Upf2-Mediated Nonsense-Mediated Degradation Pathway Involved in Genetic Compensation of TrpA1 Knockout Mutant Silkworm (Bombyx mori)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Silkworm, B. mori
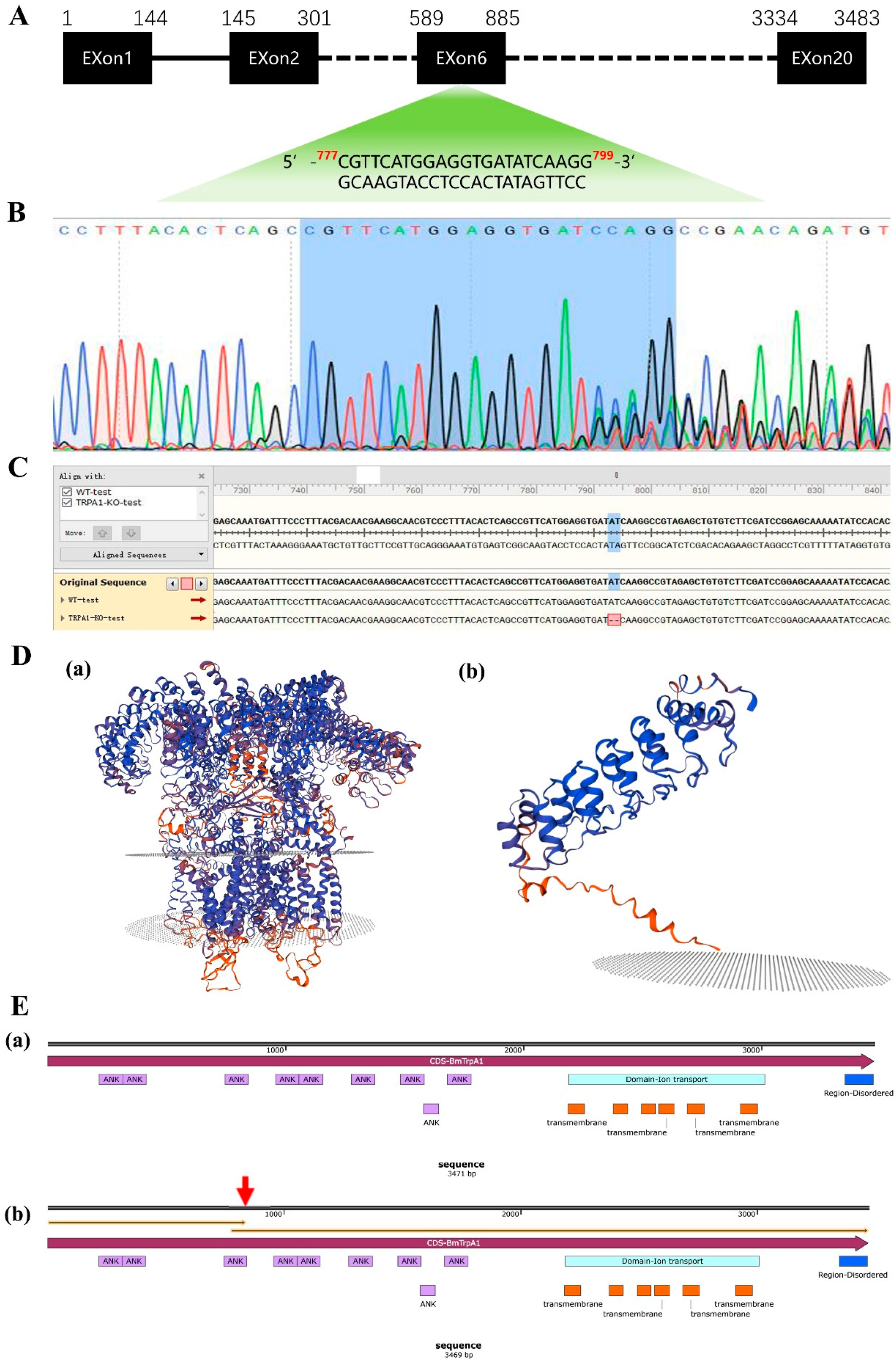
2.2. Construction of the BmTrpA1−/− Mutant and Prediction of the Three-Dimensional Structure of the Protein
2.3. Mutant Screening
2.4. Investigation of Larval Growth and Development and Cocoon Economic Traits
2.5. Extraction of Total RNA from Silkworm Tissues and RT-qPCR Analysis
2.6. Embryonic RNA Interference
3. Results
3.1. Construction of a BmTrpA1−/− Mutant Strain
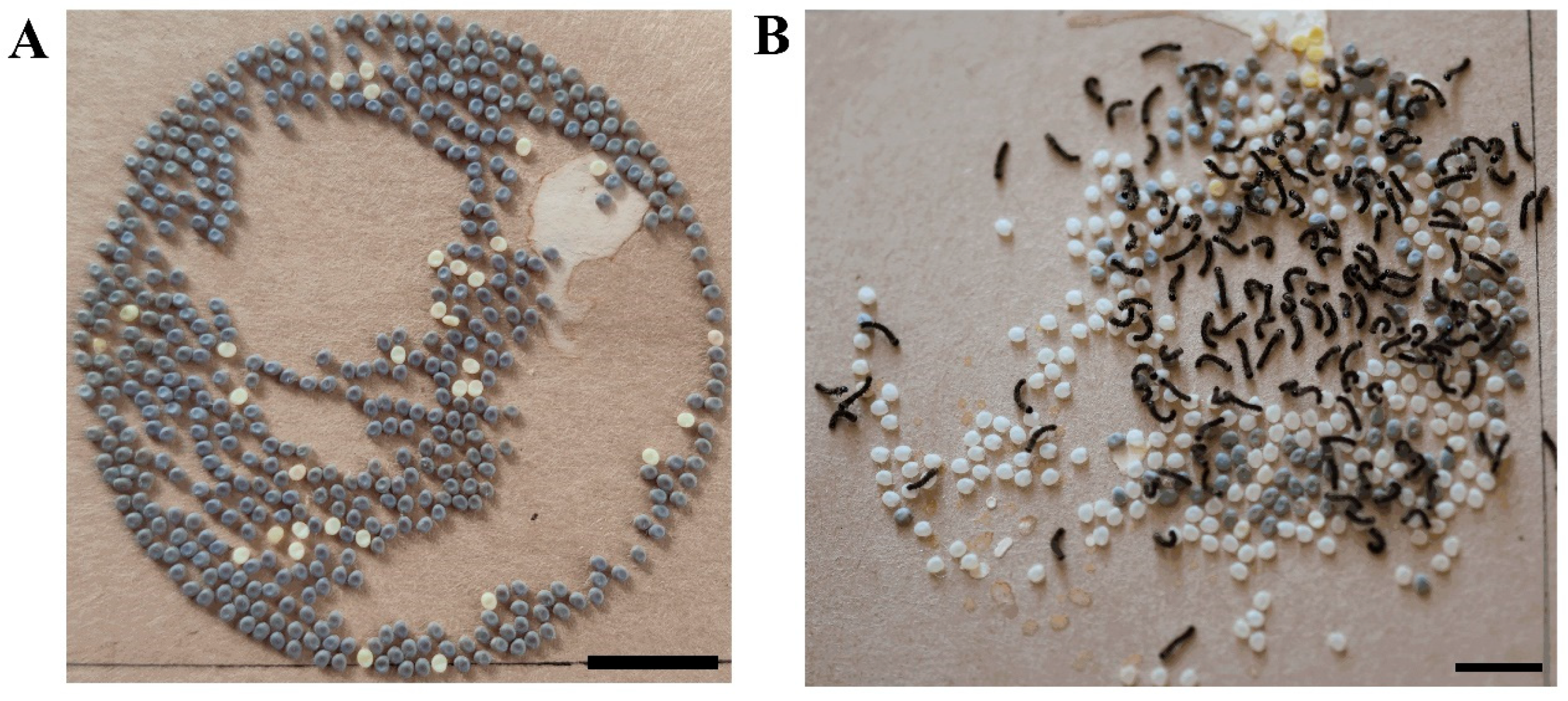
3.2. No Obvious Differences Were Observed in the Growth, Development, or Diapause Phenotype between BmTrpA1−/− and wt
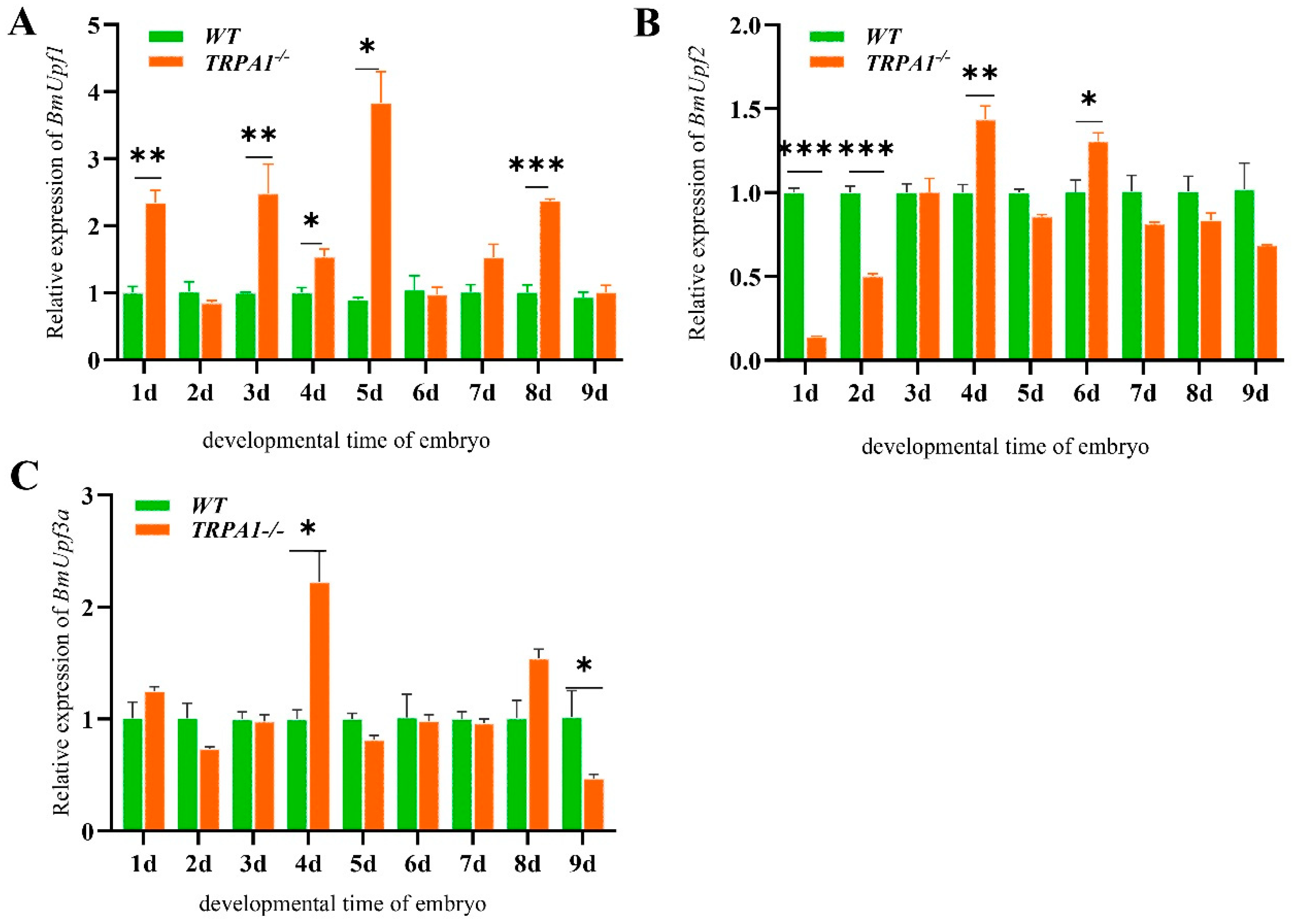
3.3. BmPyrexia, BmPainless, and BmUpf2 Showed Upregulated Expression in BmTrpA1−/− Mutant
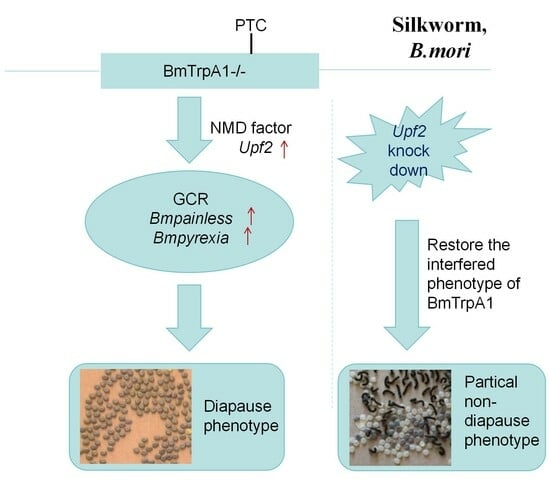
3.4. RNA Interference of the UPF2 Gene Alters the Mutant’s Diapause Phenotype
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jakutis, G.; Stainier, D.Y. Genotype–Phenotype relationships in the context of transcriptional adaptation and genetic robustness. Annu. Rev. Genet. 2021, 55, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Zhang, D.; Dai, X.; Estelle, M.; Zhao, Y. Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc. Natl. Acad. Sci. USA 2015, 112, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.T.; Dai, X.; Spencer, A.G.; Reppen, T.; Menzie, A.; Roesch, P.L.; He, Y.; Caguyong, M.J.; Bloomer, S.; Herweijer, H. Transcriptional and phenotypic comparisons of Ppara knockout and siRNA knockdown mice. Nucleic Acids Res. 2006, 34, 4486–4494. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Jaiswal, M.; Charng, W.-L.; Gambin, T.; Karaca, E.; Mirzaa, G.; Wiszniewski, W.; Sandoval, H.; Haelterman, N.A.; Xiong, B. A drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases. Cell 2014, 159, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.O.; Shin, M.; Ni, C.-W.; Gupta, A.; Grosse, A.S.; van Impel, A.; Kirchmaier, B.C.; Peterson-Maduro, J.; Kourkoulis, G.; Male, I. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 2015, 32, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Morgens, D.W.; Deans, R.M.; Li, A.; Bassik, M.C. Systematic comparison of CRISPR/Cas9 and RNAi screens for essential genes. Nat. Biotechnol. 2016, 34, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-F.; Imam, J.S.; Wilkinson, M.F. The nonsense-mediated decay RNA surveillance pathway. Annu. Rev. Biochem. 2007, 76, 51–74. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Panigrahi, G.K. Messenger RNA Surveillance: Current Understanding, Regulatory Mechanisms, and Future Implications. Mol. Biotechnol. 2024, 1–17. [Google Scholar] [CrossRef]
- El-Brolosy, M.A.; Kontarakis, Z.; Rossi, A.; Kuenne, C.; Günther, S.; Fukuda, N.; Kikhi, K.; Boezio, G.L.; Takacs, C.M.; Lai, S.-L. Genetic compensation triggered by mutant mRNA degradation. Nature 2019, 568, 193–197. [Google Scholar] [CrossRef]
- Ma, Z.; Zhu, P.; Shi, H.; Guo, L.; Zhang, Q.; Chen, Y.; Chen, S.; Zhang, Z.; Peng, J.; Chen, J. PTC-bearing mRNA elicits a genetic compensation response via Upf3a and COMPASS components. Nature 2019, 568, 259–263. [Google Scholar] [CrossRef]
- Xie, A.; Ma, Z.; Wang, J.; Zhang, Y.; Chen, Y.; Yang, C.; Chen, J.; Peng, J. Upf3a but not Upf1 mediates the genetic compensation response induced by leg1 deleterious mutations in an H3K4me3-independent manner. Cell Discov. 2023, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Meents, J.E.; Ciotu, C.I.; Fischer, M.J. TRPA1: A molecular view. J. Neurophysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Armache, J.-P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 520, 511–517. [Google Scholar] [CrossRef]
- Matsuura, H.; Sokabe, T.; Kohno, K.; Tominaga, M.; Kadowaki, T. Evolutionary conservation and changes in insect TRP channels. BMC Evol. Biol. 2009, 9, 228. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, Y.; Lee, J.; Bang, S.; Hyun, S.; Kang, J.; Hong, S.-T.; Bae, E.; Kaang, B.-K.; Kim, J. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat. Genet. 2005, 37, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J. Thermosensation and pain. J. Neurobiol. 2004, 61, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Tracey, W.D.; Wilson, R.I.; Laurent, G.; Benzer, S. painless, a Drosophila gene essential for nociception. Cell 2003, 113, 261–273. [Google Scholar] [CrossRef]
- Castillo, K.; Diaz-Franulic, I.; Canan, J.; Gonzalez-Nilo, F.; Latorre, R. Thermally activated TRP channels: Molecular sensors for temperature detection. Phys. Biol. 2018, 15, 021001. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Sokabe, T.; Kashio, M.; Yasukochi, Y.; Tominaga, M.; Shiomi, K. Embryonic thermosensitive TRPA1 determines transgenerational diapause phenotype of the silkworm, Bombyx mori. Proc. Natl. Acad. Sci. USA 2014, 111, E1249–E1255. [Google Scholar] [CrossRef]
- Huang, Z. A Novel Regulatory Mechanism Underlying the Regulation of Ambient Temperature on Larval Growth and Development in Silkworm. Master’s Thesis, Southwest University, Chongqing, China, 2023. [Google Scholar]
- Chen, Y.-r.; Jiang, T.; Zhu, J.; Xie, Y.-c.; Tan, Z.-c.; Chen, Y.-h.; Tang, S.-m.; Hao, B.-f.; Wang, S.-p.; Huang, J.-s. Transcriptome sequencing reveals potential mechanisms of diapause preparation in bivoltine silkworm Bombyx mori (Lepidoptera: Bombycidae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2017, 24, 68–78. [Google Scholar]
- Zhu, J.; Chen, Y.-R.; Geng, T.; Tang, S.-M.; Zhao, Q.-l.; Shen, X.-J. A 14-amino acids deletion in BmShadow results to non-moult on the 2nd instar in the bivoltine silkworm, Bombyx mori. Gene 2021, 777, 145450. [Google Scholar] [CrossRef] [PubMed]
- Bienert, S.; Waterhouse, A.; De Beer, T.A.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository—New features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Shiotsuki, T.; Wang, Z.; Xu, X.; Huang, Y.; Li, M.; Li, K.; Tan, A. Depletion of juvenile hormone esterase extends larval growth in Bombyx mori. Insect Biochem. Mol. Biol. 2017, 81, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Masumoto, M.; Yaginuma, T.; Niimi, T. Functional analysis of Ultrabithorax in the silkworm, Bombyx mori, using RNAi. Dev. Genes Evol. 2009, 219, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Tian, Y.; Peng, Y.; Zheng, S. Knock down of target genes by RNA interference in the embryos of lepidopteran insect, Bombyx mori. STAR Protoc. 2022, 3, 101219. [Google Scholar] [CrossRef]
- Tsuchiya, R.; Kaneshima, A.; Kobayashi, M.; Yamazaki, M.; Takasu, Y.; Sezutsu, H.; Tanaka, Y.; Mizoguchi, A.; Shiomi, K. Maternal GABAergic and GnRH/corazonin pathway modulates egg diapause phenotype of the silkworm Bombyx mori. Proc. Natl. Acad. Sci. USA 2021, 118, e2020028118. [Google Scholar] [CrossRef]
- Clerici, M.; Deniaud, A.; Boehm, V.; Gehring, N.H.; Schaffitzel, C.; Cusack, S. Structural and functional analysis of the three MIF4G domains of nonsense-mediated decay factor UPF2. Nucleic Acids Res. 2014, 42, 2673–2686. [Google Scholar] [CrossRef] [PubMed]
- Colón, E.M.; Haddock, L.A., III; Lasalde, C.; Lin, Q.; Ramírez-Lugo, J.S.; González, C.I. Characterization of the mIF4G Domains in the RNA Surveillance Protein Upf2p. Curr. Issues Mol. Biol. 2023, 46, 244–261. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequence (5′–3′) | Primer Purpose |
|---|---|---|
| TrpA1-582-F | GATAACCGATCTGGACGCTGAATG | Mutation site sequencing detection |
| TrpA1-880-R | CTTGAGCGCAAGCTAAGTGCACAG | |
| gRNA-771-793 | TtctaatacgactcactatagCGTTCATGGAGGTGATA TCAgttttagagctaga | Synthesis of sgRNA in vitro |
| Primer Name | Primer Sequence (5′–3′) | Gene Accession No. |
|---|---|---|
| BmPainless-F | CGGTCTTGCGGTTAGTGACA | NM_001309624.1 |
| BmPainless-R | GCTACCGATAAGCACGCTCT | |
| BmPyrexia-F | ATGATGGCCGCTTACGACAT | NM_001309607.1 |
| BmPyrexia-R | TCCGAGTCCTGAGTAACCGT | |
| BmUpf1-F | GCGAGAGGCAATGGAGTCTT | XM_004929604.4 |
| BmUpf1-R | CACCGGCTTCTTCCTTGAGT | |
| BmUpf2-F | CATTGCTGTCCCGATGACCT | XM_038010585.1 |
| BmUpf2-R | AACGAATTCCACGCCCTCTT | |
| BmUpf3a-F | GGATCGGAAGAGACAGACACA | NM_001046855.2 |
| BmUpf3a-R | TTCTTTCGCGAGACGCTGTT | |
| BmActin3-F | CGGCTACTCGTTCACTACC | NM_001126254.1 |
| BmActin3-R | CCGTCGGGAAGTTCGTAAG | |
| BmRp49-F | TCAATCGGATCGCTATGACA | NM_001098282.2 |
| BmRp49-R | ATGACGGGTCTTCTTGTTGG |
| Primer Name | Primer Sequence (5′–3′) |
|---|---|
| dsGFP-F | ACGTAAACGGCCACAAGTTC |
| T7dsGFP-F | TAATACGACTCACTATAGGGACGTAAACGGCCACAAGTTC |
| dsGFP-R | TGTTCTGCTGGTAGTGGTCG |
| T7dsGFP-R | TAATACGACTCACTATAGGGTGTTCTGCTGGTAGTGGTCG |
| dsUpf1-F | CTCGCAATCGCTCACGTTTC |
| T7dsUpf1-F | TAATACGACTCACTATAGGGCTCGCAATCGCTCACGTTTC |
| dsUpf1-R | ACATTCCGAGCACCACATGA |
| T7dsUpf1-R | TAATACGACTCACTATAGGGACATTCCGAGCACCACATGA |
| DsUpf2-F | TCATCAAAACTGCGGGTGGAT |
| T7dsUpf2-F | TAATACGACTCACTATAGGGTCATCAAAACTGCGGGTGGAT |
| DsUpf2-R | TGAGAGTTCTCCTCTGGTGTG |
| T7dsUpf2-R | TAATACGACTCACTATAGGGTGAGAGTTCTCCTCTGGTGTG |
| Group | Whole Cocoon Weight of Females (g) | Whole Cocoon Weight of Males (g) | Cocoon Shell Weight of Females (g) | Cocoon Shell Weight of Males (g) |
|---|---|---|---|---|
| WT (n = 30) | 1.673 ± 0.1385 | 1.300 ± 0.06543 | 0.3189 ± 0.03326 | 0.2973 ± 0.03197 |
| TRPA1−/−(n = 30) | 1.744 ± 0.1559 | 1.479 ± 0.1320 | 0.3014 ± 0.02375 | 0.299 ± 0.02042 |
| p Value | 0.0683 | <0.0001 | 0.0225 | 0.8145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.-Y.; Zhu, J.; Zhang, Y.-Z.; Cui, Q.-Y.; Wang, S.-S.; Ning, Y.-W.; Shen, X.-J. Upf2-Mediated Nonsense-Mediated Degradation Pathway Involved in Genetic Compensation of TrpA1 Knockout Mutant Silkworm (Bombyx mori). Insects 2024, 15, 313. https://doi.org/10.3390/insects15050313
Wang D-Y, Zhu J, Zhang Y-Z, Cui Q-Y, Wang S-S, Ning Y-W, Shen X-J. Upf2-Mediated Nonsense-Mediated Degradation Pathway Involved in Genetic Compensation of TrpA1 Knockout Mutant Silkworm (Bombyx mori). Insects. 2024; 15(5):313. https://doi.org/10.3390/insects15050313
Chicago/Turabian StyleWang, Dong-Yue, Juan Zhu, Yi-Zhong Zhang, Qian-Yi Cui, Shan-Shan Wang, Yang-Wei Ning, and Xing-Jia Shen. 2024. "Upf2-Mediated Nonsense-Mediated Degradation Pathway Involved in Genetic Compensation of TrpA1 Knockout Mutant Silkworm (Bombyx mori)" Insects 15, no. 5: 313. https://doi.org/10.3390/insects15050313
APA StyleWang, D.-Y., Zhu, J., Zhang, Y.-Z., Cui, Q.-Y., Wang, S.-S., Ning, Y.-W., & Shen, X.-J. (2024). Upf2-Mediated Nonsense-Mediated Degradation Pathway Involved in Genetic Compensation of TrpA1 Knockout Mutant Silkworm (Bombyx mori). Insects, 15(5), 313. https://doi.org/10.3390/insects15050313