CRISPR/Cas9-Based Functional Characterization of SfUGT50A15 Reveals Its Roles in the Resistance of Spodoptera frugiperda to Chlorantraniliprole, Emamectin Benzoate, and Benzoxazinoids
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Insecticides and Plants
2.3. Characterization and Cluster Analysis of SfUGT50A15 Sequences
2.4. Extraction of Total RNA and Synthesis of cDNA
2.5. Cloning and Analysis of the SfUGT50A15 Gene
2.6. Quantitative Real-Time PCR
2.7. Design and Preparation of sgRNA for CRISPR/Cas9
2.8. Egg Collection and Microinjection
2.9. Phenotypic Observation and Mutation Analysis
2.10. Fitness Cost Analysis
2.11. Insect Bioassay
2.12. Statistical Analysis
3. Results
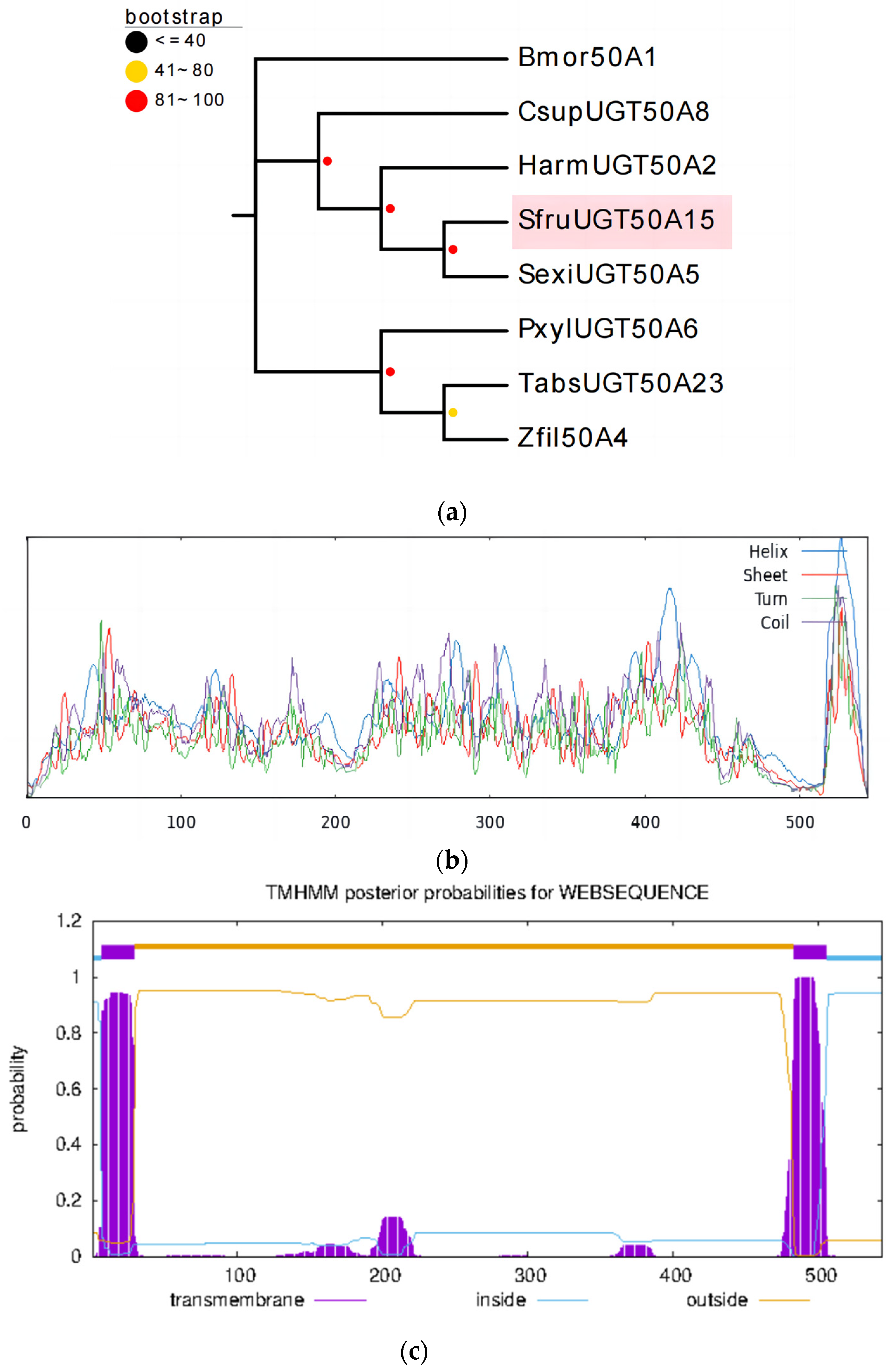
3.1. Sequence and Phylogenetic Analysis of the UGT Gene
3.2. Expression Profiles of SfUGT50A15
3.3. Generation of the SfUGT50A15-KO Line
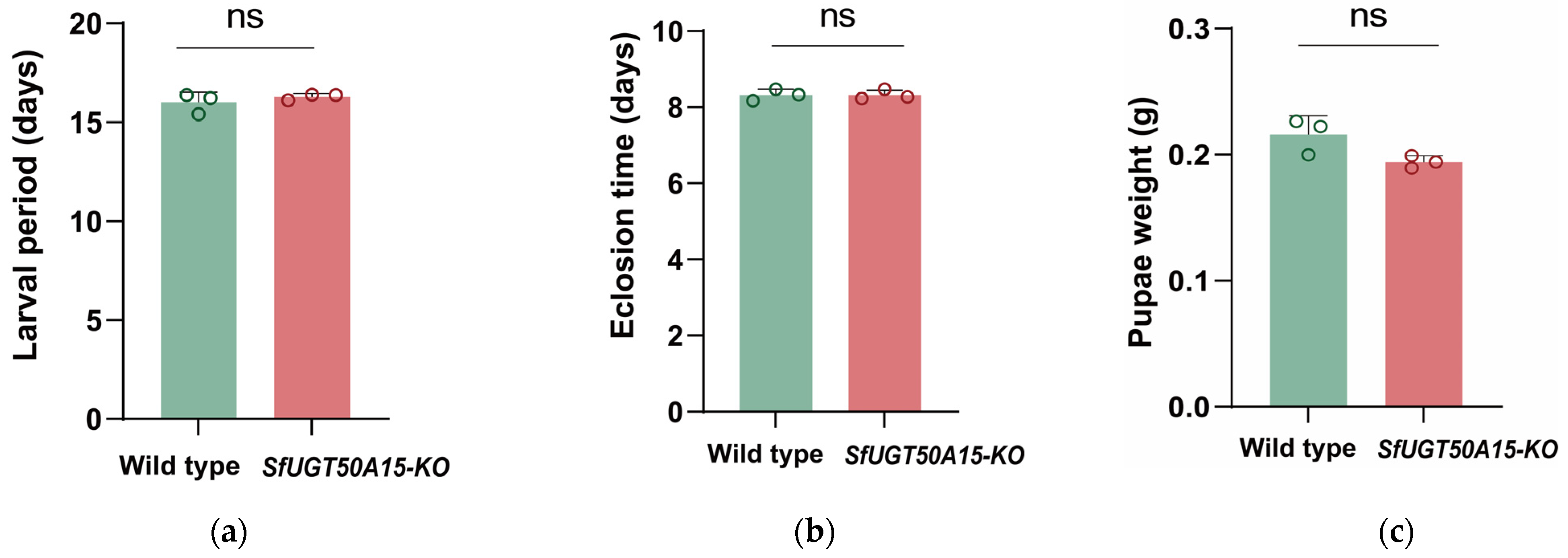
3.4. No Fitness Costs Were Associated with the Knockout
3.5. Effect of SfUGT50A15 Knockout on the Susceptibility of S. frugiperda Larvae to Insecticides
3.6. Susceptibility of Larvae to Benzoxazinoids Following SfUGT50A15 Knockout
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-Evolved Resistance of the Fall Armyworm (Lepidoptera: Noctuidae) to Synthetic Insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, J.K.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J.; Jairam, S. Modeling seasonal migration of fall armyworm moths. Int. J. Biometeorol. 2016, 60, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall Armyworm: Impacts and Implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Sun, X.-X.; Hu, C.-X.; Jia, H.-R.; Wu, Q.-L.; Shen, X.-J.; Zhao, S.-Y.; Jiang, Y.-Y.; Wu, K.-M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Pitre, H.N. Chemical control of the fall armyworm (Lepidoptera: Noctuidae): An update. Fla. Entomol. 1986, 69, 570–578. [Google Scholar] [CrossRef]
- Zhao, J.; Fang, H.; Zhang, D. Expanding application of CRISPR-Cas9 system in microorganisms. Synth. Syst. Biotechnol. 2020, 5, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-D.; Xiao, Y.-T.; Xu, P.-J.; Yang, X.-M.; Wu, Q.-L.; Wu, K.-M. Insecticide resistance monitoring for the invasive populations of fall armyworm, Spodoptera frugiperda in China. J. Integr. Agric. 2021, 20, 783–791. [Google Scholar] [CrossRef]
- Burchell, B.; Coughtrie, M.W. UDP-glucuronosyltransferases. Pharmacol. Ther. 1989, 43, 261–289. [Google Scholar] [CrossRef]
- Ahn, S.J.; Vogel, H.; Heckel, D.G. Comparative analysis of the UDP-glycosyltransferase multigene family in insects. Insect Biochem. Mol. Biol. 2012, 42, 133–147. [Google Scholar] [CrossRef]
- Bozzolan, F.; Siaussat, D.; Maria, A.; Durand, N.; Pottier, M.A.; Chertemps, T.; Maïbèche-Coisne, M. Antennal uridine diphosphate (UDP)-glycosyltransferases in a pest insect: Diversity and putative function in odorant and xenobiotics clearance. Insect Mol. Biol. 2014, 23, 539–549. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Ma, J.-F.; Xu, L.; Dong, Z.-P.; Xu, J.-W.; Li, M.-Y.; Zhu, X.-Y. Identification and expression patterns of UDP-glycosyltransferase (UGT) genes from insect pest Athetis lepigone (Lepidoptera: Noctuidae). J. Asia-Pac. Entomol. 2017, 20, 253–259. [Google Scholar] [CrossRef]
- Kennedy, C.J.; Tierney, K.B. Xenobiotic Protection/Resistance Mechanisms in Organisms. In Environmental Toxicology: Selected Entries from the Encyclopedia of Sustainability Science and Technology; Laws, E.A., Ed.; Springer: New York, NY, USA, 2013; pp. 689–721. [Google Scholar]
- Li, X.; Shi, H.; Gao, X.; Liang, P. Characterization of UDP-glucuronosyltransferase genes and their possible roles in multi-insecticide resistance in Plutella xylostella (L.). Pest Manag. Sci. 2018, 74, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Heidel-Fischer, H.M.; Vogel, H. Molecular mechanisms of insect adaptation to plant secondary compounds. Curr. Opin. Insect Sci. 2015, 8, 8–14. [Google Scholar] [CrossRef]
- Su, X.N.; Li, C.Y.; Zhang, Y.P. Chlorpyrifos and chlorfenapyr resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) relies on UDP-glucuronosyltransferases. J. Econ. Entomol. 2023, 116, 1329–1341. [Google Scholar] [CrossRef]
- Wouters, F.C.; Reichelt, M.; Glauser, G.; Bauer, E.; Erb, M.; Gershenzon, J.; Vassão, D.G. Reglucosylation of the benzoxazinoid DIMBOA with inversion of stereochemical configuration is a detoxification strategy in lepidopteran herbivores. Angew. Chem. (Int. Ed. Engl.) 2014, 53, 11320–11324. [Google Scholar] [CrossRef]
- Chen, W.; Hasegawa, D.K.; Kaur, N.; Kliot, A.; Pinheiro, P.V.; Luan, J.; Stensmyr, M.C.; Zheng, Y.; Liu, W.; Sun, H.; et al. The draft genome of whitefly Bemisia tabaci MEAM1, a global crop pest, provides novel insights into virus transmission, host adaptation, and insecticide resistance. BMC Biol. 2016, 14, 110. [Google Scholar] [CrossRef]
- You, M.; Yue, Z.; He, W.; Yang, X.; Yang, G.; Xie, M.; Zhan, D.; Baxter, S.W.; Vasseur, L.; Gurr, G.M.; et al. A heterozygous moth genome provides insights into herbivory and detoxification. Nat. Genet. 2013, 45, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Esen, A. Purification and Partial Characterization of Maize (Zea mays L.) beta-Glucosidase. Plant Physiol. 1992, 98, 174–182. [Google Scholar] [CrossRef]
- Oikawa, A.; Ebisui, K.; Sue, M.; Ishihara, A.; Iwamura, H. Purification and Characterization of a β-Glucosidase Specific for 2,4-Dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA) Glucoside in Maize. Z. Für Naturforschung C 1999, 54, 181–185. [Google Scholar] [CrossRef]
- Israni, B.; Wouters, F.C.; Luck, K.; Seibel, E.; Ahn, S.J.; Paetz, C.; Reinert, M.; Vogel, H.; Erb, M.; Heckel, D.G.; et al. The Fall Armyworm Spodoptera frugiperda Utilizes Specific UDP-Glycosyltransferases to Inactivate Maize Defensive Benzoxazinoids. Front. Physiol. 2020, 11, 604754. [Google Scholar] [CrossRef]
- Jin, T.; Lin, Y.Y.; Chi, H.; Xiang, K.P.; Ma, G.C.; Peng, Z.Q.; Yi, K.X. Comparative Performance of the Fall Armyworm (Lepidoptera: Noctuidae) Reared on Various Cereal-Based Artificial Diets. J. Econ. Entomol. 2020, 113, 2986–2996. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- Toni, L.S.; Garcia, A.M.; Jeffrey, D.A.; Jiang, X.; Stauffer, B.L.; Miyamoto, S.D.; Sucharov, C.C. Optimization of phenol-chloroform RNA extraction. Methods X 2018, 5, 599–608. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Idrees, A.; Qadir, Z.A.; Afzal, A.; Ranran, Q.; Li, J. Laboratory efficacy of selected synthetic insecticides against second instar invasive fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. PLoS ONE 2022, 17, e0265265. [Google Scholar] [CrossRef] [PubMed]
- Payton, M.E.; Greenstone, M.H.; Schenker, N. Overlapping Confidence Intervals or Standard Error Intervals: What Do they Mean in Terms of Statistical Significance. J. Insect Sci. 2003, 3, 34. [Google Scholar] [CrossRef]
- Sachdev, B.; Khan, Z.; Zarin, M.; Malhotra, P.; Seth, R.K.; Bhatnagar, R.K. Irradiation influence on the phenoloxidase pathway and an anti-oxidant defense mechanism in Spodoptera litura (Lepidoptera: Noctuidae) and its implication in radio-genetic ‘F1 sterility’ and biorational pest suppression tactics. Bull. Entomol. Res. 2017, 107, 281–293. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, S.H.; Ren, M.M.; Tian, X.R.; Wei, Q.; Mburu, D.K.; Su, J.Y. The expression of Spodoptera exigua P450 and UGT genes: Tissue specificity and response to insecticides. Insect Sci. 2019, 26, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Yang, W.; Zhao, H.; Wang, B.J.; Shi, Y.; Wang, M.Y.; Liu, S.Q.; Liao, X.L.; Shi, L. Functional analysis of UDP-glycosyltransferase genes conferring indoxacarb resistance in Spodoptera litura. Pestic. Biochem. Physiol. 2023, 196, 105589. [Google Scholar] [CrossRef]
- Gui, F.; Lan, T.; Zhao, Y.; Guo, W.; Dong, Y.; Fang, D.; Liu, H.; Li, H.; Wang, H.; Hao, R.; et al. Genomic and transcriptomic analysis unveils population evolution and development of pesticide resistance in fall armyworm Spodoptera frugiperda. Protein Cell 2022, 13, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Ohta, K.; Tashiro, S.; Shono, T. Metabolic resistance mechanisms of the housefly (Musca domestica) resistant to pyraclofos. Pestic. Biochem. Physiol. 2006, 85, 76–83. [Google Scholar] [CrossRef]
- Silva, A.X.; Jander, G.; Samaniego, H.; Ramsey, J.S.; Figueroa, C.C. Insecticide resistance mechanisms in the green peach aphid Myzus persicae (Hemiptera: Aphididae) I: A transcriptomic survey. PLoS ONE 2012, 7, e36366. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Xie, W.; Yang, X.; Wang, S.; Wu, Q.; Li, R.; Pan, H.; Liu, B.; Shi, X.; Fang, Y.; et al. Transcriptomic and proteomic responses of sweetpotato whitefly, Bemisia tabaci, to thiamethoxam. PLoS ONE 2013, 8, e61820. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, B.; Gao, X.; Liang, P. Over-expression of UDP-glycosyltransferase gene UGT2B17 is involved in chlorantraniliprole resistance in Plutella xylostella (L.). Pest Manag. Sci. 2017, 73, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xiao, T.; Lu, K. Contribution of UDP-glycosyltransferases to chlorpyrifos resistance in Nilaparvata lugens. Pestic. Biochem. Physiol. 2023, 190, 105321. [Google Scholar] [CrossRef] [PubMed]
- Krempl, C.; Sporer, T.; Reichelt, M.; Ahn, S.J.; Heidel-Fischer, H.; Vogel, H.; Heckel, D.G.; Joußen, N. Potential detoxification of gossypol by UDP-glycosyltransferases in the two Heliothine moth species Helicoverpa armigera and Heliothis virescens. Insect Biochem. Mol. Biol. 2016, 71, 49–57. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, P.; Zeng, X.; Liu, X.; Shang, Q. Characterization of UDP-Glucuronosyltransferases and the Potential Contribution to Nicotine Tolerance in Myzus persicae. Int. J. Mol. Sci. 2019, 20, 3637. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E. The hormetic response of heart rate of fish embryos to contaminants—Implications for research and policy. Sci. Total Environ. 2022, 815, 152911. [Google Scholar] [CrossRef]
- Wu, K.; Shirk, P.D.; Taylor, C.E.; Furlong, R.B.; Shirk, B.D.; Pinheiro, D.H.; Siegfried, B.D. CRISPR/Cas9 mediated knockout of the abdominal-A homeotic gene in fall armyworm moth (Spodoptera frugiperda). PLoS ONE 2018, 13, e0208647. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Zeng, B.; Wang, Y.; James, A.A.; Gurr, G.M.; Yang, G.; Lin, X.; Huang, Y.; You, M. CRISPR/Cas9 mediated knockout of the abdominal-A homeotic gene in the global pest, diamondback moth (Plutella xylostella). Insect Biochem. Mol. Biol. 2016, 75, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.H.; Xu, J.; Cui, Z.; Dong, X.T.; Ye, Z.F.; Niu, D.J.; Huang, Y.P.; Dong, S.L. Functional characterization of SlitPBP3 in Spodoptera litura by CRISPR/Cas9 mediated genome editing. Insect Biochem. Mol. Biol. 2016, 75, 1–9. [Google Scholar] [CrossRef]
- Wei, W.; Xin, H.; Roy, B.; Dai, J.; Miao, Y.; Gao, G. Heritable genome editing with CRISPR/Cas9 in the silkworm, Bombyx mori. PLoS ONE 2014, 9, e101210. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.A.; Davies, S.A. The Malpighian tubule: Rapid insights from post-genomic biology. J. Insect Physiol. 2006, 52, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhu, L.; Guo, L.; Wang, S.; Wu, Q.; Crickmore, N.; Zhou, X.; Bravo, A.; Soberón, M.; Guo, Z.; et al. A versatile contribution of both aminopeptidases N and ABC transporters to Bt Cry1Ac toxicity in the diamondback moth. BMC Biol. 2022, 20, 33. [Google Scholar] [CrossRef]
- Guo, L.; Cheng, Z.; Qin, J.; Sun, D.; Wang, S.; Wu, Q.; Crickmore, N.; Zhou, X.; Bravo, A.; Soberón, M.; et al. MAPK-mediated transcription factor GATAd contributes to Cry1Ac resistance in diamondback moth by reducing PxmALP expression. PLoS Genet. 2022, 18, e1010037. [Google Scholar] [CrossRef]





| Primer Name | Primer Sequence (5′-3′) | Primer Purpose |
|---|---|---|
| SfUGT50A15.qF | ACAGAGCCCTGAATGCCATC | RT-qPCR |
| SfUGT50A15.qR | TGAAGCTCACATTCTTCGCC | |
| SfruActinF | TACTCCTAAGCCTGTTGATG | RT-qPCR |
| SfruActinR | TTATGTCATGGTGCCGAAT | |
| SfUGT50A15.F1 | ACGTAGTAGGGGCAGCCC | Cloning the SfUGT50A15 gene |
| SfUGT50A15.R1 | TATGTACATTACATTTTATAAACTGAACATCGATC | |
| SfUGT50A15.sgF | GAAATTAATACGACTCACTATAGGACTCCGTCACCCCATTCTT | Preparation of sgRNA templates |
| SfUGT50A15.sgR | TTCTAGCTCTAAAACAAGAATGGGGTGACGGAGT | |
| SfUGT50A15.F2 | TCAGGAAGGACTTTTGATCTCG | Identification of somatic mutations |
| SfUGT50A15.R2 | CATTCTGGAGTATGAAGCTCACAT |
| Insecticide | Strain | LC50 (µg/g) a | 95% FL b | N c | Toxicity Ratio d |
|---|---|---|---|---|---|
| Chlorantraniliprole | WT | 19.305 | 11.08–33.636 | 192 | 5.67 e |
| SfUGT50A15-KO | 3.402 | 1.919–6.032 | 192 | ||
| Emamectin benzoate | WT | 15.988 | 7.803–32.757 | 192 | 5.00 e |
| SfUGT50A15-KO | 3.196 | 1.046–6.228 | 192 | ||
| Spinetoram | WT | 0.244 | 0.244–8.815 | 192 | 3.87 |
| SfUGT50A15-KO | 0.063 | 0.063–8.601 | 192 | ||
| Lufenuron | WT | 0.137 | 0.137–5.097 | 192 | 1.28 |
| SfUGT50A15-KO | 0.107 | 0.107–3.737 | 192 | ||
| Chlorfenapyr | WT | 4.081 | 4.081–31.146 | 192 | 1.12 |
| SfUGT50A15-KO | 3.642 | 3.642–27.171 | 192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Luo, M.; Yuan, J.; Gao, B.; Yang, M.; Wang, G. CRISPR/Cas9-Based Functional Characterization of SfUGT50A15 Reveals Its Roles in the Resistance of Spodoptera frugiperda to Chlorantraniliprole, Emamectin Benzoate, and Benzoxazinoids. Insects 2024, 15, 314. https://doi.org/10.3390/insects15050314
Shi Z, Luo M, Yuan J, Gao B, Yang M, Wang G. CRISPR/Cas9-Based Functional Characterization of SfUGT50A15 Reveals Its Roles in the Resistance of Spodoptera frugiperda to Chlorantraniliprole, Emamectin Benzoate, and Benzoxazinoids. Insects. 2024; 15(5):314. https://doi.org/10.3390/insects15050314
Chicago/Turabian StyleShi, Zhan, Mei Luo, Jinxi Yuan, Bin Gao, Minghuan Yang, and Guirong Wang. 2024. "CRISPR/Cas9-Based Functional Characterization of SfUGT50A15 Reveals Its Roles in the Resistance of Spodoptera frugiperda to Chlorantraniliprole, Emamectin Benzoate, and Benzoxazinoids" Insects 15, no. 5: 314. https://doi.org/10.3390/insects15050314
APA StyleShi, Z., Luo, M., Yuan, J., Gao, B., Yang, M., & Wang, G. (2024). CRISPR/Cas9-Based Functional Characterization of SfUGT50A15 Reveals Its Roles in the Resistance of Spodoptera frugiperda to Chlorantraniliprole, Emamectin Benzoate, and Benzoxazinoids. Insects, 15(5), 314. https://doi.org/10.3390/insects15050314




