Climate as a Predictive Factor for Invasion: Unravelling the Range Dynamics of Carpomya vesuviana Costa
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Geographical Distribution Data
2.2. Environmental Variables
2.3. Accuracy Evaluation of Single and Ensemble Models
2.4. Migration of Centres of Potential Geographical Distributions and Overlapping Distribution Areas
2.5. Quantification of the Ecological Niche
2.6. Analysis of MESS and Most Dissimilar Variable (MoD)
3. Results
3.1. Model Accuracy Evaluation
3.2. Current Potential Global Geographic Distribution of C. vesuviana
3.3. Ecological Niche Analysis
3.4. Future Potential Global Geographic Distribution of C. vesuviana
3.5. Changes in Spatial Patterns of C. vesuviana
3.6. MESS and MoD Variable Analysis
3.7. Environmental Factor Response Curves for C. vesuviana
3.8. Centres of Potential Geographical Distributions of C. vesuviana
4. Discussion
4.1. Impact of Environmental and Distributional Data on the Performance of SDMs
4.2. Relationships between Environmental Variables and Changes in the Potential Geographical Distribution of C. vesuviana
4.3. Ecological Niche Dynamics of C. vesuviana
4.4. Applicability and Limitations of Model Predictions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcrof, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rohr, J.; Cui, R.; Xin, Y.; Han, L.; Yang, X.; Gu, S.; Du, Y.; Liang, J.; Wang, X.; et al. Biological invasions facilitate zoonotic disease emergences. Nat. Commun. 2022, 13, 1762. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Climate change and biological invasions: Evidence, expectations, and response options. Biol. Rev. 2017, 92, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; Kleunen, M.; Kühn, I.; et al. Projecting the continental accumulation of alien species through to 2050. Glob. Change Biol. 2021, 27, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Kenis, M.; Auger-Rozenberg, M.A.; Roques, A.; Timms, L.; Péré, C.; Cock, M.J.W.; Settele, J.; Augustin, S.; Lopez-Vaamonde, C. Ecological effects of invasive alien insects. Biol. Invasions 2009, 11, 21–45. [Google Scholar] [CrossRef]
- Mclaughlin, G.M.; Dearden, P.K. Invasive insects: Management methods explored. J. Insect Sci. 2019, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Unwelcome exchange: International trade as a direct and indirect driver of biological invasions worldwide. One Earth 2021, 4, 666–679. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.U.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef]
- Gyi, M.M.; Lai, O.P.; Dikshit, A.K.; Sharma, V.P. Eficacy of insecticides for controlling ber fruit fly. Ann. Plant Prot. Sci. 2003, 11, 152–153. [Google Scholar]
- He, S. Study on the Bioecology of Carpomya vesuviana Costa and Theforecast of Its Potential Geographic Distribution in China. Master’s Thesis, Beijing Forestry University, Beijing, China, 2009; p. 11. [Google Scholar]
- Stonehouse, J.M.; Mumford, J.D.; Mustafa, G. Economic losses to Tephritid fruit flies (Diptera: Tephritidae) in Pakistan. Crop Prot. 1998, 17, 159–164. [Google Scholar] [CrossRef]
- Dashad, S.S.; Chaudhary, O.P.; Rakesh, O. Studies on the incidence of ber fruit fly (Carpomyia vesuviana Costa) in southwestern Haryana. Crop Res. 1999, 18, 115–118. [Google Scholar]
- Pareek, S.; Fagera, M.S.; Dhaka, R.S. Genetic variability and association analysis for fruit fly (Carpomyia vesuviana Costa) infestation in ber. Indian J. Plant Prot. 2003, 31, 89–90. [Google Scholar]
- Zhang, R.; Wang, X.; Adil, S. Identification and precaution of the ber fruit fly, Carpomya vesuviana, a quarantine pest insect in China. Chin. Bull. Entomol. 2007, 6, 928–930+951. [Google Scholar]
- Azam, K.M.; Al-Ansari, M.S.; Al-Raeesi, A. Fruit flies of Oman with a new record of Carpomya vasuviana Costa (Diptera: Tephritidae). Res. Crops 2004, 5, 274–277. [Google Scholar]
- Farrar, N.; Asadi, G.H.; Golestaneh, S.R. Damage and host ranges of Ber Fruit fly Carpomyia vesuviana Costa (Tephritidae) and its rate of parasitism. J. Agric. Sci. 2004, 5, 120–130. [Google Scholar]
- Adil, S.; He, S.; Tian, C.; Luo, Y.; Yu, F.; Feng, X. Theoccurrence of Carpomya vesuviana Costa in Turpan and the distribution of pupae. Plant Quar. 2008, 22, 295–297. [Google Scholar]
- Duncan, R.P.; Blackburn, T.M.; Veltman, C.J. Determinants of geographical range sizes: A test using introduced New Zealand birds. J. Anim. Ecol. 1999, 68, 963–975. [Google Scholar] [CrossRef]
- Duncan, R.P.; Bomford, M.; Forsyth, D.M.; Conibear, L. High predictability in introduction outcomes and the geographical range size of introduced Australian birds: A role for climate. J. Anim. Ecol. 2001, 70, 621–632. [Google Scholar] [CrossRef]
- Forsyth, D.M.; Duncan, R.P.; Bomford, M.; Moore, G. Climatic suitability, life-history traits, introduction effort, and the establishment and spread of introduced mammals in Australia. Conserv. Biol. 2004, 18, 557–569. [Google Scholar] [CrossRef]
- He, S.; Zhu, Y.; Adil, S.; Yu, F.; Wen, J.; Tian, C. Occurrence of Carpomya vesuviana in Turpan region. Chin. Bull. Entomol. 2009, 46, 930–934+822. [Google Scholar]
- Soberon, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Wiens, J.A.; Stralberg, D.; Jongsomjit, D.; Howell, C.A.; Snyder, M.A. Niches, models, and climate change: Assessing the assumptions and uncertainties. Proc. Natl. Acad. Sci. USA 2009, 106, 19729–19736. [Google Scholar] [CrossRef] [PubMed]
- Schickele, A.; Leroy, B.; Beaugrand, G.; Goberville, E.; Hattab, T.; Francour, P.; Raybaud, V. Modelling European small pelagic fish distribution: Methodological insights. Ecol. Model. 2020, 416, 108902. [Google Scholar] [CrossRef]
- Ramasamy, M.; Das, B.; Ramesh, R. Predicting climate change impacts on potential worldwide distribution of fall armyworm based on CMIP6 projections. J. Pest Sci. 2022, 95, 841–854. [Google Scholar] [CrossRef]
- Herrera, C.; Jurado-Rivera, J.A.; Leza, M. Ensemble of small models as a tool for alien invasive species management planning: Evaluation of Vespa velutina (Hymenoptera: Vespidae) under Mediterranean Island conditions. J. Pest Sci. 2023, 96, 359–371. [Google Scholar] [CrossRef]
- Finch, E.A.; Beale, T.; Chellappan, M.; Goergen, G.; Gadratagi, B.G.; Khan, M.A.M.; Rehman, A.; Rwomushana, I.; Sarma, A.K.; Wyckhuys, K.A.J.P.M.S. The potential global distribution of the papaya mealybug, Paracoccus marginatus, a polyphagous pest. Pest Manag. Sci. 2021, 77, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Dormann, C.F.; Calabrese, J.M.; Guillera-Arroita, G.; Matechou, E.; Bahn, V.; Bartoń, K.; Beale, C.M.; Ciuti, S.; Elith, J.; Gerstner, K.; et al. Model averaging in ecology: A review of Bayesian, information theoretic and tactical approaches. Ecol. Monogr. 2018, 88, 485–504. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Y.; Wang, T.; Jiang, X.; Hu, X.; Feng, J. Double-edged effects of climate change on plant invasions: Ecological niche modeling global distributions of two invasive alien plants. Sci. Total Environ. 2020, 740, 139933. [Google Scholar] [CrossRef]
- Lázaro-Lobo, A.; Ramirez-Reyes, C.; Lucardi, R.D.; Ervin, G.N. Multivariate analysis of invasive plant species distributions in southern US forests. Landsc. Ecol. 2021, 36, 3539–3555. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Ma, P.; Kumar, S.; Rocca, M.; Morisette, J.T.; Jarnevich, C.S.; Benson, N. Ensemble habitat mapping of invasive plant species. Risk Anal. Agric. Technol. 2010, 30, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, H.; Lv, Z. Evaluating the performance of species distribution models Biomod 2 and MaxEnt using the giant panda distribution data. Chin. J. Appl. Ecol. 2017, 28, 4001–4006. [Google Scholar]
- Zhao, J.; Jiang, C.; Ding, Y.; Li, G.; Li, Q. Analyzing potential distribution and disturbance intensity of plateau pika in the source region of the Yellow River via BIOMOD2 integrated model. Chin. J. Ecol. 2024, 43, 1192–1201. [Google Scholar]
- Aldo, P.; Pierco, C. Carpomya vesuviana A. Costa (Diptera Tephritidae Trypetinae Carpomyini) from jujube tree in Emilia-Romagna (Northern Italy). Redia 2014, 97, 177–178. [Google Scholar]
- Alieh, A.; Arkady, S.L.; Hussein, S.; Javad, K. First record of the velvet ants (Hymenoptera: Mutillidae) reared from puparia of the ber fruit fly Carpomya vesuviana Costa (Diptera: Tephritidae) in Iran. Zootaxa 2014, 3861, 585–590. [Google Scholar]
- Amini, A.; Sadeghi, H.; Lotfalizadeh, H.; Notton, D.G. Parasitoids (Hymenoptera: Pteromalidae, Diapriidae) of Carpomya vesuviana Costa (Diptera: Tephritidae) in South Khorasan province of Iran. Biharean Biol. 2014, 8, 141206. [Google Scholar]
- Haldhar, S.M.; Bhargava, R.; Krishna, H.; Berwal, M.K.; Saroj, P.L. Bottom-up effects of different host plant resistance cultivars on ber (Ziziphus mauritiana)-fruit fly (Carpomyia vesuviana) interactions. Crop Prot. 2018, 106, 117–124. [Google Scholar] [CrossRef]
- Jayant, Y.; Ram, K.G.; Meenakshi, D. Varietal/germplasm screening of Ber, Ziziphus mauritiana (Lamk) against Ber fruit fly, Carpomyia vesuviana (Costa) under semi-arid conditions of Haryana. J. Entomol. Zool. Stud. 2018, 6, 908–911. [Google Scholar]
- Shivbhagvan; Acharya, V.S.; Sharma, M. Efficacy of Insecticides/Botanicals against Ber Fruit Fly, Carpomyia vesuviana Costa on Ber. Ann. Biol. 2018, 34, 79–83. [Google Scholar]
- Chonticha, K.; Pairot, P. DNA barcoding of fruit flies (Diptera: Tephritidae) in Thailand: Ambiguity, misidentification and cryptic diversity. Mitochondrial DNA Part A 2019, 30, 861–873. [Google Scholar]
- Gaur, R.K.; Kumar, M.; Sharma, S.; Yadav, B.S. Survey studies on insects and non insect pest associated with ber crop in South West Haryana. J. Entomol. Zool. Stud. 2020, 8, 856–863. [Google Scholar]
- Jayant, Y.; Ram, K.G.; Yogesh, K.; Nisha, K.; Surender, S.Y.; Mujahid, K.; Yadav, S. Evaluation of antixenotic and allelochemical traits of ber (Ziziphus mauritiana Lamk.) fruits as source of host plant resistance against fruit fly (Carpomyia vesuviana Costa) (Diptera: Tephritidae) in semi-arid region of India. Phytoparasitica 2020, 48, 607–620. [Google Scholar]
- Haldhar, S.M. Biology of Ber Fruit Fly Carpomyia Vesuviana Costa. Indian J. Entomol. 2021, 83, 423–426. [Google Scholar] [CrossRef]
- Filiz, A.; Uludağ, A.; Kütük, H.; Jabran, K. A preliminary study on arthropods as potential biological control agents for management of alien ornamental plants in Turkey. Acta Herbol. 2022, 31, 27–42. [Google Scholar] [CrossRef]
- He, Y.; Xu, Y.; Chen, X. Biology, ecology and management of Tephritid fruit flies in China: A review. Insects 2023, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Alieh, A.; Hossein, L.; Francisco, J.P.F.; Jean-Yvse, R. Potential Parasitoids for Biocontrol of the Ber Fruit Fly, Carpomya vesuviana Costa (Diptera: Tephritidae). Life 2024, 14, 50. [Google Scholar]
- Karuppaiah, V. Biology and management of ber fruit fly, Carpomyia vesuviana Costa (Diptera: Tephritidae): A review. Afr. J. Agric. Res. 2014, 9, 1310–1317. [Google Scholar]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Ye, Y.; Zhou, G.; Yin, X. Changes in distribution and productivity of steppe vegetation in Inner Mongolia during 1961 to 2010: Analysis based on MaxEnt model and synthetic model. Acta Ecol. Sin. 2006, 36, 4718–4728. [Google Scholar]
- Wang, R.; Li, Q.; Feng, C.; Shi, Z. Predicting potential ecological distribution of Locusta migratoria tibetensis in China using MaxEnt ecological niche modeling. Acta Ecol. Sin. 2017, 37, 8556–8566. [Google Scholar]
- Sayit, H.; Nurbay, A.; Xu, Z.L.; Arman, J. Analysis of Potential Distribution and Suitable Area of Calligonum mongolicumin Xinjiang Based on MaxEnt Model. J. Northwest A&F Univ. 2018, 33, 71–77. [Google Scholar]
- Zhang, X.; Li, G.; Du, S. Predicting the influence of future climate change on the suitable distribution areas of Elaeagnus angustifolia. Chin. J. Appl. Ecol. 2018, 29, 3213–3220. [Google Scholar]
- Lakra, R.K.; Zile Singh, Z.S. Seasonal fluctuations in incidence of ber fruitfly Carpomyia vesuviana Costa (Diptera: Tephritidae) under Agro-climatic conditions of Hisar. Haryana Agric Univ. J. Res. 1985, 15, 42–50. [Google Scholar]
- Peng, S.; Zhang, J.; Zhang, X.; Li, Y.; Liu, Y.; Wang, Z. Conservation of woody species in China under future climate and land-cover changes. J. Appl. Ecol. 2021, 59, 141–152. [Google Scholar] [CrossRef]
- Peng, S.; Hu, R.; Santiago, J.E.V.; Luo, Y.; Tong, L.; Zhang, X.; Zhang, J.; Wang, Z. Preserving the woody plant tree of life in China under future climate andland-cover changes. Proc. R. Soc. B 2022, 289, 20221497. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Lv, Y.; Jin, L.; Huang, J.; Su, B.; Tao, H. Overview of the new scenario of CMIP6 model (SSP-RCP) and its application in the HuaiHe river basin. Adv. Meteorol. Sci. Technol. 2020, 10, 102–109. [Google Scholar]
- Akinwande, M.O.; Dikko, H.G.; Samson, A. Variance inflation factor: As a condition for the inclusion of suppressor variable(s) in regression analysis. Open J. Stat. 2015, 5, 62189. [Google Scholar] [CrossRef]
- Vu, D.H.; Muttaqi, K.M.; Agalgaonkar, A.P. A variance inflation factor and backward elimination based robust regression model for forecasting monthly electricity demand using climatic variables. Appl. Energy 2015, 140, 385–394. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitao, P.J. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Zhao, G.; Cui, X.; Sun, J.; Li, T.; Wang, Q.; Ye, X.; Fan, B. Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized Biomod2 and MaxEnt models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Xian, X.; Zhao, H.; Wang, R.; Huang, H.; Chen, B.; Zhang, G.; Liua, W.; Wan, F. Climate change has increased the global threats posed by three ragweeds (Ambrosia L.) in the Anthropocene. Sci. Total Environ. 2023, 859, 160252. [Google Scholar] [CrossRef]
- Brown, J. SDM toolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, Y.; Ma, S.; Wang, C.; Zhang, D. Simulation of suitable distribution of goji berries in XinJiang and local environmental adaptation differentiation. Chin. J. Plant Ecol. 2021, 45, 1221–1230. [Google Scholar] [CrossRef]
- Dan, L.; Richard, E.; Michael, T. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2713–2951. [Google Scholar]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Zhang, Y. Prediction of Global Potential Suitable Habitats of Nicotiana alata Link et Otto Based on MaxEnt Model. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2023. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Fang, Y. Responses of the distribution pattern of Quercus Chenii to climate change following the Last Glacial Maximum. Chin. J. Plant Ecol. 2016, 40, 1164–1178. [Google Scholar]
- Araújo, M.B.; Peterson, A.T. Uses and misuses of bioclimatic envelope modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef]
- Abdulwahab, U.A.; Hammill, E.; Hawkins, C.P. Choice of climate data affects the performance and interpretation of species distribution models. Ecol. Model. 2022, 471, 110042. [Google Scholar] [CrossRef]
- Petitpierre, B.; Broennimann, O.; Kueffer, C.; Daehler, C.; Guisan, A. Selecting predictors to maximize the transferability of species distribution models: Lessons from cross- continental plant invasions. Glob. Ecol. Biogeogr. 2017, 26, 275–287. [Google Scholar] [CrossRef]
- Roberts, D.R.; Bahn, V.; Ciuti, S.; Boyce, M.S.; Elith, J.; Guillera-Arroita, G.; Hauenstein, S.; Lahoz-Monfort, J.J.; Schröder, B.; Thuiller, W.; et al. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography 2017, 40, 913–929. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V. Residence time determines the distribution of alien plants. In Invasive Plants: Ecological and Agricultural Aspects; Birkhäuser Basel: Basel, Switzerland, 2005; pp. 77–96. [Google Scholar]
- Schmidt, J.P.; Drake, J.M.; Stephens, P. Residence time, native range size, and genome size predict naturalization among angiosperms introduced to Australia. Ecol. Evol. 2017, 7, 10289–10300. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, C.; Gentili, R.; Smith, M.; Guarino, M.F.; Citterio, S. The worldwide spread, success, and impact of ragweed (Ambrosia spp.). Crit. Rev. Plant Sci. 2017, 36, 139–178. [Google Scholar] [CrossRef]
- Cao, P.L.; Axmanová, I.; Chytrý, M.; Malavasi, M.; Acosta, A.T.R.; Giulio, S.; Attorre, F.; Bergmeier, E.; Biurrun, I.; Campos, J.A.; et al. The biogeography of alien plant invasions in the Mediterranean Basin. J. Veg. Sci. 2021, 32, e12980. [Google Scholar]
- Lü, W.; Lin, W.; Li, Z.; Geng, J.; Wan, F.; Wang, Z. Potential geographic distribution of Ber fruit fly, Carpomya vesuviana Costa, in China. Plant Quar. 2008, 6, 343–347. [Google Scholar]
- Adil, S.; Wen, J.; Luo, Y.; He, S.; Yu, F.; Chen, M. Impacts of main meteorological factors on emergence rate of Carpomyia vesuviana overwinter generation adult in turpan. For. Res. 2012, 25, 540–544. [Google Scholar]
- Guo, S.; Ge, X.; Zou, Y.; Zhou, Y.; Wang, T.; Zong, S. Projecting the Potential Global Distribution of Carpomya vesuviana (Diptera: Tephritidae), Considering Climate Change and Irrigation Patterns. Forests 2019, 10, 355. [Google Scholar] [CrossRef]
- Zachariah, A.D.; Barney, J.N. Climatic niche shifts in 815 introduced plant species affect their predicted distributions. Glob. Ecol. Biogeogr. 2021, 30, 1671–1684. [Google Scholar] [CrossRef]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of consensus methods in predictive species distribution modelling. Divers. Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Wen, X.; Fang, G.; Chai, S.; He, C.; Sun, S.; Zhao, G.; Lin, X. Can ecological niche models be used to accurately predict the distribution of invasive insects? A case study of Hyphantria cunea in China. Ecol. Evol. 2024, 14, e11159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dang, Y.; Wang, X. Risk analysis of dispersal and outbreak of Massicus raddei (Coleoptera: Cerambycidae) in China based on climate and host distribution. Sci. Silvae Sin. 2022, 58, 95–109. [Google Scholar]
- Han, L.; Zhang, Z.; Tu, W.; Zhang, Q.; Hong, Y.; Chen, S.; Liu, X. Preferred prey reduce species realized niche shift and improve range expansion prediction. Sci. Total Environ. 2023, 859, 160370. [Google Scholar] [CrossRef]
- Miller, J.R.; Turner, M.G.; Smithwick, E.A.; Dent, C.L.; Stanley, E.H. Spatial extrapolation: The science of predicting ecological patterns and processes. BioScience 2004, 54, 310–320. [Google Scholar] [CrossRef]
- Sheth, S.N.; Angert, A.L. The evolution of environmental tolerance and range size: A comparison of geographically restricted and widespread Mimulus. Evolution 2014, 68, 2917–2931. [Google Scholar] [CrossRef]
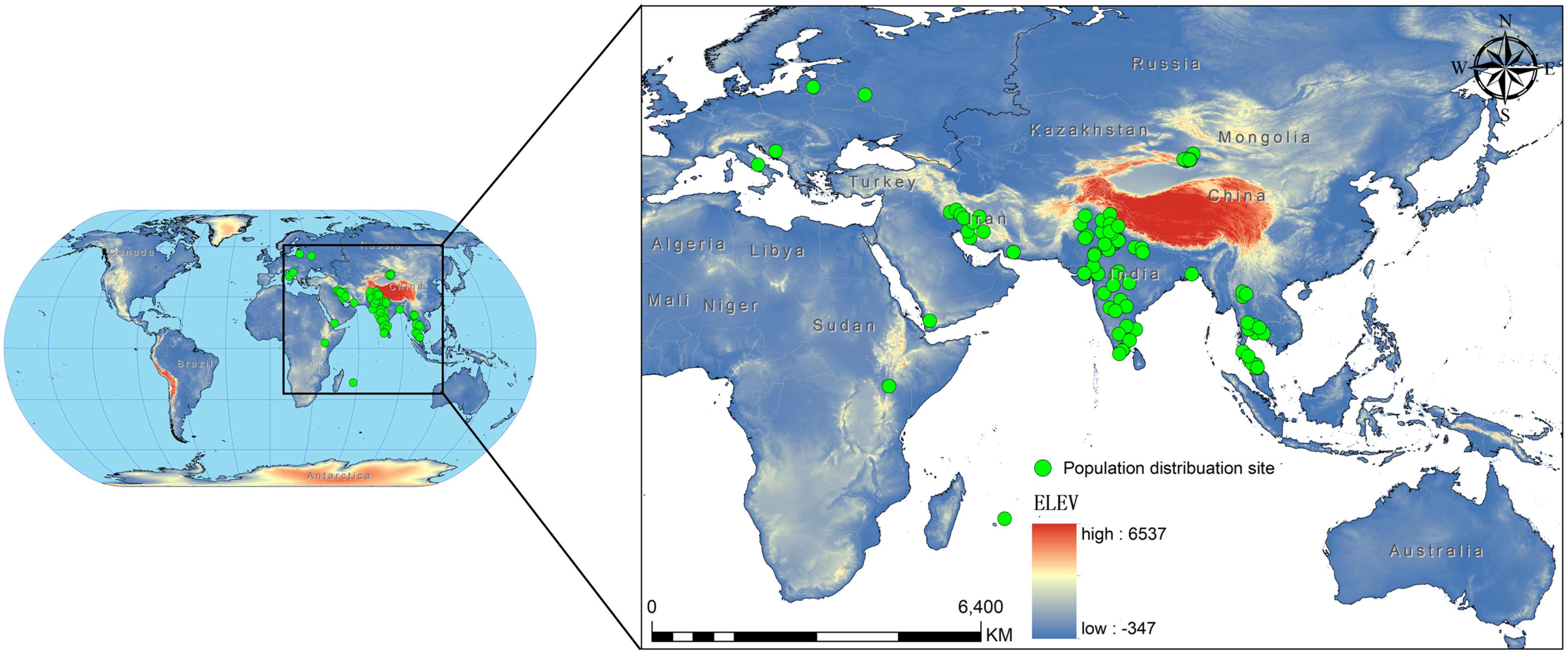

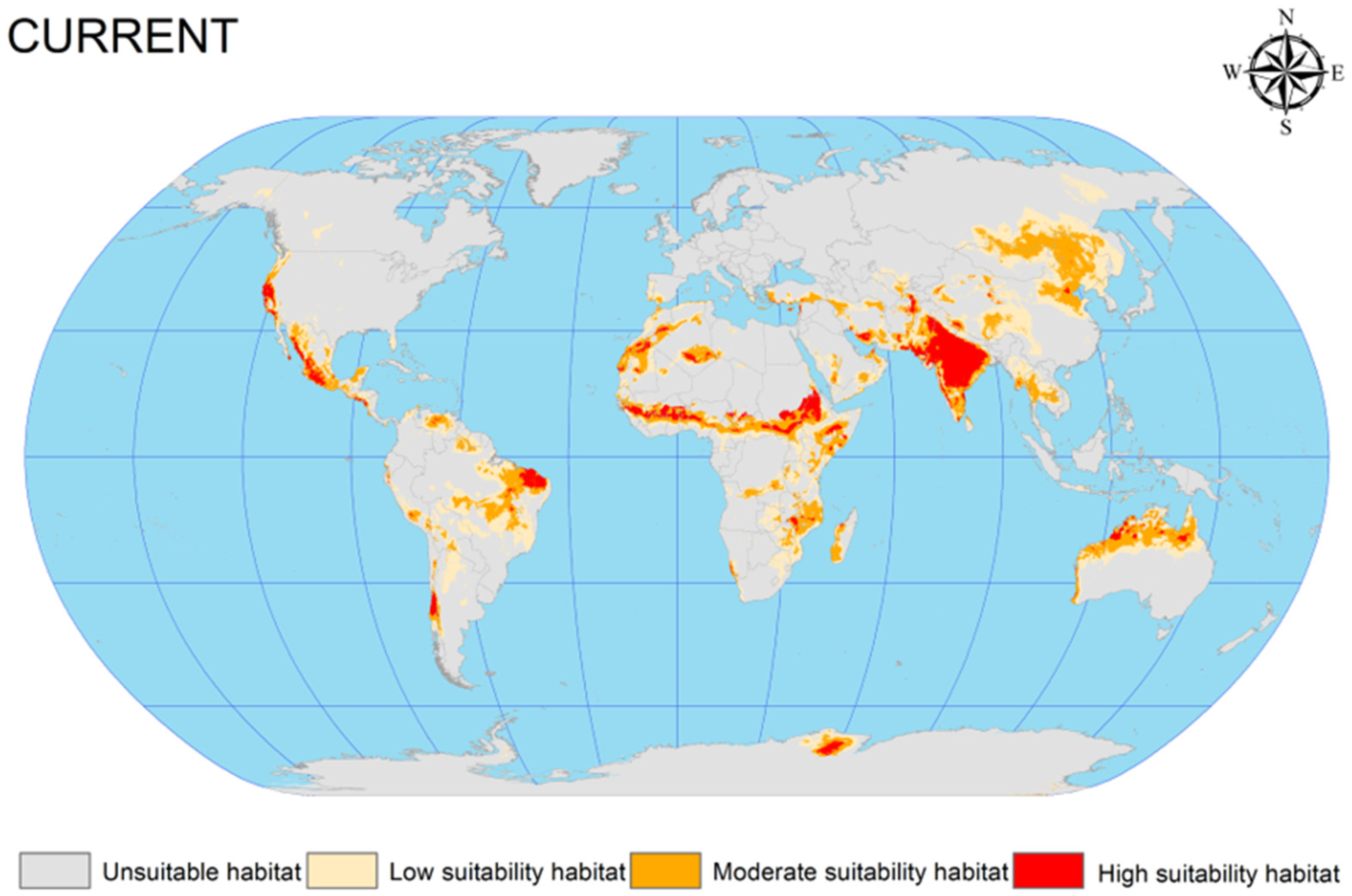
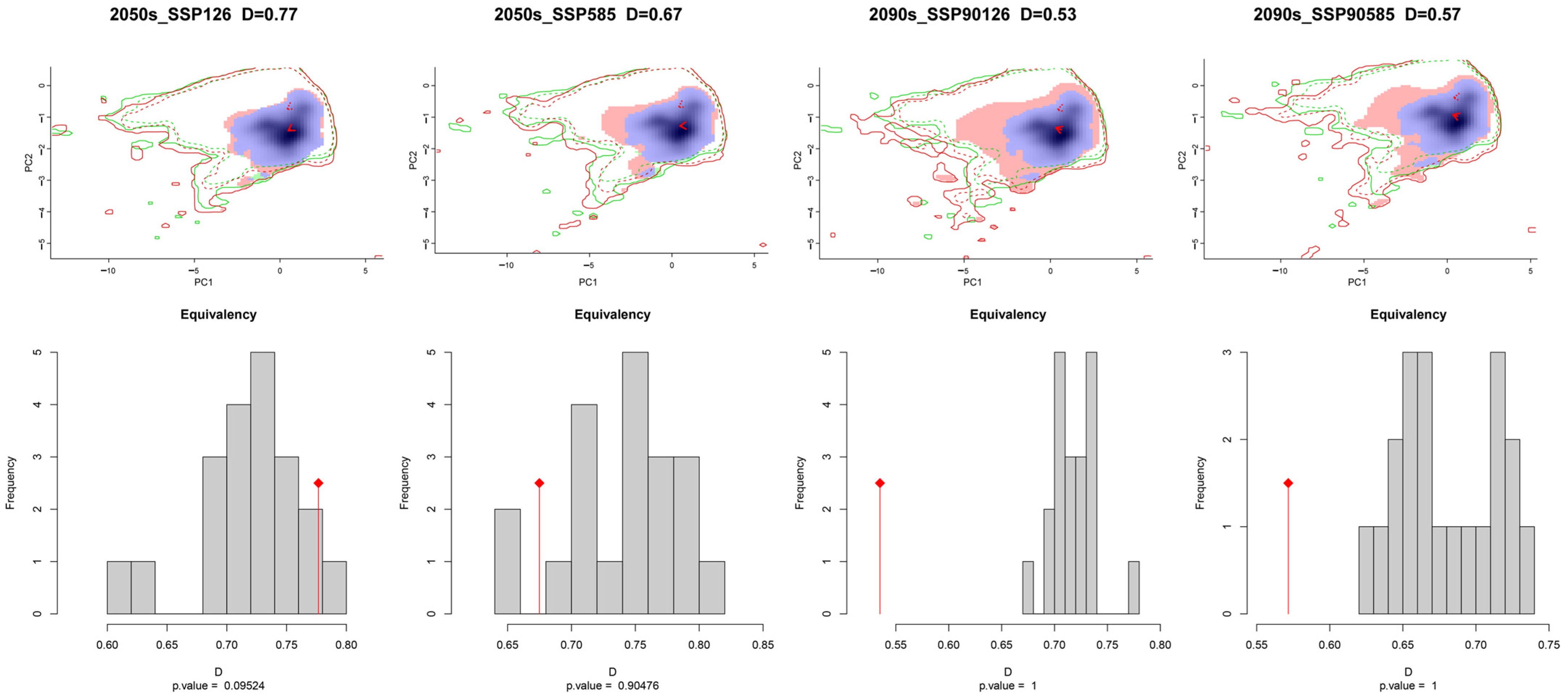
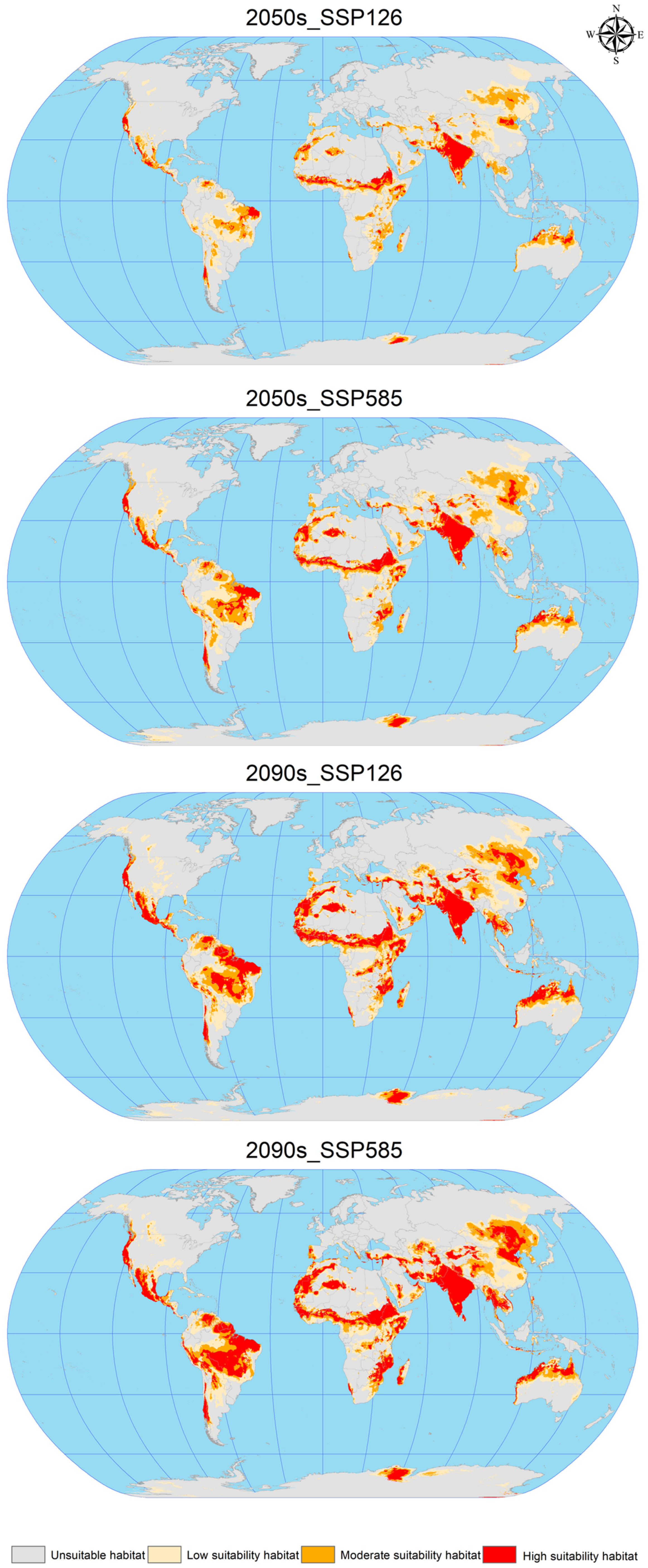

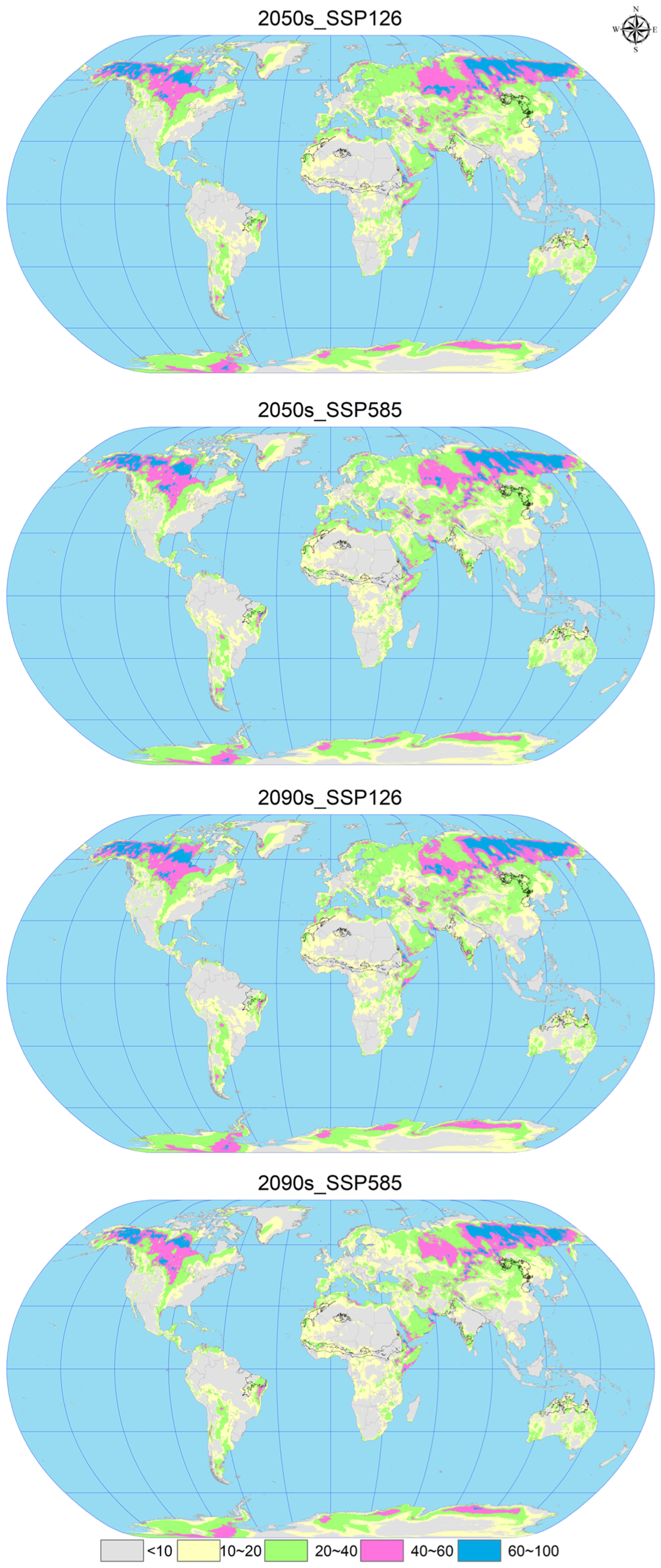
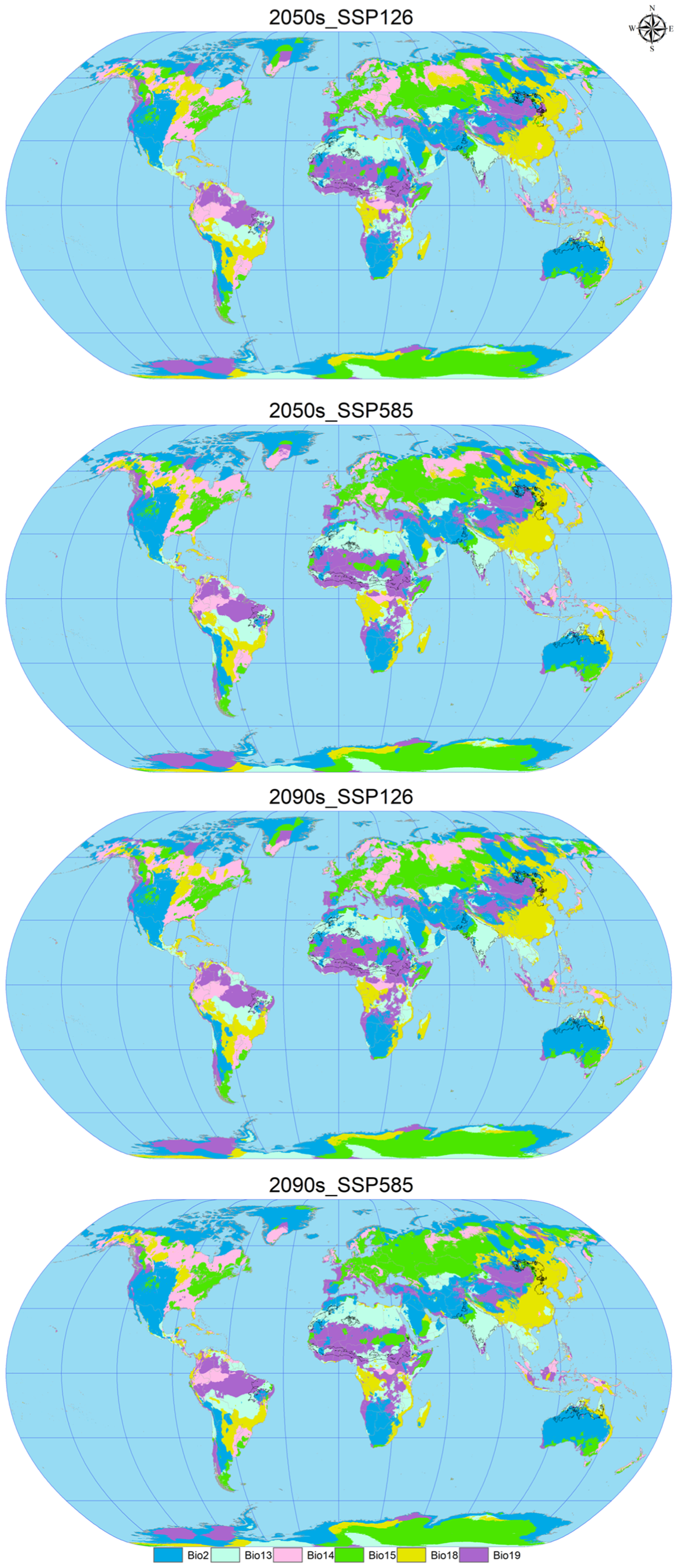
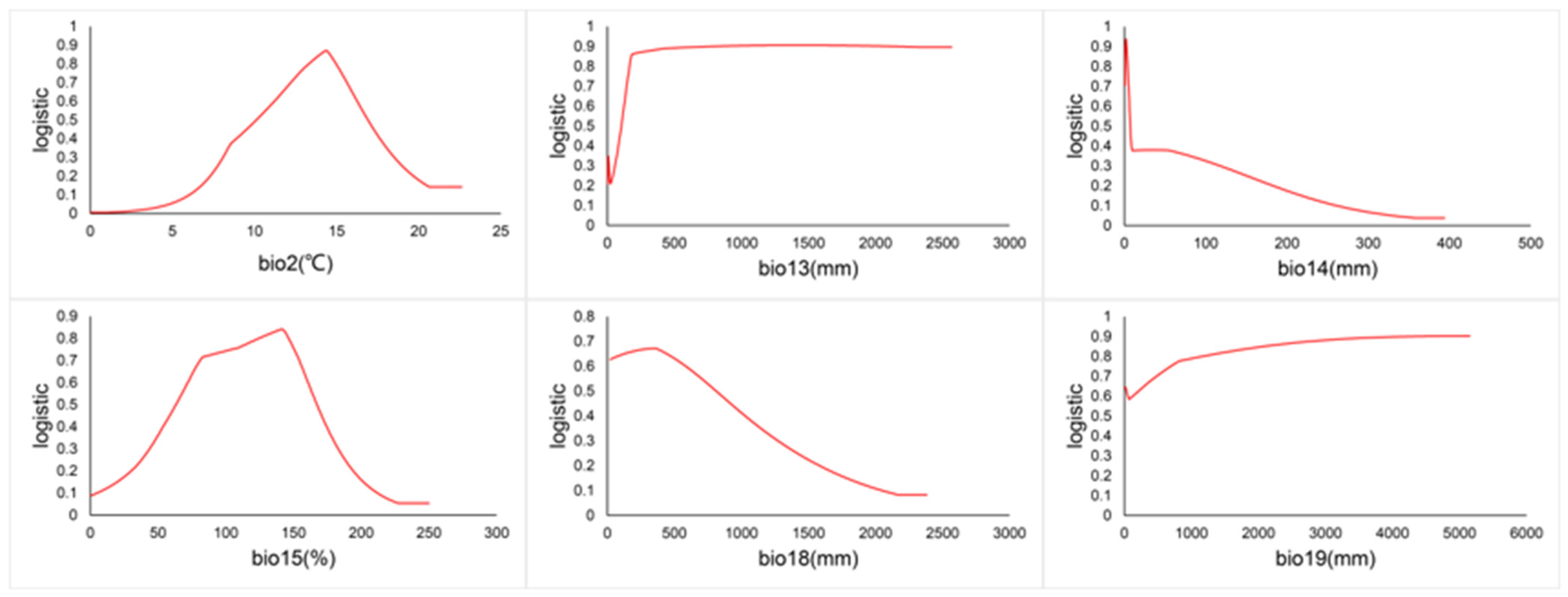

| Environmental Factors | Factor Name | Variable Name | Work Unit | VIF | Contribution | Permutation Importance |
|---|---|---|---|---|---|---|
| Bioclimatic factors | Bio2 | Mean Diurnal Range | °C | 3.8343 | 8.1146 | 1.4312 |
| Bio13 | Precipitation of Wettest Month | mm | 6.3589 | 27.4789 | 13.6867 | |
| Bio14 | Precipitation of Driest Month | mm | 3.8343 | 7.4252 | 6.0334 | |
| Bio15 | Precipitation Seasonality | % | 5.4600 | 27.1387 | 32.7995 | |
| Bio18 | Precipitation of Warmest Quarter | mm | 3.3828 | 21.0308 | 34.1939 | |
| Bio19 | Precipitation of Coldest Quarter | mm | 4.6173 | 8.8118 | 11.8552 |
| Habitat Type | Unsuitable | Low | Moderate | High |
|---|---|---|---|---|
| Area | 12,516.24 | 1237.66 | 862.36 | 283.72 |
| Percentage (%) | 84.00 | 8.30 | 5.78 | 1.90 |
| Climate Scenarios | Niche Width | |
|---|---|---|
| B1 | B2 | |
| Proj50126 | 0.3814 | 0.9606 |
| Proj50585 | 0.4353 | 0.9662 |
| Proj90126 | 0.4808 | 0.9705 |
| Proj90585 | 0.4612 | 0.9681 |
| Projcurrent | 0.3735 | 0.9601 |
| Climate Scenarios | Unsuitable | Low | Moderate | High | ALL |
|---|---|---|---|---|---|
| 2050s_SSP126 | 12,293.47 | 1336.58 | 898.67 | 371.27 | 2606.52 |
| 2050s_SSP585 | 11,477.49 | 1598.69 | 1143.23 | 680.58 | 3422.50 |
| 2090s_SSP126 | 10,802.94 | 1722.09 | 1204.86 | 1170.09 | 4097.51 |
| 2090s_SSP585 | 10,776.49 | 1642.67 | 1077.86 | 1402.96 | 4123.50 |
| Climate Scenarios | Range Expansion | No Occupancy | No Change | Range Contraction | Gain (%) | Loss (%) |
|---|---|---|---|---|---|---|
| 2050s_SSP126 | 369.85 | 13,294.76 | 1210.24 | 72.87 | 15.48 | 3.05 |
| 2050s_SSP585 | 1396.02 | 12,268.60 | 1258.45 | 24.65 | 58.58 | 1.03 |
| 2090s_SSP126 | 2413.06 | 11,251.55 | 1277.33 | 5.78 | 101.25 | 0.24 |
| 2090s_SSP585 | 2956.58 | 10,708.03 | 1270.28 | 12.8360 | 124.04 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Guo, F.; Gao, G. Climate as a Predictive Factor for Invasion: Unravelling the Range Dynamics of Carpomya vesuviana Costa. Insects 2024, 15, 374. https://doi.org/10.3390/insects15060374
Feng C, Guo F, Gao G. Climate as a Predictive Factor for Invasion: Unravelling the Range Dynamics of Carpomya vesuviana Costa. Insects. 2024; 15(6):374. https://doi.org/10.3390/insects15060374
Chicago/Turabian StyleFeng, Chuangju, Facheng Guo, and Guizhen Gao. 2024. "Climate as a Predictive Factor for Invasion: Unravelling the Range Dynamics of Carpomya vesuviana Costa" Insects 15, no. 6: 374. https://doi.org/10.3390/insects15060374
APA StyleFeng, C., Guo, F., & Gao, G. (2024). Climate as a Predictive Factor for Invasion: Unravelling the Range Dynamics of Carpomya vesuviana Costa. Insects, 15(6), 374. https://doi.org/10.3390/insects15060374




