Molecular Data Confirm Interspecific Limits of Four Alloxysta and One Phaenoglyphis Species of Parasitic Wasps within the Subfamily Charipinae (Cynipoidea: Figitidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
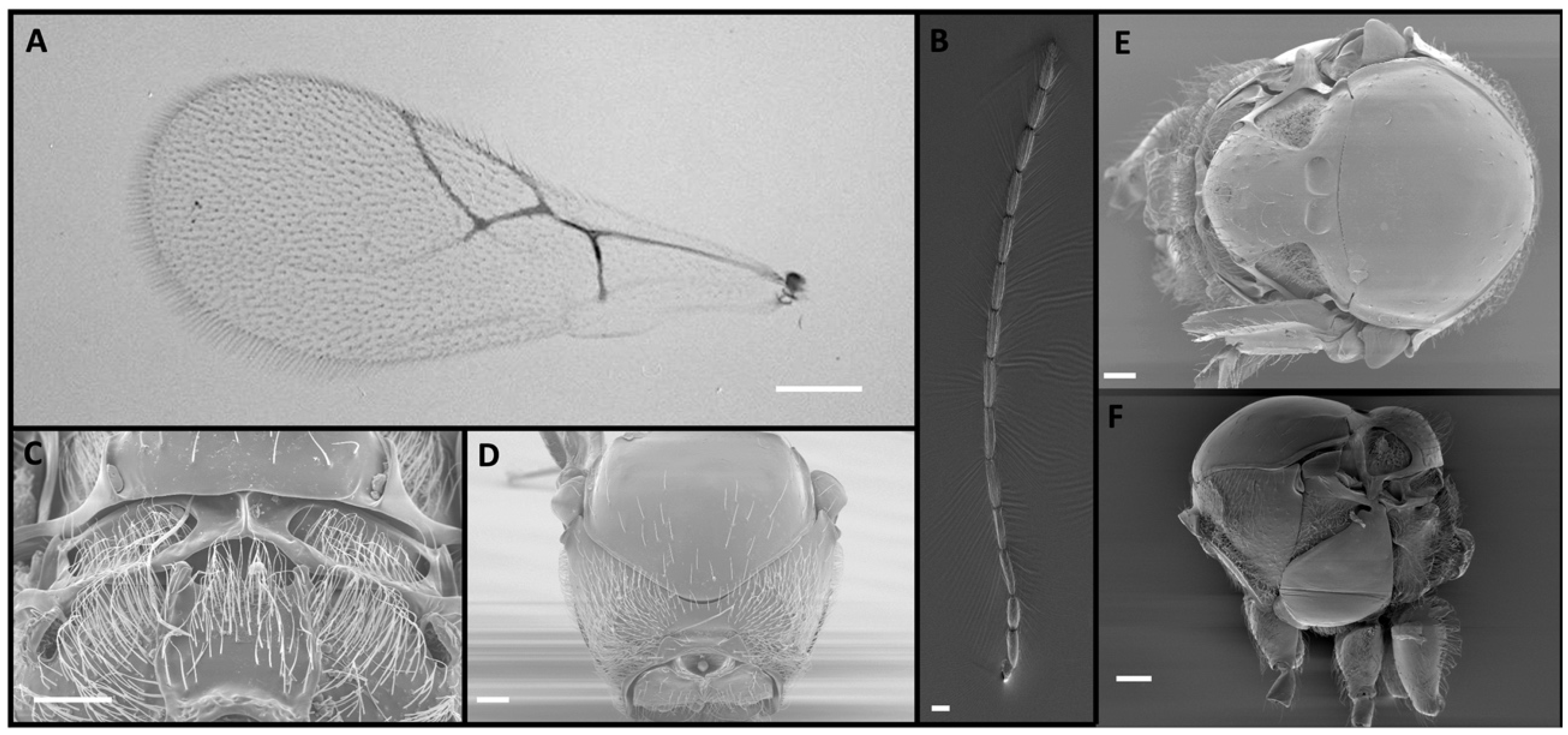
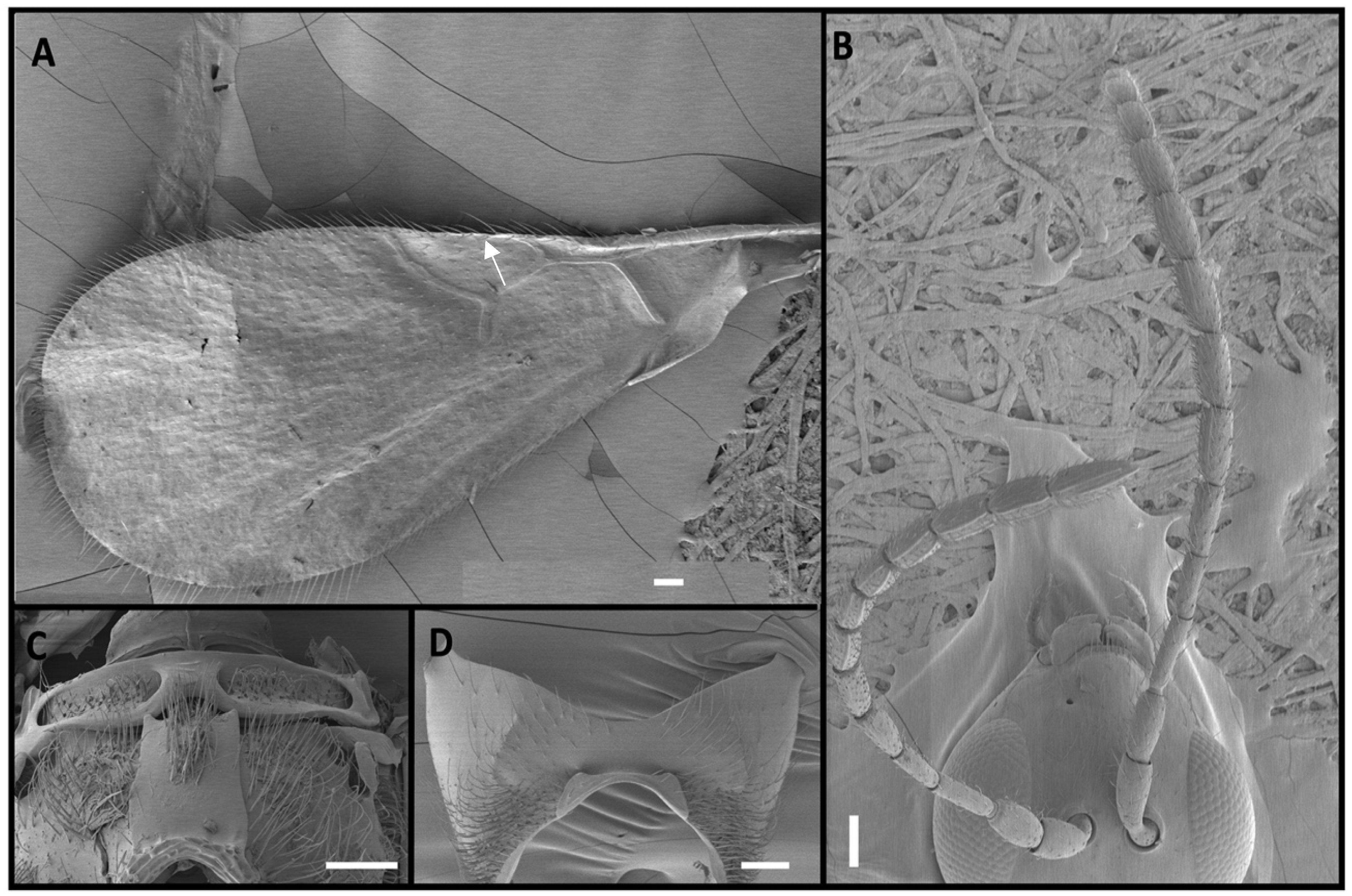
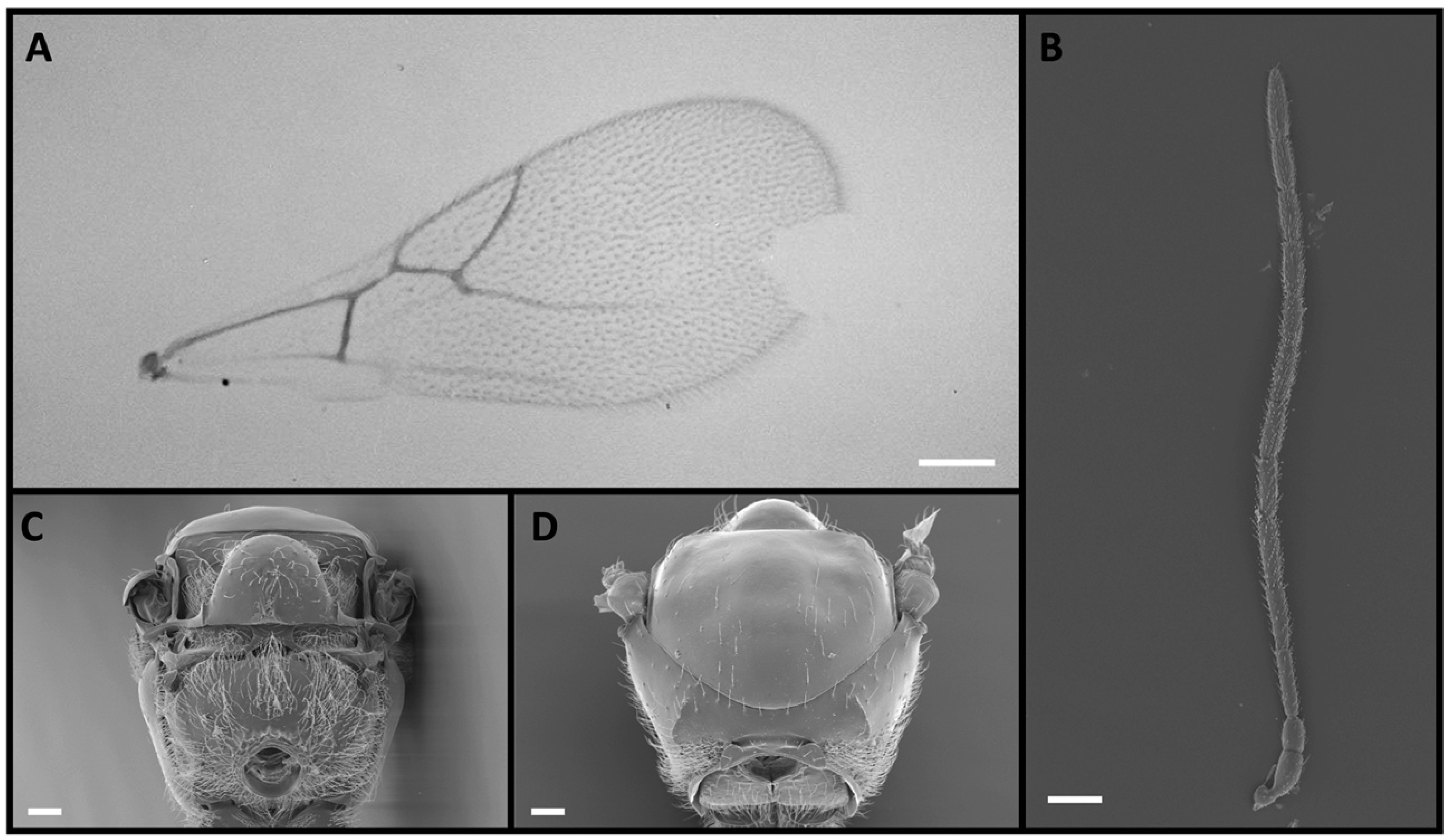
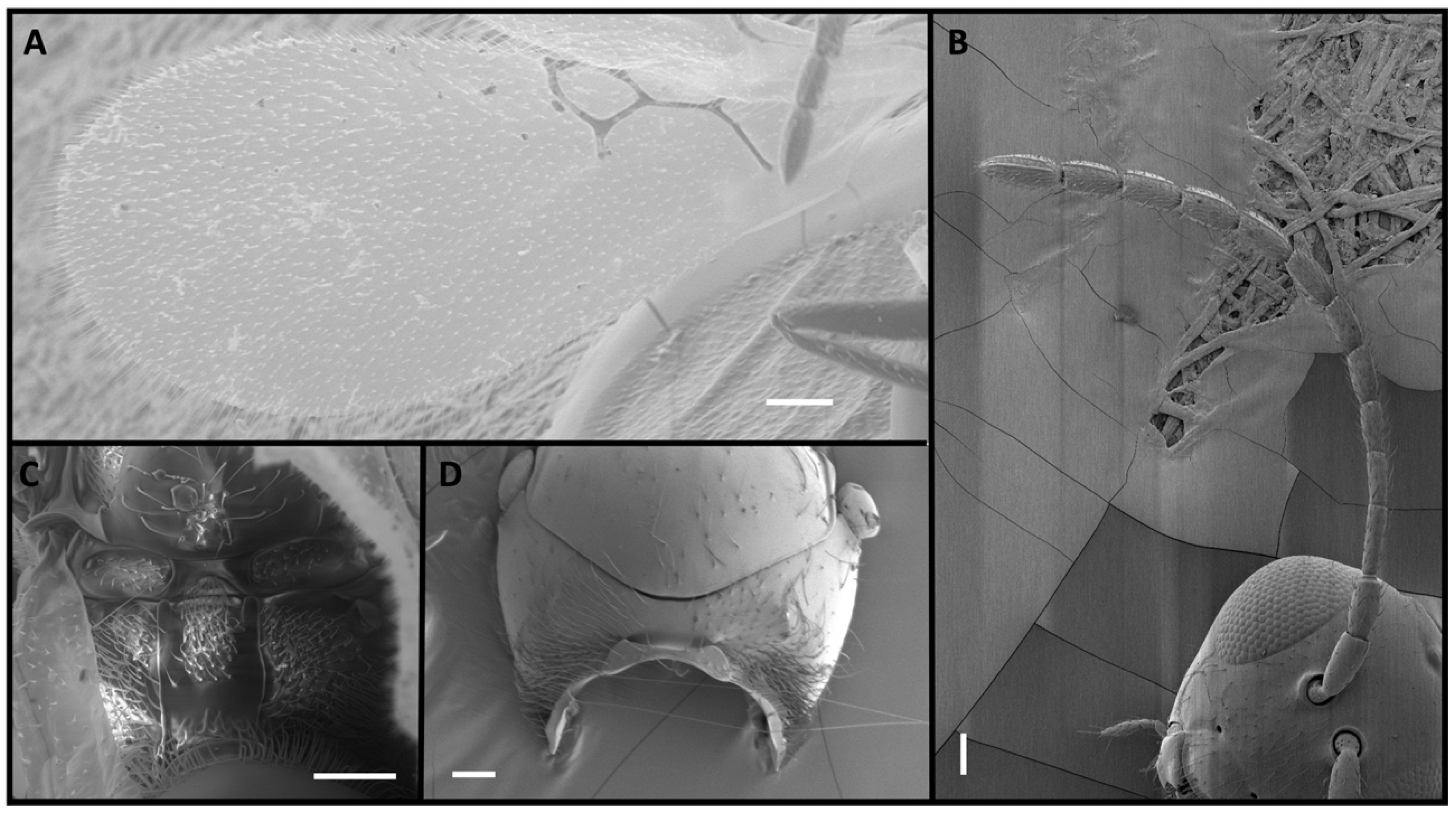
2.1. Morphological Identification
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Phylogenetic Analyses
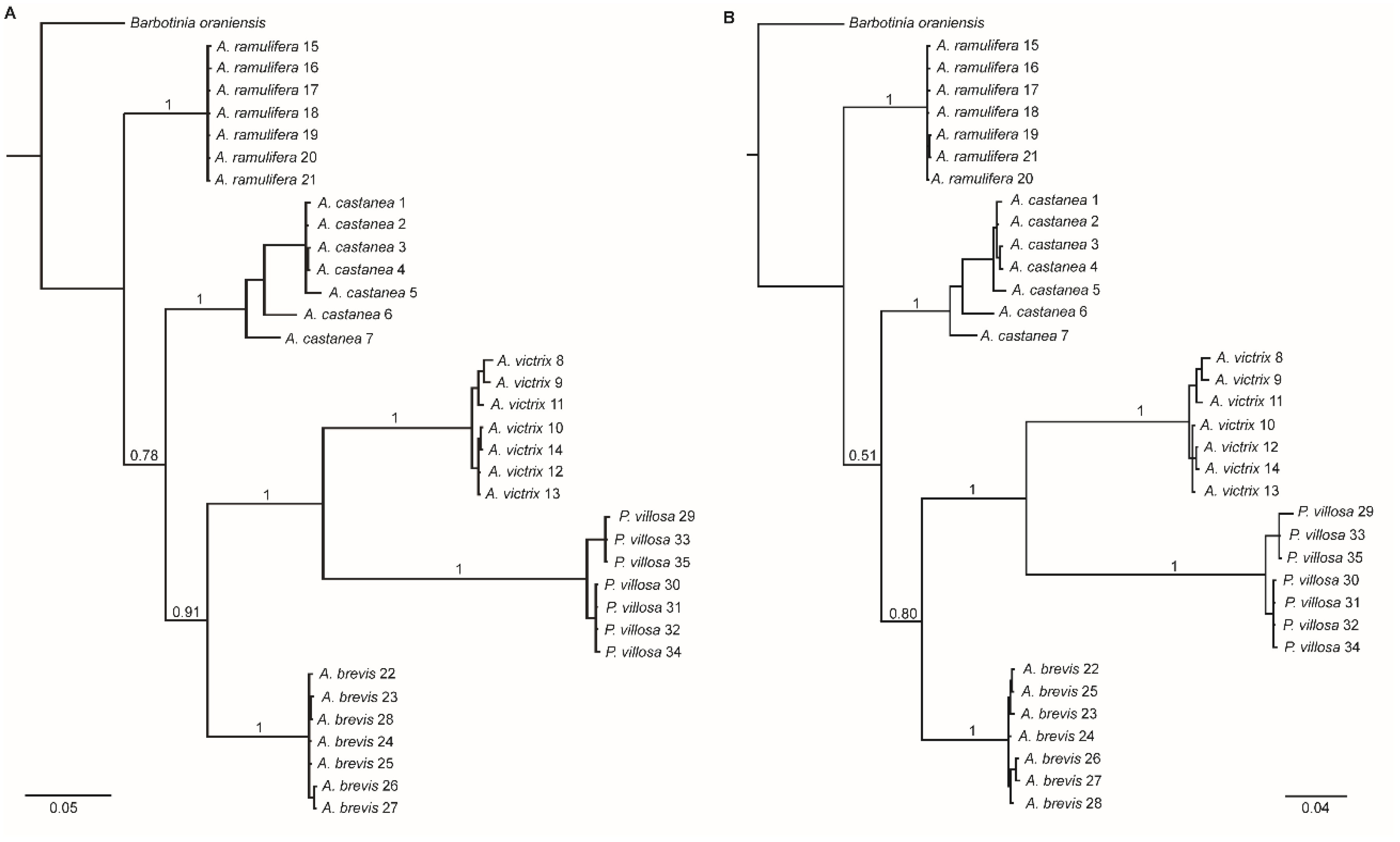
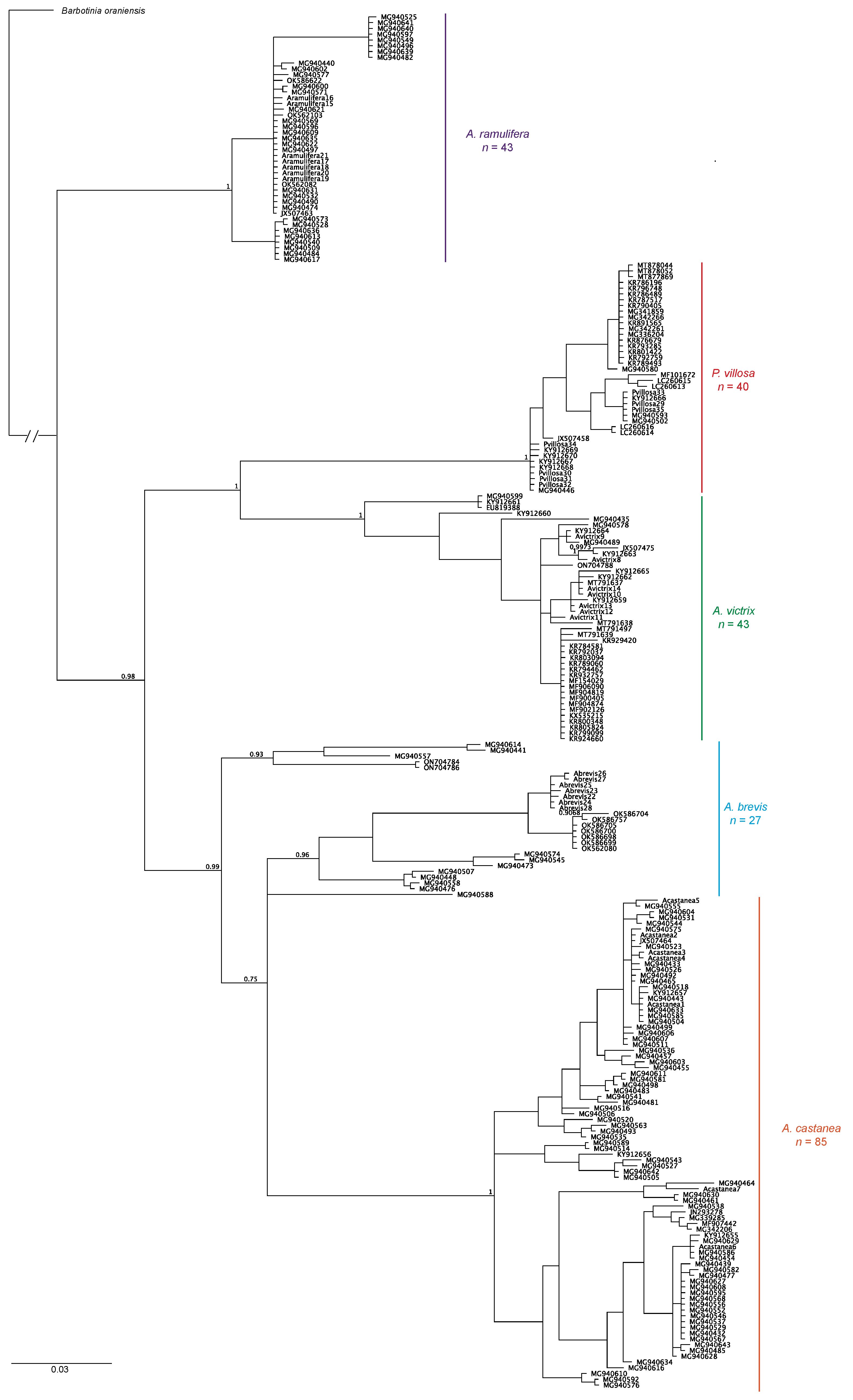
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassell, M.P. The Spatial and Temporal Dynamics of Host-Parasitoid Interactions; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Murdoch, W.W.; Briggs, C.J. Nisbet Consumer-Resource Dynamics; Princeton University Press: Princeton, NJ, USA, 2003. [Google Scholar]
- Heimpel, G.E.; Mills, N.J. Biological Control: Ecology and Applications; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Ovruski, S.M.; Fidalgo, P. Use of parasitoids (Hym.) in the control of fruit flies (Dip.: Tephritidae) in Argentina: Bibliographic review (1937–1991). IOBC-WPRS Bull. 1994, 17, 84–92. [Google Scholar]
- Rathman, R.J.; Johnson, M.W.; Tabashnik, B.E. Production of Ganaspidium utilis (Hymenoptera: Eucoilidae) for biological control of Liriomyza spp. (Diptera: Agromyzidae). Biol. Control 1991, 1, 256–260. [Google Scholar] [CrossRef]
- Villeneuve, F.; Trottin Caudal, Y. La protection integree des cultures legumieres en France: Acquis et perspectives. Infos 1997, 135, 40–44. [Google Scholar]
- Heraty, J. Molecular systematics, Chalcidoidea and biological control. In Genetics, Evolution and Biological Control; Ehler, L.E., Sforza, R., Mateille, T., Eds.; CABI Publishing: Oxon, UK, 2003; pp. 39–72. [Google Scholar]
- Heraty, J.M.; Woolley, J.B.; Hopper, K.R.; Hawks, D.L.; Kim, J.W.; Buffington, M. Molecular phylogenetics and reproductive incompatibility in a complex of cryptic species of aphid parasitoids. Mol. Phylogenet. Evol. 2007, 45, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Stary, P.; Delebeque, C.J.; Gariepy, T.D.; Barta, R.J.; Hoelmer, K.A.; Heimpel, G.E. Cryptic species of parasitoids attacking the soybean aphid (Hemiptera: Aphididae) in Asia: Binodoxys communis (Gahan) and Binodoxys koreanus (Hymenoptera: Braconidae: Aphidiinae). Ann. Entomol. Soc. Am. 2009, 102, 925–936. [Google Scholar] [CrossRef]
- Lue, C.H.; Abram, P.K.; Hrcek, J.; Buffington, M.L.; Staniszenko, P.P.A. Metabarcoding and applied ecology with hyper-diverse organisms: Recommendations for biological control research. Mol. Ecol. 2024, 32, 6461–6473. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.S.; Evenhuis, H.H. North American Charipidae: Key to genera, nomenclature, species checklists, and a new species of Dilyta Förster (Hymenoptera: Cynipoidea). Proc. Entomol. Soc. Wash. 1991, 93, 136–158. [Google Scholar]
- Gomez-Marco, F.; Urbaneja, A.; Jaques, J.A.; Rugman-Jones, P.F.; Stouthamer, R.; Tena, A. Untangling the aphid-parasitoid food web in citrus: Can hyperparasitoids disrupt biological control? Biol. Control 2015, 81, 111–121. [Google Scholar] [CrossRef]
- Villa-Ayala, P.; Sánchez-Rivera, G.; Rodríguez-Vélez, B.; Castrejón-Ayala, F. Parasitoids and Hyperparasitoids Associated with the Sugarcane Aphid, Melanaphis sacchari (Zehntner), in Morelos, Mexico. Soc. Southwest. Entomol. 2020, 45, 563–566. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.C.; Cheng, J.; Liu, S.; Yuan, H.B. Population dynamics and species composition of maize field parasitoids attacking aphids in northeastern China. PLoS ONE 2020, 15, e0241530. [Google Scholar] [CrossRef]
- Bandyan, S.K.; Peters, R.S.; Kadir, N.B.; Ferrer-Suay, M.; Kirchner, W.H. A survey of aphid parasitoids and hyperparasitoids (Hymenoptera) on six crops in the Kurdistan Region of Iraq. J. Hymenopt. Res. 2021, 81, 9–21. [Google Scholar] [CrossRef]
- Satar, S.; Kavallieratos, N.G.; Tüfekli, M.; Satar, G.; Athanassiou, C.G.; Papanikolaou, N.E.; Karacaoglu, M.; Özdemir, I.; Stary, P. Capsella bursa-pastoris Is a Key Overwintering Plant for Aphids in the Mediterranean Region. Insects 2021, 12, 744. [Google Scholar] [CrossRef]
- Burton, R.L.; Starks, K.J. Control of a Primary Parasite of the Greenbug with a Secondary Parasite in Greenhouse Screening for Plant Resistance. J. Econ. Entomol. 1977, 70, 219–220. [Google Scholar] [CrossRef]
- Nagasaka, K.; Takahasi, N.; Okabayashi, T. Impact of secondary parasitism on Aphidius colemani in the banker plant system on aphid control in commercial greenhouses in Kochi, Japan. Appl. Entomol. Zool. 2010, 45, 541–550. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.A.; Morelli, K.A.; Jansen, A.M. Cytochrome c oxidase subunit 1 gene as a DNA barcode for discriminating Trypanosoma cruzi DTUs and closely related species. Parasites Vectors 2017, 10, 488. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; de Waard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.; Cicero, C. DNA barcoding: Promise and pitfalls. PLos Biol. 2004, 2, e354. [Google Scholar] [CrossRef] [PubMed]
- Dasmahapatra, K.K.; Mallet, J. DNA barcodes: Recent successes and future prospects. Heredity 2006, 97, 254–255. [Google Scholar] [CrossRef]
- Dowton, M.; Austin, A.D. Molecular phylogeny of the insect order Hymenoptera: Apocritan relationships. Proc. Natl. Acad. Sci. USA 1994, 91, 9911–9915. [Google Scholar] [CrossRef]
- Van Veen, F.F.; Belshaw, R.O.; Godfray, H.C.J. The value of the ITS2 region for the identification of species boundaries between Alloxysta hyperparasitoids (Hymenoptera: Charipidae) of aphids. Eur. J. Entomol. 2003, 100, 449–454. [Google Scholar] [CrossRef]
- Wagener, B.; Reineke, A.; Löhr, B.; Zebitz, C.P. Phylogenetic study of Diadegma species (Hymenoptera: Ichneumonidae) inferred from analysis of mitochondrial and nuclear DNA sequences. Biol. Control 2006, 37, 131–140. [Google Scholar] [CrossRef]
- Rahimi, S.; Hajizadeh, J.; Hosseini, R.; Sohani, M.M. Evaluation of COI and ITS2 sequences ability to distinguish Lysiphlebus fabarum and Lysiphlebus confusus (Hymenoptera: Aphidiidae). J. Entomol. Soc. Iran 2012, 31, 53–66. [Google Scholar]
- Ptaszyńska, A.A.; Latoch, P.; Hurd, P.J.; Polaszek, A.; Michalska-Madej, J.; Grochowalski, Ł.; Strapagiel, D.; Gnat, S.; Załuski, D.; Gancarz, M.; et al. Amplicon Sequencing of Variable 16S rRNA from Bacteria and ITS2 Regions from Fungi and Plants, Reveals Honeybee Susceptibility to Diseases Results from Their Forage Availability under Anthropogenic Landscapes. Pathogens 2021, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pike, K.S.; Greenstone, M.H.; Shufran, K.A. Molecular Markers for Identification of the Hyperparasitoids Dendrocerus carpenteri and Alloxysta xanthopsis in Lysiphlebus testaceipes Parasitizing Cereal Aphids. BioControl 2006, 51, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Suay, M.; Staverløkk, A.; Selfa, J.; Pujade-Villar, J.; Naik, S.; Ekrem, T. Nuclear and mitochondrial markers suggest new species boundaries in Alloxysta (Hymenoptera: Cynipoidea: Figitidae). Arthropod Syst. Phylogeny 2018, 76, 463–473. [Google Scholar] [CrossRef]
- Ferrer-Suay, M.; Selfa, J.; Cuesta-Porta, V.; Pujade-Villar, J. Intraspecific variation in the morphology of Alloxysta fracticornis (Thomson, 1862) (Hymenoptera: Figitidae: Charipinae). J. Nat. Hist. 2022, 56, 707–717. [Google Scholar] [CrossRef]
- Ferrer-Suay, M.; Selfa, J.; Pujade-Villar, J. A review of the subfamily Charipinae (Hymenoptera: Cynipoidea: Figitidae): Hyperparasitoids potentially affecting the biological control of aphids. Ann. Soc. Entomol. Fr. 2021, 57, 481–498. [Google Scholar] [CrossRef]
- Ferrer-Suay, M.; Selfa, J.; Pujade-Villar, J. Overview of the current status of Charipinae (Hymenoptera, Cynipoidea, Figitidae): Taxonomy, distribution, and diversity. Zootaxa 2023, 5339, 001–039. [Google Scholar] [CrossRef]
- Ferrer-Suay, M.; Paretas-Martinez, J.; Selfa, J.; Pujade-Villar, J. Taxonomic and synonymic world catalogue of the Charipinae and notes about this subfamily (Hymenoptera: Cynipoidea: Figitidae). Zootaxa 2012, 3376, 1–92. [Google Scholar] [CrossRef]
- Ferrer-Suay, M.; Janković, M.; Selfa, J.; van Veen, F.; Tomanović, Z.; Kos, K.; Rakhshani, E.; Pujade-Villar, J. Qualitative analysis of aphid and primary parasitoid trophic relations of genus Alloxysta (Hymenoptera: Cynipoidea: Figitidae: Charipinae). Environ. Entomol. 2014, 43, 1485–1495. [Google Scholar] [CrossRef]
- Ferrer-Suay, M.; Vogel, J.; Peters, R.S.; Selfa, J. Characterizing the northwestern European species of Phaenoglyphis Förster, 1869 (Hymenoptera: Figitidae) with novel insights from CO1 barcoding data. BDJ, in press.
- Ferrer-Suay, M.; Selfa, J.; Pujade-Villar, J. A proposal of Charipinae’s keys for the world fauna (Hymenoptera: Cynipoidea: Figitidae). Zookeys 2019, 822, 79–139. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Whitfield, J.B. Molecular and morphological data suggest a single origin of the polydnaviruses among braconid wasps. Naturwissenschaften 1997, 84, 502–507. [Google Scholar] [CrossRef]
- Borschsenius, F. FastGap 1.2. Department of Biosciences, Aarhus University, Denmark. 2009. Available online: http://www.aubot.dk/FastGap_home.htm (accessed on 1 March 2024).
- Simmons, M.P.; Ochoterena, H. Gaps as characters in sequence-based phylogenetic analyses. Syst. Biol. 2000, 49, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Proceedings of the 2nd International Symposium; Petrov, B.N., Csaki, F., Eds.; Akademia Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods); Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Ferrer-Suay, M.; Selfa, J.; Seco-Fernández, M.V.; Melika, G.; Alipour, A.; Rakhshani, E.; Talebi, A.A.; Pujade-Villar, J. A contribution to the knowledge of Charipinae (Hymenoptera: Cynipoidea: Figitidae) associated with aphids from Iran, including new records. North-West. J. Zool. 2013, 9, 30–44. [Google Scholar]
- Ferrer-Suay, M.; Paretas-Martínez, J.; Pujade-Villar, J. Revision of Apocharips Fergusson (Hymenopterza: Figitidae: Charipinae) with description of three new species from Colombia. Zootaxa 2013, 3646, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Suay, M.; Selfa, J.; Pujade-Villar, J. Revision of Thomson and Zetterstedt collections of Alloxysta genus deposited in Lund Museum of Zoology (Sweden). Entomol. Tidskr. 2013, 134, 77–102. [Google Scholar]
- Ferrer-Suay, M.; Selfa, J.; Notton, D.; Pujade-Villar, J. Revision of the types of species of Alloxysta Förster, 1869 described by Cameron and Fergusson (Hymenoptera: Figitidae: Charipinae) deposited in the Natural History Museum (London) including a key to the fauna of Great Britain. Eur. J. Taxon. 2013, 53, 1–27. [Google Scholar]
- Ferrer-Suay, M.; Selfa, J.; Seco, M.V.; Pujade-Villar, J. Revision of Hellén types of Alloxysta Förster (Hymenoptera: Figitidae, Charipinae). Entomol. Fenn. 2014, 25, 86–101. [Google Scholar] [CrossRef]
- Ferrer-Suay, M.; Selfa, J.; Pujade-Villar, J. Review of the Hartig type collection of Alloxysta (Hymenoptera: Figitidae: Charipinae) and other Alloxysta material deposited in the Zoologische Staatssammlung Museum (Munich). Fragm. Faun. 2014, 57, 75–116. [Google Scholar] [CrossRef][Green Version]
- Ferrer-Suay, M.; Selfa, J.; Pujade-Villar, J. New contribution to the knowledge of the genus Alloxysta (Hymenoptera: Cynipoidea: Figitidae): Revision of some type material. Ann. Naturhist. Mus. Wien Ser. B 2015, 117, 23–36. [Google Scholar]
- Casiraghi, A.; Dregni, J.S.; Pérez Hidalgo, N.; Heimpel, G.E.; Selfa, J.; Ferrer-Suay, M. Brachyptery analysis in Alloxysta (Hymenoptera: Figitidae). Proc. Entomol. Soc. Wash. 2022, 124, 1–12. [Google Scholar] [CrossRef]
- Dietz, L.; Eberle, J.; Mayer, C.; Kukowka, S.; Bohacz, C.; Baur, H.; Espeland, M.; Huber, B.A.; Hutter, C.; Mengual, X.; et al. Standardized nuclear markers improve and homogenize species delimitation in Metazoa. Methods Ecol. Evol. 2023, 14, 543–555. [Google Scholar] [CrossRef]
- Gokhman, V.E. Chromosome Study of the Hymenoptera: History, Current State, Perspectives. Biol. Bull. Rev. 2023, 13, 247–257. [Google Scholar] [CrossRef]
- Ferrer-Suay, M.; Selfa, J.; Mata-Casanova, N.; Hidalgo, N.P.; Pujade-Villar, J. Worldwide revision of the genus Phaenoglyphis Förster, 1869 (Hymenoptera, Cynipoidea, Figitidae, Charipinae). Insect Syst. Evol. 2019, 50, 235–296. [Google Scholar] [CrossRef]
- Zhang, Y.; Gates, M.; Shorthouse, J. Testing species limits of Eurytomidae (Hymenoptera) associated with galls induced by Diplolepis (Hymenoptera: Cynipidae) in Canada using an integrative approach. Can. Entomol. 2014, 146, 321–334. [Google Scholar] [CrossRef]
- Kocic, K.; Petrovic, A.; Ckrkic, J.; Mitrovic, M.; Tomanovic, Z. Phylogenetic relationships and subgeneric classification of European Ephedrus species (Hymenoptera, Braconidae, Aphidiinae). Zookeys 2019, 878, 1–22. [Google Scholar] [CrossRef] [PubMed]

| A. castanea | A. victrix | A. ramulifera | A. brevis | P. villosa | |
|---|---|---|---|---|---|
| A. castanea | 0.0247 ± 0.0329 | ||||
| A. victrix | 0.1452 ± 0.0017 | 0.0176 ± 0.0045 | |||
| A. ramulifera | 0.1248 ± 0.0008 | 0.1409 ± 0.0009 | 0.0022 ± 0.0008 | ||
| A. brevis | 0.1237 ± 0.0012 | 0.1367 ± 0.0011 | 0.1324 ± 0.0025 | 0.0035 ± 0.0035 | |
| P. villosa | 0.1640 ± 0.0012 | 0.1299 ± 0.0068 | 0.1431 ± 0.0093 | 0.1293 ± 0.0012 | 0.0145 ± 0.0113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer-Suay, M.; Bulgarella, M.; Heimpel, G.E.; Rakhshani, E.; Selfa, J. Molecular Data Confirm Interspecific Limits of Four Alloxysta and One Phaenoglyphis Species of Parasitic Wasps within the Subfamily Charipinae (Cynipoidea: Figitidae). Insects 2024, 15, 354. https://doi.org/10.3390/insects15050354
Ferrer-Suay M, Bulgarella M, Heimpel GE, Rakhshani E, Selfa J. Molecular Data Confirm Interspecific Limits of Four Alloxysta and One Phaenoglyphis Species of Parasitic Wasps within the Subfamily Charipinae (Cynipoidea: Figitidae). Insects. 2024; 15(5):354. https://doi.org/10.3390/insects15050354
Chicago/Turabian StyleFerrer-Suay, Mar, Mariana Bulgarella, George E. Heimpel, Ehsan Rakhshani, and Jesús Selfa. 2024. "Molecular Data Confirm Interspecific Limits of Four Alloxysta and One Phaenoglyphis Species of Parasitic Wasps within the Subfamily Charipinae (Cynipoidea: Figitidae)" Insects 15, no. 5: 354. https://doi.org/10.3390/insects15050354
APA StyleFerrer-Suay, M., Bulgarella, M., Heimpel, G. E., Rakhshani, E., & Selfa, J. (2024). Molecular Data Confirm Interspecific Limits of Four Alloxysta and One Phaenoglyphis Species of Parasitic Wasps within the Subfamily Charipinae (Cynipoidea: Figitidae). Insects, 15(5), 354. https://doi.org/10.3390/insects15050354








