Different Sensitivity of Flower-Visiting Diptera to a Neonicotinoid Insecticide: Expanding the Base for a Multiple-Species Risk Assessment Approach
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species, Populations, and Test Conditions
2.2. Imidacloprid Solutions
2.3. Experimental Design, Exposure, and Mortality Assessment
2.4. Sublethal Effect Assessment: Reproduction (Oviposition Rate and Fecundity)
2.5. Ecotoxicological Data from Literature
2.6. Statistical Analysis
3. Results
3.1. Species Sensitivity Distribution: Mortality
3.2. Sublethal Effects: Reproduction (Oviposition Rate and Fecundity)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of Pollinators in Changing Landscapes for World Crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J.; Winfree, R.; Tarrant, S. How Many Flowering Plants Are Pollinated by Animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Rader, R.; Bartomeus, I.; Garibaldi, L.A.; Garratt, M.P.D.; Howlett, B.G.; Winfree, R.; Cunningham, S.A.; Mayfield, M.M.; Arthur, A.D.; Andersson, G.K.S.; et al. Non-Bee Insects Are Important Contributors to Global Crop Pollination. Proc. Natl. Acad. Sci. USA 2016, 113, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Skevington, J.H.; Dang, P.T. Exploring the Diversity of Flies (Diptera). Biodiversity 2002, 3, 3–27. [Google Scholar] [CrossRef]
- Ssymank, A.; Kearns, C.A.; Pape, T.; Thompson, F.C. Pollinating Flies (Diptera): A Major Contribution to Plant Diversity and Agricultural Production. Biodiversity 2008, 9, 86–89. [Google Scholar] [CrossRef]
- Courtney, G.W.; Pape, T.; Skevington, J.H.; Sinclair, B.J. Biodiversity of Diptera. In Insect Biodiversity; Wiley: Hoboken, NJ, USA, 2017; pp. 229–278. [Google Scholar]
- Woodcock, T.S.; Larson, B.M.H.; Kevan, P.G.; Inouye, D.W.; Lunau, K. Flies and Flowers II: Floral Attractants and Rewards. J. Pollinat. Ecol. 2014, 12, 63–94. [Google Scholar] [CrossRef]
- Inouye, D.W.; Larson, B.M.H.; Ssymank, A.; Kevan, P.G. Flies and Flowers III: Ecology of Foraging and Pollination. J. Pollinat. Ecol. 2015, 16, 115–133. [Google Scholar] [CrossRef]
- Roquer-Beni, L.; Arnan, X.; Rodrigo, A.; Bosch, J. What Makes a Good Pollinator? Relationship between Pollinator Traits and Pollination Effectiveness in Apple Flowers. Entomol. Gen. 2022, 42, 875–882. [Google Scholar] [CrossRef]
- Rodríguez-Gasol, N.; Alins, G.; Veronesi, E.R.; Wratten, S. The Ecology of Predatory Hoverflies as Ecosystem-Service Providers in Agricultural Systems. Biol. Control 2020, 151, 104405. [Google Scholar] [CrossRef]
- Larson, B.M.H.; Kevan, P.G.; Inouye, D.W. Flies and Flowers: Taxonomic Diversity of Anthophiles and Pollinators. Can. Entomol. 2001, 133, 439–465. [Google Scholar] [CrossRef]
- Raguso, R.A. Don’t Forget the Flies: Dipteran Diversity and Its Consequences for Floral Ecology and Evolution. Appl. Entomol. Zool. 2020, 55, 1–7. [Google Scholar] [CrossRef]
- Rader, R.; Cunningham, S.A.; Howlett, B.G.; Inouye, D.W. Non-Bee Insects as Visitors and Pollinators of Crops: Biology, Ecology, and Management. Annu. Rev. Entomol. 2020, 65, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Al-Dobai, S.; Reitz, S.; Sivinski, J. Tachinidae (Diptera) Associated with Flowering Plants: Estimating Floral Attractiveness. Biol. Control 2012, 61, 230–239. [Google Scholar] [CrossRef]
- Orford, K.A.; Vaughan, I.P.; Memmott, J. The Forgotten Flies: The Importance of Non-Syrphid Diptera as Pollinators. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142934. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual Ecosystem Services of Syrphid Flies (Diptera: Syrphidae): Pollinators and Biological Control Agents. Pest Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef]
- Dindo, M.L.; Grenier, S. Production of Dipteran Parasitoids. In Mass Production of Beneficial Organisms; Elsevier: Amsterdam, The Netherlands, 2023; pp. 71–100. [Google Scholar]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemuller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Zattara, E.E.; Aizen, M.A. Worldwide Occurrence Records Suggest a Global Decline in Bee Species Richness. One Earth 2021, 4, 114–123. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 Percent Decline over 27 Years in Total Flying Insect Biomass in Protected Areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Ssymank, A.; Sorg, M.; de Kroon, H.; Jongejans, E. Insect Biomass Decline Scaled to Species Diversity: General Patterns Derived from a Hoverfly Community. Proc. Natl. Acad. Sci. USA 2021, 118, e2002554117. [Google Scholar] [CrossRef]
- Barendregt, A.; Zeegers, T.; van Steenis, W.; Jongejans, E. Forest Hoverfly Community Collapse: Abundance and Species Richness Drop over Four Decades. Insect Conserv. Divers. 2022, 15, 510–521. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Rundlöf, M.; Andersson, G.K.S.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed Coating with a Neonicotinoid Insecticide Negatively Affects Wild Bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-Specific Effects of Neonicotinoid Pesticides on Honey Bees and Wild Bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA); Health Canada Pest Management Regulatory Agency (PMRA); California Departament of Pesticide Regulatio. USEPA Guidance for Assessing Pesticide Risks to Bees, USEPA: Washington, DC, USA, 2014.
- EFSA Guidance on the Risk Assessment of Plant Protection Products on Bees (Apis Mellifera, Bombus Spp. and Solitary Bees). EFSA J. 2013, 11, 3295. [CrossRef]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Maini, S.; Porrini, C.; Simon-Delso, N.; Bosch, J. Bees and Pesticide Regulation: Lessons from the Neonicotinoid Experience. Biol. Conserv. 2020, 241, 108356. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Goka, K. Pesticide Residues and Bees–A Risk Assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef]
- Azpiazu, C.; Bosch, J.; Bortolotti, L.; Medrzycki, P.; Teper, D.; Molowny-Horas, R.; Sgolastra, F. Toxicity of the Insecticide Sulfoxaflor Alone and in Combination with the Fungicide Fluxapyroxad in Three Bee Species. Sci. Rep. 2021, 11, 6821. [Google Scholar] [CrossRef]
- Arena, M.; Sgolastra, F. A Meta-Analysis Comparing the Sensitivity of Bees to Pesticides. Ecotoxicology 2014, 23, 324–334. [Google Scholar] [CrossRef]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Renzi, M.T.; Tosi, S.; Bogo, G.; Teper, D.; Porrini, C.; Molowny-Horas, R.; Bosch, J. Synergistic Mortality between a Neonicotinoid Insecticide and an Ergosterol-Biosynthesis-Inhibiting Fungicide in Three Bee Species. Pest Manag. Sci. 2017, 73, 1236–1243. [Google Scholar] [CrossRef]
- Biddinger, D.J.; Robertson, J.L.; Mullin, C.; Frazier, J.; Ashcraft, S.A.; Rajotte, E.G.; Joshi, N.K.; Vaughn, M. Comparative Toxicities and Synergism of Apple Orchard Pesticides to Apis Mellifera (L.) and Osmia Cornifrons (Radoszkowski). PLoS ONE 2013, 8, e72587. [Google Scholar] [CrossRef]
- Heard, M.S.; Baas, J.; Dorne, J.-L.; Lahive, E.; Robinson, A.G.; Rortais, A.; Spurgeon, D.J.; Svendsen, C.; Hesketh, H. Comparative Toxicity of Pesticides and Environmental Contaminants in Bees: Are Honey Bees a Useful Proxy for Wild Bee Species? Sci. Total Environ. 2017, 578, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Hesketh, H.; Lahive, E.; Horton, A.A.; Svendsen, C.; Rortais, A.; Dorne, J.L.; Baas, J.; Heard, M.S.; Spurgeon, D.J. Comparing Bee Species Responses to Chemical Mixtures: Common Response Patterns? PLoS ONE 2017, 12, e0176289. [Google Scholar] [CrossRef] [PubMed]
- Linguadoca, A.; Jürison, M.; Hellström, S.; Straw, E.A.; Šima, P.; Karise, R.; Costa, C.; Serra, G.; Colombo, R.; Paxton, R.J.; et al. Intra-Specific Variation in Sensitivity of Bombus Terrestris and Osmia Bicornis to Three Pesticides. Sci. Rep. 2022, 12, 17311. [Google Scholar] [CrossRef] [PubMed]
- de Assis, J.C.; Tadei, R.; Menezes-Oliveira, V.B.; Silva-Zacarin, E.C.M. Are Native Bees in Brazil at Risk from the Exposure to the Neonicotinoid Imidacloprid? Environ. Res. 2022, 212, 113127. [Google Scholar] [CrossRef] [PubMed]
- Lourencetti, A.P.S.; Azevedo, P.; Miotelo, L.; Malaspina, O.; Nocelli, R.C.F. Surrogate Species in Pesticide Risk Assessments: Toxicological Data of Three Stingless Bees Species. Environ. Pollut. 2023, 318, 120842. [Google Scholar] [CrossRef] [PubMed]
- Beneficial Arthropod Regulatory Testing Group; European and Mediterranean Plant Protection Organisation; Council of Europe; Organisation for Economic Co-operation and Development; International Organisation for Biological and Integrated Control of Noxious Animals and Plants; Society of Environmental Toxicology and Chemistry-Europe; Commission of the European Communities. Guidance Document on Regulatory Testing and Risk Assessment Procedures for Plant Protection Products with Non-Target Arthropods: From the ESCORT 2 Workshop (European Standard Characteristics of Non-Target Arthropod Regulatory Testing), Candolfi, M.P., Barrett, K.L., Campbell, P.J., Forster, R., Grandy, N., Huet, M.-C., Lewis, G., Oomen, P.A., Schmuck, R., Vog, H., Eds.; SETAC: Brussels, Belgium, 2001; ISBN 9781880611524.
- Williams, J.H.; Bordoni, A.; Bednarska, A.; Pinto, A.; Martins, C.A.H.; Henriques, D.; Sgolastra, F.; Knapp, J.; Loureiro, J.; Sousa, J.P.; et al. Roadmap for Action on the Environmental Risk Assessment of Chemicals for Insect Pollinators (IPol-ERA). EFSA Support. Publ. 2023, 20, 8431E. [Google Scholar] [CrossRef]
- Basley, K.; Davenport, B.; Vogiatzis, K.; Goulson, D. Effects of Chronic Exposure to Thiamethoxam on Larvae of the Hoverfly Eristalis Tenax (Diptera, Syrphidae). PeerJ 2018, 6, e4258. [Google Scholar] [CrossRef] [PubMed]
- Moens, J.; De Clercq, P.; Tirry, L. Side Effects of Pesticides on the Larvae of the Hoverfly Episyrphus Balteatus in the Laboratory. Phytoparasitica 2011, 39, 1–9. [Google Scholar] [CrossRef]
- Nagloo, N.; Rigosi, E.; O’Carroll, D.C. Acute and Chronic Toxicity of Imidacloprid in the Pollinator Fly, Eristalis Tenax L., Assessed Using a Novel Oral Bioassay. Ecotoxicol. Environ. Saf. 2023, 251, 114505. [Google Scholar] [CrossRef]
- Benelli, M.; Tóth, F.; Dindo, M.L. Low-temperature Storage of Exorista Larvarum Puparia as a Tool for Assisting Parasitoid Production. Entomol. Exp. Appl. 2018, 166, 914–924. [Google Scholar] [CrossRef]
- Mellini, E.; Coulibaly, A.K. Un Decennio Di Sperimentazione Sul Sistema Ospite-Parassita Galleria Mellonella L.-Pseudogonia rufifrons Wied: Sintesi dei risultati. Boll. Ist. Ent. “G. Grandi” Univ. Bologna 1991, 45, 191–249. [Google Scholar]
- Amorós-Jiménez, R.; Pineda, A.; Fereres, A.; Marcos-García, M.Á. Prey Availability and Abiotic Requirements of Immature Stages of the Aphid Predator Sphaerophoria Rueppellii. Biol. Control 2012, 63, 17–24. [Google Scholar] [CrossRef]
- Amorós-Jiménez, R.; Pineda, A.; Fereres, A.; Marcos-García, M.Á. Feeding Preferences of the Aphidophagous Hoverfly Sphaerophoria Rueppellii Affect the Performance of Its Offspring. BioControl 2014, 59, 427–435. [Google Scholar] [CrossRef]
- Pekas, A.; De Craecker, I.; Boonen, S.; Wäckers, F.L.; Moerkens, R. One Stone; Two Birds: Concurrent Pest Control and Pollination Services Provided by Aphidophagous Hoverflies. Biol. Control 2020, 149, 104328. [Google Scholar] [CrossRef]
- Sánchez, M.; Belliure, B.; Montserrat, M.; Gil, J.; Velásquez, Y. Pollination by the Hoverfly Eristalinus Aeneus (Diptera: Syrphidae) in Two Hybrid Seed Crops: Celery and Fennel (Apiaceae). J. Agric. Sci. 2022, 160, 194–206. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic Insecticides (Neonicotinoids and Fipronil): Trends, Uses, Mode of Action and Metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- van der Sluijs, J.P.; Amaral-Rogers, V.; Belzunces, L.P.; Bijleveld van Lexmond, M.F.I.J.; Bonmatin, J.-M.; Chagnon, M.; Downs, C.A.; Furlan, L.; Gibbons, D.W.; Giorio, C.; et al. Conclusions of the Worldwide Integrated Assessment on the Risks of Neonicotinoids and Fipronil to Biodiversity and Ecosystem Functioning. Environ. Sci. Pollut. Res. 2015, 22, 148–154. [Google Scholar] [CrossRef]
- Maini, S.; Medrzycki, P.; Porrini, C. The Puzzle of Honey Bee Losses: A Brief Review. Bull. Insectology 2010, 63, 153–160. [Google Scholar]
- Lundin, O.; Rundlöf, M.; Smith, H.G.; Fries, I.; Bommarco, R. Neonicotinoid Insecticides and Their Impacts on Bees: A Systematic Review of Research Approaches and Identification of Knowledge Gaps. PLoS ONE 2015, 10, e0136928. [Google Scholar] [CrossRef]
- OJEU Commission Implementing Regulation (EU) 2018/783 of 29 May 2018 Amending Implementing Regulation (EU) No 540/2011 as Regards the Conditions of Approval of the Active Substance Imidacloprid. Off. J. Eur. Union 2018, 132, 31–34.
- Goulson, D. Pesticides, Corporate Irresponsibility, and the Fate of Our Planet. One Earth 2020, 2, 302–305. [Google Scholar] [CrossRef]
- OECD. Test No. 214: Honeybees, Acute Contact Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 1998; ISBN 9789264070189.
- OECD. Test No. 213: Honeybees, Acute Oral Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 1998; ISBN 9789264070165.
- OECD. Test No. 246: Bumblebee, Acute Contact Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 2017; ISBN 9789264284104.
- OECD. Test No. 247: Bumblebee, Acute Oral Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 2017; ISBN 9789264284128.
- Posthuma, L.; Suter II, G.W.; Traas, T.P. Species Sensitivity Distributions in Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2001; ISBN 1420032313. [Google Scholar]
- Wheeler, J.; Grist, E.P.; Leung, K.M.; Morritt, D.; Crane, M. Species Sensitivity Distributions: Data and Model Choice. Mar. Pollut. Bull. 2002, 45, 192–202. [Google Scholar] [CrossRef] [PubMed]
- van Straalen, N.M. Biodiversity of Ecotoxicological Responses in Animals. Netherlands J. Zool. 1994, 44, 112–129. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Flatt, T. Endocrine Uncoupling of the Trade-off between Reproduction and Somatic Maintenance in Eusocial Insects. Curr. Opin. Insect Sci. 2016, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Hinarejos, S.; Pitts-Singer, T.L.; Boyle, N.K.; Joseph, T.; Lūckmann, J.; Raine, N.E.; Singh, R.; Williams, N.M.; Bosch, J. Pesticide Exposure Assessment Paradigm for Solitary Bees. Environ. Entomol. 2019, 48, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Dindo, M.L.; Modesto, M.; Rossi, C.; Di Vito, M.; Burgio, G.; Barbanti, L.; Mattarelli, P. Monarda Fistulosa Hydrolate as Antimicrobial Agent in Artificial Media for the in Vitro Rearing of the Tachinid Parasitoid Exorista Larvarum. Entomol. Exp. Appl. 2021, 169, 79–89. [Google Scholar] [CrossRef]
- Dindo, M.L.; Marchetti, E.; Baronio, P. In Vitro Rearing of the Parasitoid Exorista Larvarum (Diptera: Tachinidae) from Eggs Laid Out of Host. J. Econ. Entomol. 2007, 100, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Dindo, M.L.; Rezaei, M.; De Clercq, P. Improvements in the Rearing of the Tachinid Parasitoid Exorista Larvarum (Diptera: Tachinidae): Influence of Adult Food on Female Longevity and Reproduction Capacity. J. Insect Sci. 2019, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Branquart, E.; Hemptinne, J.-L.; Bauffe, C.; Benfekih, L. Cannibalism In Episyrphus Balteatus (Ditp: Syrphidae). BioControl 1997, 42, 145–152. [Google Scholar] [CrossRef]
- Campoy, A.; Lutsyk, M.; Pérez-Bañón, C.; Rojo, S. Age-Stage Two-Sex Life Table Analysis of Eristalinus Aeneus (Diptera, Syrphidae) Reared with Two Different Larval Media. Bull. Entomol. Res. 2022, 112, 13–20. [Google Scholar] [CrossRef]
- Gladis, T. Laborzucht Einiger Eristalinen (Diptera, Syrphidae) Und Möglichkeiten Für Ihren Einsatz in Der Pflanzenzüchtung [Laboratory Rearing of Some Eristalines (Diptera, Syrphidae) and the Possibility of Their Use in Plant Cultures]. Verhandlungen der Westdtsch. Entomol. 1994, 1993, 139–152. [Google Scholar]
- Straw, E.A.; Carpentier, E.N.; Brown, M.J.F. Roundup Causes High Levels of Mortality Following Contact Exposure in Bumble Bees. J. Appl. Ecol. 2021, 58, 1167–1176. [Google Scholar] [CrossRef]
- Straw, E.A.; Brown, M.J.F. Co-Formulant in a Commercial Fungicide Product Causes Lethal and Sub-Lethal Effects in Bumble Bees. Sci. Rep. 2021, 11, 21653. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.r-project.org/ (accessed on 30 March 2022).
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Suchail, S.; Guez, D.; Belzunces, L.P. Characteristics of Imidacloprid Toxicity in Two Apis Mellifera Subspecies. Environ. Toxicol. Chem. 2000, 19, 1901–1905. [Google Scholar] [CrossRef]
- Thorley, J.; Schwarz, C. Ssdtools: An R Package to Fit Species Sensitivity Distributions. J. Open Source Softw. 2018, 3, 1082. [Google Scholar] [CrossRef]
- OECD. Current Approaches in the Statistical Analysis of Ecotoxicity Data: A Guidance to Application, OECD Environment Health and Safety Publications Series on Testing and Assessment; OECD: Paris, France, 2016.
- Laskowski, R. Some Good Reasons to Ban the Use of NOEC, LOEC and Related Concepts in Ecotoxicology. Oikos 1995, 73, 140–144. [Google Scholar] [CrossRef]
- USEPA Benchmark Dose Tools (BMDS) Online. Available online: https://bmdsonline.epa.gov/ (accessed on 12 April 2023).
- Yasuda, M.; Sakamoto, Y.; Goka, K.; Nagamitsu, T.; Taki, H. Insecticide Susceptibility in Asian Honey Bees (Apis Cerana (Hymenoptera: Apidae)) and Implications for Wild Honey Bees in Asia. J. Econ. Entomol. 2017, 110, 447–452. [Google Scholar] [CrossRef]
- Soares, H.M.; Jacob, C.R.O.; Carvalho, S.M.; Nocelli, R.C.F.; Malaspina, O. Toxicity of Imidacloprid to the Stingless Bee Scaptotrigona Postica Latreille, 1807 (Hymenoptera: Apidae). Bull. Environ. Contam. Toxicol. 2015, 94, 675–680. [Google Scholar] [CrossRef]
- da Costa, L.M.; Grella, T.C.; Barbosa, R.A.; Malaspina, O.; Nocelli, R.C.F. Determination of Acute Lethal Doses (LD50 and LC50) of Imidacloprid for the Native Bee Melipona Scutellaris Latreille, 1811 (Hymenoptera: Apidae). Sociobiology 2015, 62. [Google Scholar] [CrossRef]
- Kueh Tai, F.; Pattemore, D.E.; Jochym, M.; Beggs, J.R.; Northcott, G.L.; Mortensen, A.N. Honey Bee Toxicological Responses Do Not Accurately Predict Environmental Risk of Imidacloprid to a Solitary Ground-Nesting Bee Species. Sci. Total Environ. 2022, 839, 156398. [Google Scholar] [CrossRef]
- Youn, Y.N.; Seo, M.J.; Shin, J.G.; Jang, C.; Yu, Y.M. Toxicity of Greenhouse Pesticides to Multicolored Asian Lady Beetles, Harmonia Axyridis (Coleoptera: Coccinellidae). Biol. Control 2003, 28, 164–170. [Google Scholar] [CrossRef]
- Lucas, É.; Giroux, S.; Demougeot, S.; Duchesne, R.-M.; Coderre, D. Compatibility of a Natural Enemy, Coleomegilla Maculata Lengi (Col., Coccinellidae) and Four Insecticides Used against the Colorado Potato Beetle (Col., Chrysomelidae). J. Appl. Entomol. 2004, 128, 233–239. [Google Scholar] [CrossRef]
- Hartfelder, K.; Engels, W. Allometric and Multivariate Analysis of Sex and Caste Polymorphism in the Neotropical Stingless Bee, Scaptotrigona Postica. Insectes Soc. 1992, 39, 251–266. [Google Scholar] [CrossRef]
- Lourenço, C.T.; Carvalho, S.M.; Malaspina, O.; Nocelli, R.C.F. Oral Toxicity of Fipronil Insecticide Against the Stingless Bee Melipona Scutellaris (Latreille, 1811). Bull. Environ. Contam. Toxicol. 2012, 89, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H. Extrapolation of Acute Toxicity across Bee Species. Integr. Environ. Assess. Manag. 2016, 12, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Beadle, K.; Singh, K.S.; Troczka, B.J.; Randall, E.; Zaworra, M.; Zimmer, C.T.; Hayward, A.; Reid, R.; Kor, L.; Kohler, M.; et al. Genomic Insights into Neonicotinoid Sensitivity in the Solitary Bee Osmia Bicornis. PLoS Genet. 2019, 15, e1007903. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.T.; Joshi, N.K.; Rajotte, E.G.; López-Uribe, M.M.; Zhu, F.; Biddinger, D.J. A New Ingestion Bioassay Protocol for Assessing Pesticide Toxicity to the Adult Japanese Orchard Bee (Osmia Cornifrons). Sci. Rep. 2020, 10, 9517. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Awanbor, O.; Schulz, R.S.; Brühl, C.A. Osmia Bicornis Is Rarely an Adequate Regulatory Surrogate Species. Comparing Its Acute Sensitivity towards Multiple Insecticides with Regulatory Apis Mellifera Endpoints. PLoS ONE 2019, 14, e0201081. [Google Scholar] [CrossRef]
- ECOTOX Curated Toxicity Data Were Retrieved from the ECOTOXicology Knowledgebase. U.S. Environmental Protection Agency. Available online: http://www.epa.gov/ecotox/ (accessed on 1 November 2022).
- Bortolotti, L.; Porrini, C.; Sbrenna, G. Effetti Dell’imidacloprid Nei Confronti Di Bombus Terrestris (L.). Prove Di Laboratorio. Inf. Fitopatol. 2002, 3, 66–71. [Google Scholar]
- Hagen, M.; Wikelski, M.; Kissling, W.D. Space Use of Bumblebees (Bombus Spp.) Revealed by Radio-Tracking. PLoS ONE 2011, 6, e19997. [Google Scholar] [CrossRef] [PubMed]
- Suchail, S.; Debrauwer, L.; Belzunces, L.P. Metabolism of Imidacloprid in Apis Mellifera. Pest Manag. Sci. 2004, 60, 291–296. [Google Scholar] [CrossRef]
- Calvo-Agudo, M.; González-Cabrera, J.; Picó, Y.; Calatayud-Vernich, P.; Urbaneja, A.; Dicke, M.; Tena, A. Neonicotinoids in Excretion Product of Phloem-Feeding Insects Kill Beneficial Insects. Proc. Natl. Acad. Sci. USA 2019, 116, 16817–16822. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Démares, F.J.; Nicolson, S.W.; Medrzycki, P.; Pirk, C.W.W.; Human, H. Effects of a Neonicotinoid Pesticide on Thermoregulation of African Honey Bees (Apis Mellifera Scutellata). J. Insect Physiol. 2016, 93–94, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.L.; Harmon-Threatt, A.N. Chronic Contact with Realistic Soil Concentrations of Imidacloprid Affects the Mass, Immature Development Speed, and Adult Longevity of Solitary Bees. Sci. Rep. 2019, 9, 3724. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.; Clarke, R.M.; Oldfield, S.E.; Wood, L.K.; Hempel de Ibarra, N.; Cresswell, J.E. The Effect of Dietary Neonicotinoid Pesticides on Non-Flight Thermogenesis in Worker Bumble Bees (Bombus Terrestris). J. Insect Physiol. 2018, 104, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Haddi, K.; Mendes, M.V.; Barcellos, M.S.; Lino-Neto, J.; Freitas, H.L.; Guedes, R.N.C.; Oliveira, E.E. Sexual Success after Stress? Imidacloprid-Induced Hormesis in Males of the Neotropical Stink Bug Euschistus Heros. PLoS ONE 2016, 11, e0156616. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Calabrese, E.J. Environmental Toxicology and Ecotoxicology: How Clean Is Clean? Rethinking Dose-Response Analysis. Sci. Total Environ. 2020, 746, 138769. [Google Scholar] [CrossRef]
- Cutler, G.C.; Amichot, M.; Benelli, G.; Guedes, R.N.C.; Qu, Y.; Rix, R.R.; Ullah, F.; Desneux, N. Hormesis and Insects: Effects and Interactions in Agroecosystems. Sci. Total Environ. 2022, 825, 153899. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormetic Mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef] [PubMed]
- De Smet, L.; Hatjina, F.; Ioannidis, P.; Hamamtzoglou, A.; Schoonvaere, K.; Francis, F.; Meeus, I.; Smagghe, G.; de Graaf, D.C. Stress Indicator Gene Expression Profiles, Colony Dynamics and Tissue Development of Honey Bees Exposed to Sub-Lethal Doses of Imidacloprid in Laboratory and Field Experiments. PLoS ONE 2017, 12, e0171529. [Google Scholar] [CrossRef] [PubMed]
- Derecka, K.; Blythe, M.J.; Malla, S.; Genereux, D.P.; Guffanti, A.; Pavan, P.; Moles, A.; Snart, C.; Ryder, T.; Ortori, C.A. Transient Exposure to Low Levels of Insecticide Affects Metabolic Networks of Honeybee Larvae. PLoS ONE 2013, 8, e68191. [Google Scholar] [CrossRef] [PubMed]
- Erofeeva, E.A. Environmental Hormesis: From Cell to Ecosystem. Curr. Opin. Environ. Sci. Heal. 2022, 29, 100378. [Google Scholar] [CrossRef]
- Crossthwaite, A.J.; Bigot, A.; Camblin, P.; Goodchild, J.; Lind, R.J.; Slater, R.; Maienfisch, P. The Invertebrate Pharmacology of Insecticides Acting at Nicotinic Acetylcholine Receptors. J. Pestic. Sci. 2017, 42, 67–83. [Google Scholar] [CrossRef]
- Jones, A.K.; Brown, L.A.; Sattelle, D.B. Insect Nicotinic Acetylcholine Receptor Gene Families: From Genetic Model Organism to Vector, Pest and Beneficial Species. Invertebr. Neurosci. 2007, 7, 67–73. [Google Scholar] [CrossRef]
- Maloney, E.M.; Taillebois, E.; Gilles, N.; Morrissey, C.A.; Liber, K.; Servent, D.; Thany, S.H. Binding Properties to Nicotinic Acetylcholine Receptors Can Explain Differential Toxicity of Neonicotinoid Insecticides in Chironomidae. Aquat. Toxicol. 2021, 230, 105701. [Google Scholar] [CrossRef]
- Pamminger, T. Extrapolating Acute Contact Bee Sensitivity to Insecticides Based on Body Weight Using a Phylogenetically Informed Interspecies Scaling Framework. Environ. Toxicol. Chem. 2021, 40, 2042–2050. [Google Scholar] [CrossRef]
- Bailey, E.; Field, L.; Rawlings, C.; King, R.; Mohareb, F.; Pak, K.-H.; Hughes, D.; Williamson, M.; Ganko, E.; Buer, B.; et al. A Near-Chromosome Level Genome Assembly of the European Hoverfly, Sphaerophoria Rueppellii (Diptera: Syrphidae), Provides Comparative Insights into Insecticide Resistance-Related Gene Family Evolution. BMC Genom. 2022, 23, 198. [Google Scholar] [CrossRef]
- Doyle, T.; Jimenez-Guri, E.; Hawkes, W.L.S.; Massy, R.; Mantica, F.; Permanyer, J.; Cozzuto, L.; Hermoso Pulido, T.; Baril, T.; Hayward, A.; et al. Genome-wide Transcriptomic Changes Reveal the Genetic Pathways Involved in Insect Migration. Mol. Ecol. 2022, 31, 4332–4350. [Google Scholar] [CrossRef]
- Yuan, H.; Gao, B.; Wu, C.; Zhang, L.; Li, H.; Xiao, Y.; Wu, K. Genome of the Hoverfly Eupeodes Corollae Provides Insights into the Evolution of Predation and Pollination in Insects. BMC Biol. 2022, 20, 157. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Hayward, A.; Buer, B.; Maiwald, F.; Nebelsiek, B.; Glaubitz, J.; Bass, C.; Nauen, R. Phylogenomic and Functional Characterization of an Evolutionary Conserved Cytochrome P450-Based Insecticide Detoxification Mechanism in Bees. Proc. Natl. Acad. Sci. USA 2022, 119, e2205850119. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Franke, L.A.; Rehberg, C.; Wollmann, C.; Stahlschmidt, P.; Jeker, L.; Brühl, C.A. Interspecific Sensitivity of Bees towards Dimethoate and Implications for Environmental Risk Assessment. Sci. Rep. 2016, 6, 34439. [Google Scholar] [CrossRef] [PubMed]
- Belanger, S.; Barron, M.; Craig, P.; Dyer, S.; Galay-Burgos, M.; Hamer, M.; Marshall, S.; Posthuma, L.; Raimondo, S.; Whitehouse, P. Future Needs and Recommendations in the Development of Species Sensitivity Distributions: Estimating Toxicity Thresholds for Aquatic Ecological Communities and Assessing Impacts of Chemical Exposures. Integr. Environ. Assess. Manag. 2017, 13, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Schmolke, A.; Galic, N.; Feken, M.; Thompson, H.; Sgolastra, F.; Pitts-Singer, T.; Elston, C.; Pamminger, T.; Hinarejos, S. Assessment of the Vulnerability to Pesticide Exposures across Bee Species. Environ. Toxicol. Chem. 2021, 40, 2640–2651. [Google Scholar] [CrossRef]
- Topping, C.J.; Aldrich, A.; Berny, P. Overhaul Environmental Risk Assessment for Pesticides. Science 2020, 367, 360–363. [Google Scholar] [CrossRef]
- Hätönen, M.; Kantner, C.; Lopez Losada, R.; Ludwig, N.; Benavent González, A.; Riedhammer, C.; Kunz, P.; Panico, S.C.; Laakkonen, E.; Parramon Dolcet, L.; et al. European Arthropods and Their Role in Pollination: Scientific Report of Their Biodiversity, Ecology and Sensitivity to Biocides; European Chemicals Agency: Helsinki, Finland, 2022; ISBN 978-92-9468-131-7. [Google Scholar]

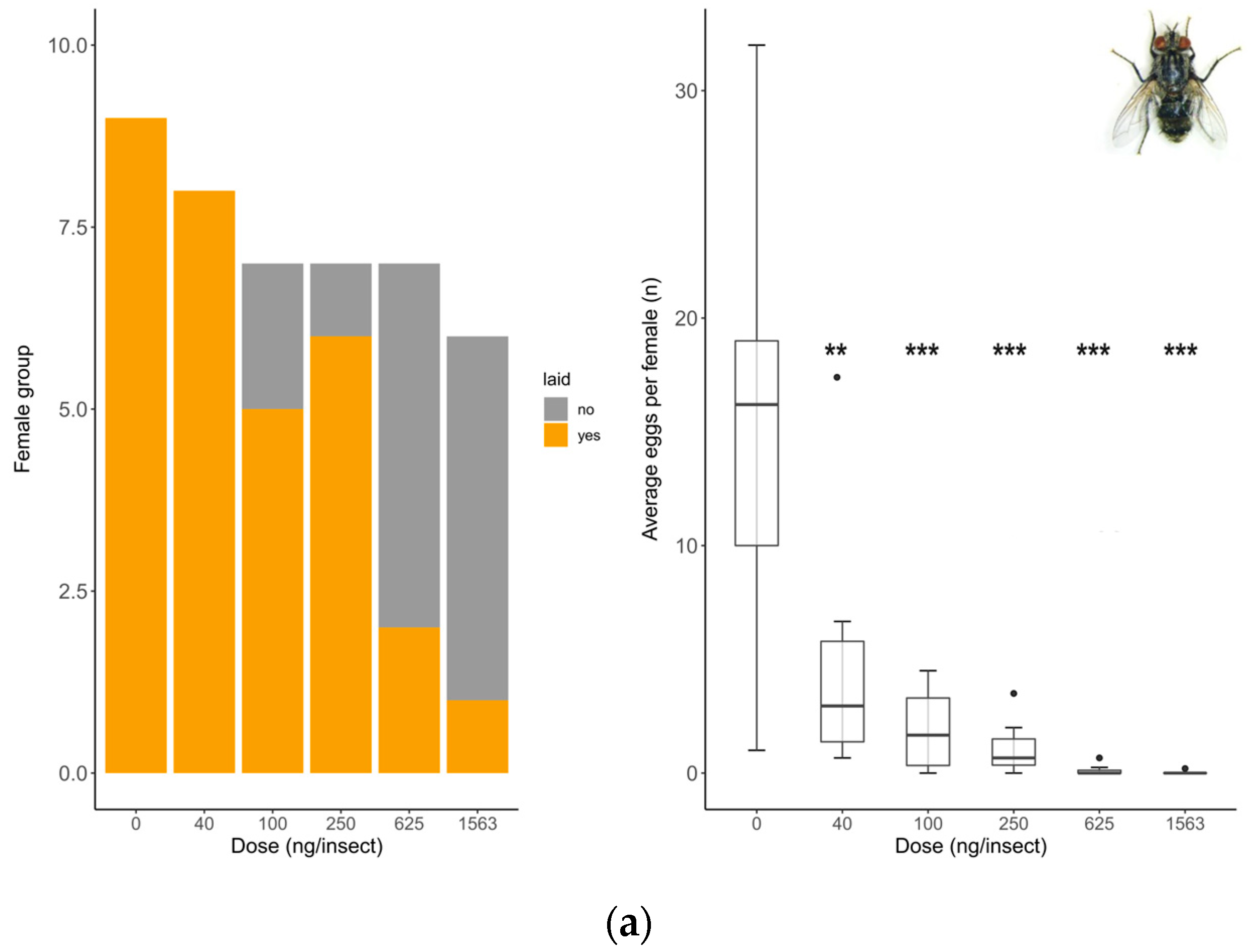
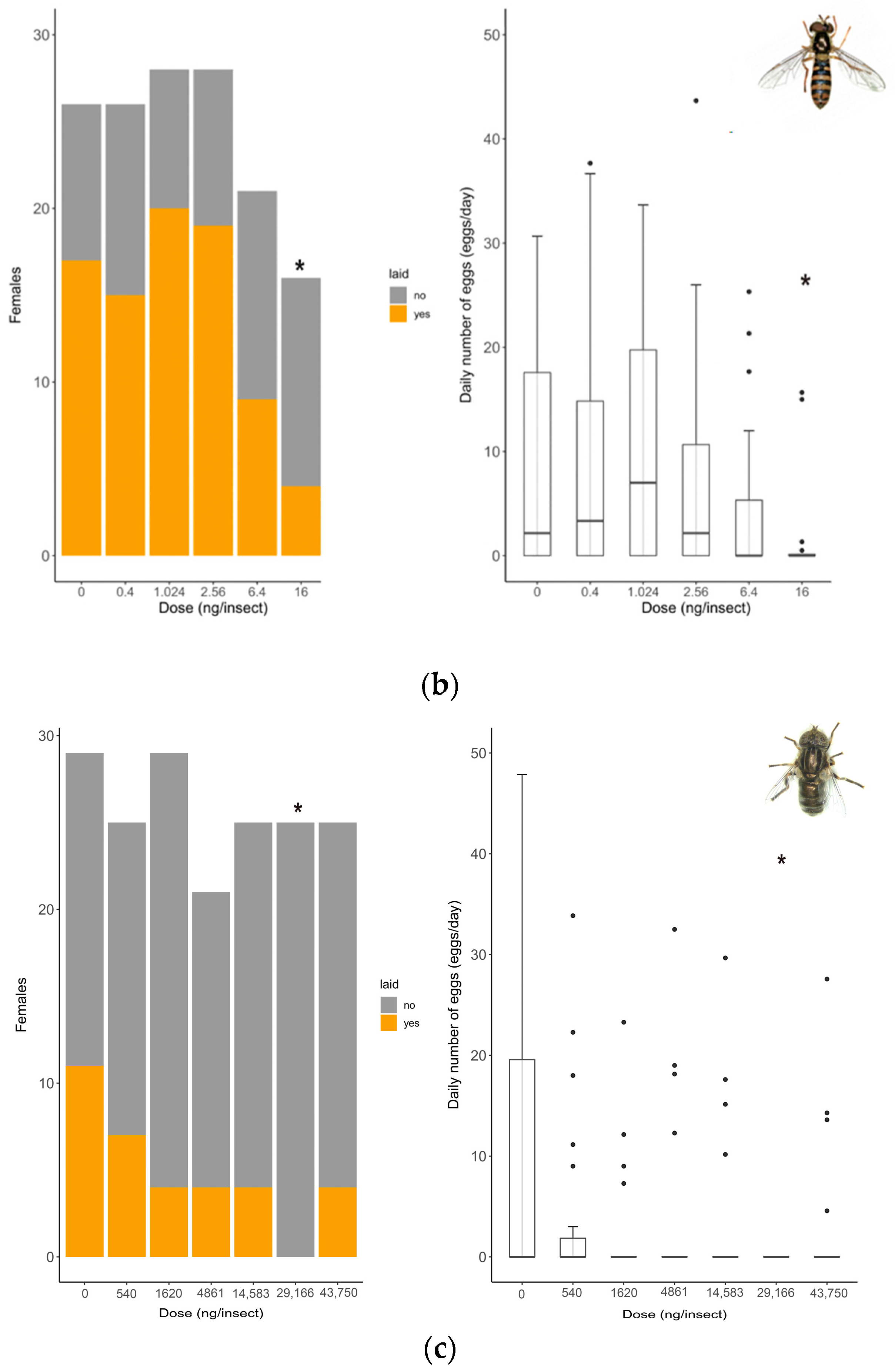
| Species | n | Model | Slope | Log-Likelihood | Residual Standard Error (df) | AIC | p-Value | 48 h LD50 | 95% CI | 48 h LD50 | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (ng/Insect) | (µg/g Insect) | ||||||||||
| Exorista larvarum | 233 | Log-logistic | −0.765 | −14.87 | 4.89 (3) | 37.75 | 0.0087 | 467.46 | 302.28–632.65 | 11.66 | 7.54–15.79 |
| Sphaerophoria rueppellii | 205 | Log-logistic | −1.668 | −19.35 | 5.11 (3) | 46.85 | 0.012 | 10.23 | 7.81–12.65 | 1.35 | 0.83–1.86 |
| Eristalinus aeneus * | 193 | Log-logistic | −1.062 | −9.86 | 2.75 (2) | 27.74 | 0.036 | 18,176.20 | 8005.6–28,346.9 | 344.77 | 151.85–537.69 |
| Species | n | Model | BMDL | BMD | BMDU | p-Value | AIC |
|---|---|---|---|---|---|---|---|
| (ng/Insect) | (ng/Insect) | (ng/Insect) | |||||
| Exorista larvarum | 178 | Multistage 1° | 47.08 | 75.11 | 127.12 | 0.639 | 33.535 |
| Sphaerophoria rueppellii | 174 | Logistic | 1.70 | 2.50 | 4.69 | 0.649 | 190.797 |
| Eristalinus aeneus | 209 | Log-Logistic | 356.79 | 1080.91 | 8498.47 | 0.178 | 165.298 |
| Species | LD50 | BMDL | SSI |
|---|---|---|---|
| (ng/Insect) | (ng/Insect) | ||
| Exorista larvarum | 467.46 | 47.08 | 9.93 |
| Sphaerophoria rueppellii | 10.23 | 1.70 | 6.01 |
| Eristalinus aeneus | 18,176.20 | 356.79 | 50.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henriques Martins, C.A.; Azpiazu, C.; Bosch, J.; Burgio, G.; Dindo, M.L.; Francati, S.; Sommaggio, D.; Sgolastra, F. Different Sensitivity of Flower-Visiting Diptera to a Neonicotinoid Insecticide: Expanding the Base for a Multiple-Species Risk Assessment Approach. Insects 2024, 15, 317. https://doi.org/10.3390/insects15050317
Henriques Martins CA, Azpiazu C, Bosch J, Burgio G, Dindo ML, Francati S, Sommaggio D, Sgolastra F. Different Sensitivity of Flower-Visiting Diptera to a Neonicotinoid Insecticide: Expanding the Base for a Multiple-Species Risk Assessment Approach. Insects. 2024; 15(5):317. https://doi.org/10.3390/insects15050317
Chicago/Turabian StyleHenriques Martins, Cátia Ariana, Celeste Azpiazu, Jordi Bosch, Giovanni Burgio, Maria Luisa Dindo, Santolo Francati, Daniele Sommaggio, and Fabio Sgolastra. 2024. "Different Sensitivity of Flower-Visiting Diptera to a Neonicotinoid Insecticide: Expanding the Base for a Multiple-Species Risk Assessment Approach" Insects 15, no. 5: 317. https://doi.org/10.3390/insects15050317
APA StyleHenriques Martins, C. A., Azpiazu, C., Bosch, J., Burgio, G., Dindo, M. L., Francati, S., Sommaggio, D., & Sgolastra, F. (2024). Different Sensitivity of Flower-Visiting Diptera to a Neonicotinoid Insecticide: Expanding the Base for a Multiple-Species Risk Assessment Approach. Insects, 15(5), 317. https://doi.org/10.3390/insects15050317










