 251–257. [Google Scholar]
- Lefcort, H.; Durden, L. The effect of infection with Lyme disease spirochetes (Borrelia burgdorferi) on the phototaxis, activity, and questing height of the tick vector Ixodes scapularis. Parasitology 1996, 113, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Faulde, M.K.; Robbins, R.G. Tick infestation risk and Borrelia burgdorferi s.l. infection-induced increase in host-finding efficacy of female Ixodes ricinus under natural conditions. Exp. Appl. Acarol. 2008, 44, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Estrada-Peña, A.; Rego, R.O.; De la Fuente, J. Tick-pathogen ensembles: Do molecular interactions lead ecological innovation? Front. Cell. Infect. Microbiol. 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Alberdi, P.; Ayllón, N.; Valdés, J.J.; Pierce, R.; Villar, M.; De la Fuente, J. Anaplasma phagocytophilum increases the levels of histone modifying enzymes to inhibit cell apoptosis and facilitate pathogen infection in the tick vector Ixodes scapularis. Epigenetics 2016, 11, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Alberdi, P.; Valdes, J.J.; Villar, M.; De la Fuente, J. Anaplasma phagocytophilum infection subverts carbohydrate metabolic pathways in the tick vector, Ixodes scapularis. Front. Cell. Infect. Microbiol. 2017, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Gaines, D.N.; Operario, D.J.; Stroup, S.; Stromdahl, E.; Wright, C.; Gaff, H.; Broyhill, J.; Smith, J.; Norris, D.E.; Henning, T.; et al. Ehrlichia and spotted fever group Rickettsiae surveillance in Amblyomma americanum in Virginia through use of a novel six-plex real-time PCR assay. Vector Borne Zoonotic Dis. 2014, 14, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.Y.; Chung, I.H.; Robinson, L.K.; Austin, A.L.; Dasch, G.A.; Massung, R.F. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J. Clin. Microbiol. 2013, 51, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Stromdahl, E.Y.; Richards, A.L. Detection of Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Gulf Coast ticks collected from humans in the United States. Vector Borne Zoonotic Dis. 2012, 12, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.B.; Maes, S.E.; Hojgaard, A.; Fleshman, A.C.; Sheldon, S.W.; Eisen, R.J. A molecular algorithm to detect and differentiate human pathogens infecting Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae). Ticks Tick-Borne Dis. 2018, 9, 390–403. [Google Scholar] [CrossRef]
- Courtney, J.W.; Kostelnik, L.M.; Zeidner, N.S.; Massung, R.F. Multiplex Real-Time PCR for Detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J. Clin. Microbiol. 2004, 42, 3164–3168. [Google Scholar] [CrossRef]
- Dinkel, K.D.; Herndon, D.R.; Noh, S.M.; Lahmers, K.K.; Todd, S.M.; Ueti, M.W.; Scoles, G.A.; Mason, K.L.; Fry, L.M. A US isolate of Theileria orientalis, Ikeda genotype, is transmitted to cattle by the invasive Asian longhorned tick, Haemaphysalis longicornis. Parasites Vectors 2021, 14, 157. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).