The Expression and Function of Notch Involved in Ovarian Development and Fecundity in Basilepta melanopus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Sample Collection and RNA Extraction
2.3. Bioinformatics Analyses
2.4. Temporal and Spatial Expression Analysis
2.5. dsRNA Synthesis
2.6. RNA Interference
3. Results
3.1. Bioinformatics Analyses of the Notch Gene in B. melanopus
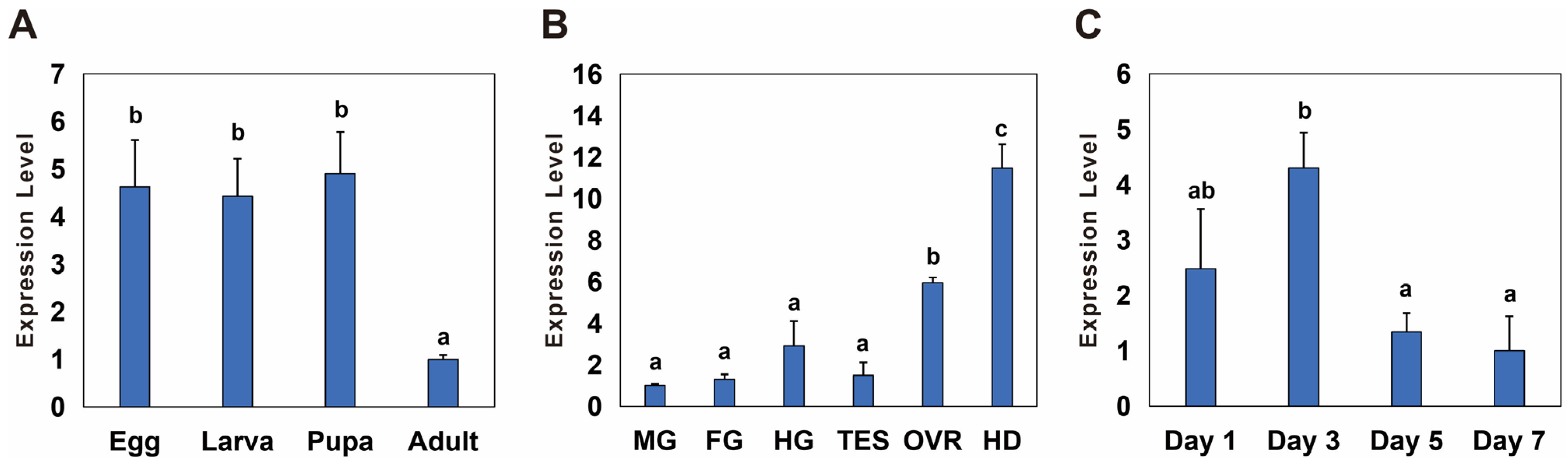
3.2. Temporal and Spatial Expression of Bmnotch
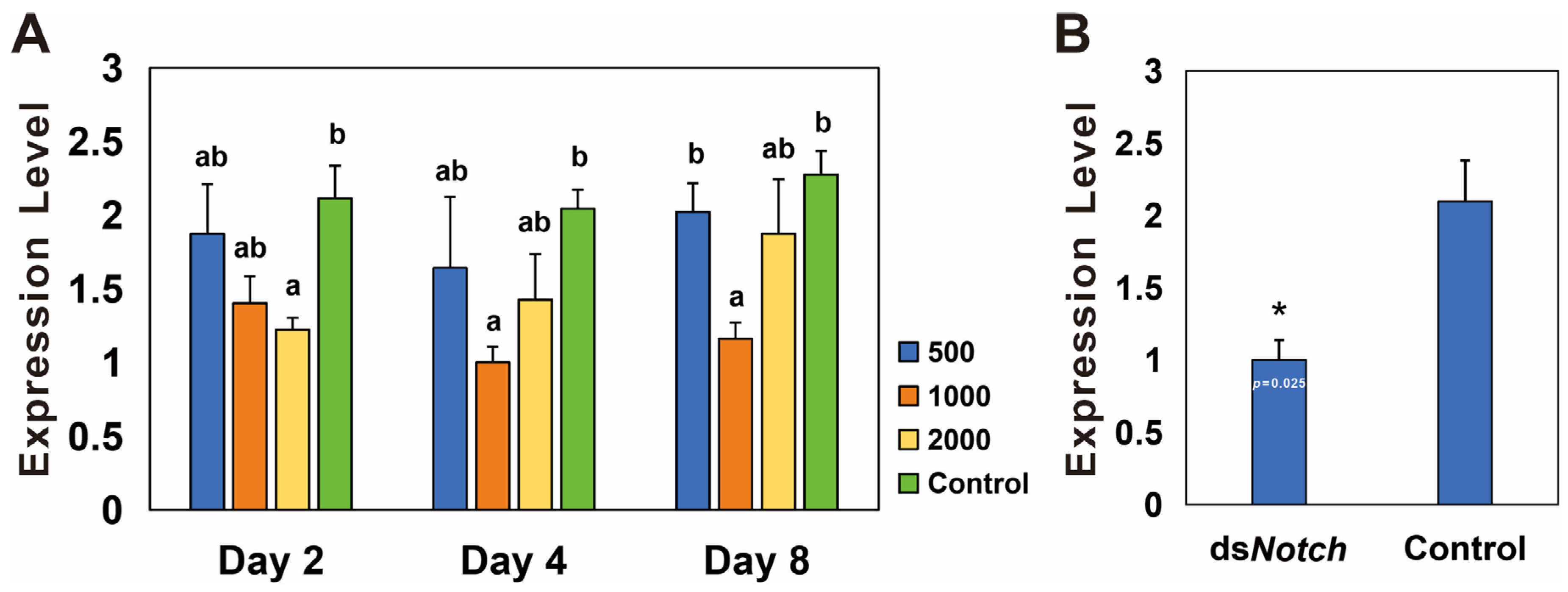
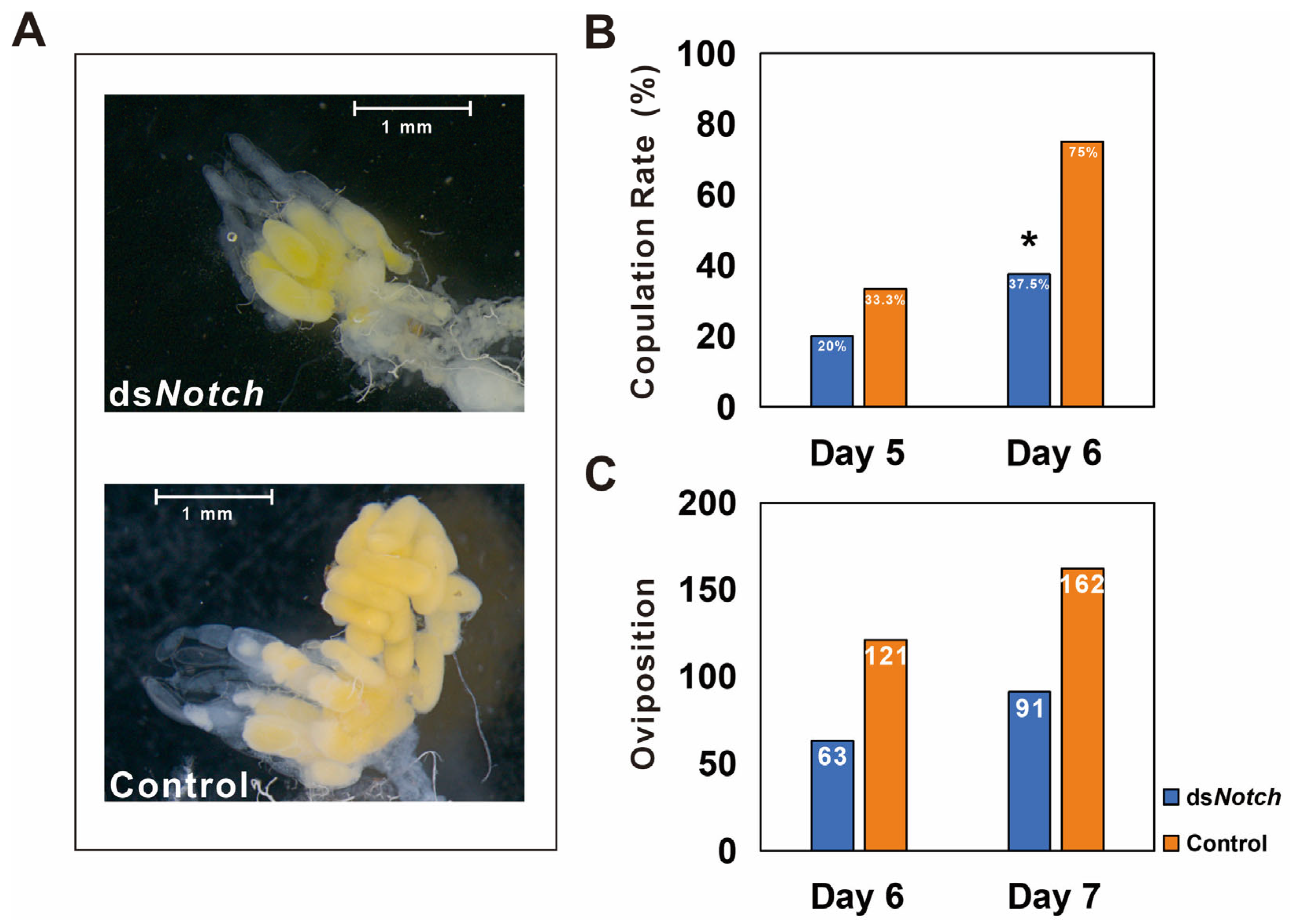
3.3. Silencing of Bmnotch and Its Effect on Ovary Development
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopan, R.; Ilagan, M.X.G. The canonical notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S. Making sense out of missense mutations: Mechanistic dissection of Notch receptors through structure-function studies in Drosophila. Dev. Growth Differ. 2020, 62, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.W.; Posakony, J.W. Lateral inhibition: Two modes of non-autonomous negative autoregulation by neuralized. PLoS Genet. 2018, 14, e1007528. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Kim, W.S.; Mandal, L.; Banerjee, U. Interaction between Notch and hif-α in development and survival of Drosophila blood cells. Science 2011, 332, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Chung, A.Y.; Wu, I.; Foldi, J.; Chen, J.; Ji, J.D.; Tateya, T.; Kang, Y.J.; Han, J.H.; Gessler, M.; et al. Integrated Regulation of Toll-like Receptor Responses by Notch and Interferon-γ Pathways. Immunity 2008, 29, 691–703. [Google Scholar] [CrossRef]
- Sachan, N.; Mishra, A.K.; Mutsuddi, M.; Mukherjee, A. The Drosophila importin-α3 is required for nuclear import of notch in vivo and it displays synergistic effects with Notch receptor on cell proliferation. PLoS ONE 2013, 8, e68247. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Wilkin, M.B.; Woodcock, S.A.; Bonfini, A.; Hung, Y.; Mazaleyrat, S.; Baron, M. The Drosophila ZO-1 protein Polychaetoid suppresses Deltex-regulated Notch activity to modulate germline stem cell niche formation. Open Biol. 2017, 7, 160322. [Google Scholar] [CrossRef]
- Ng, C.L.; Qian, Y.; Schulz, C. Notch and Delta are required for survival of the germline stem cell lineage in testes of Drosophila melanogaster. PLoS ONE 2019, 14, e0222471. [Google Scholar] [CrossRef]
- Bäumer, D.; Ströhlein, N.M.; Schoppmeier, M. Opposing effects of Notch-signaling in maintaining the proliferative state of follicle cells in the telotrophic ovary of the beetle Tribolium. Front. Zool. 2012, 9, 15. [Google Scholar] [CrossRef]
- Chang, C.H.; Liu, Y.T.; Weng, S.C.; Chen, I.Y.; Tsao, P.N.; Shiao, S.H. The non-canonical Notch signaling is essential for the control of fertility in Aedes aegypti. PLoS Negl. Trop. Dis. 2018, 12, e0006307. [Google Scholar] [CrossRef] [PubMed]
- Irles, P.; Elshaer, N.; Piulachs, M.D. The Notch pathway regulates both the proliferation and differentiation of follicular cells in the panoistic ovary of Blattella germanica. Open Biol. 2016, 6, 150197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Li, W.; Liu, H.Y.; Xiang, F.; Kang, Y.K.; Yin, X.; Huang, A.P.; Wang, Y.J. Systemic identification and analyses of genes potentially involved in chemosensory in the devastating tea pest Basilepta melanopus. Comp. Biochem. Physiol. D-Genom. Proteom. 2019, 31, 100586. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wang, S.; Niu, J.Z. RNAi-based pesticides: Current knowledge and potential applications for Integrated Pest Management. Entomol. Gen. 2023, 43, 1–4. [Google Scholar] [CrossRef]
- Mehlhorn, S.; Hunnekuhl, V.S.; Geibel, S.; Nauen, R.; Bucher, G. Establishing RNAi for basic research and pest control and identification of the most efficient target genes for pest control: A brief guide. Front. Zool. 2021, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Mogilicherla, K.; Chakraborty, A.; Taning, C.N.T.; Smagghe, G.; Roy, A. RNAi in termites (Isoptera): Current status and prospects for pest management. Entomol. Gen. 2023, 43, 55–68. [Google Scholar] [CrossRef]
- Li, Y.C.; Xu, H.; He, W.W.; Rong, H.L.; Li, S.C.; Kim, D.S.; Han, P.; Yang, Y.; Zhang, J. Silencing of insect dsRNase genes enhances the plastid-mediated RNAi effect on the Colorado potato beetle. Entomol. Gen. 2023, 43, 69–77. [Google Scholar] [CrossRef]
- Wang, Z.G.; Chen, R.Y.; Jiang, Y.K.; Wang, Z.W.; Wang, J.J.; Niu, J.Z. Investigation of potential non-target effects to a ladybeetle Propylea japonica in the scenario of RNAi-based pea aphid control. Entomol. Gen. 2023, 43, 79–88. [Google Scholar] [CrossRef]
- Chao, Z.J.; Ma, Z.Z.; Zhang, Y.H.; Yan, S.; Shen, J. Establishment of star polycation-based RNA interference system in all developmental stages of fall armyworm Spodoptera frugiperda. Entomol. Gen. 2023, 43, 127–137. [Google Scholar] [CrossRef]
- Heilig, M.; Armbruster, P.A. Efficient RNAi knockdown at 20 °C in Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2024, 61, 508–511. [Google Scholar] [CrossRef]
- Hoang, T.; Foquet, B.; Rana, S.; Little, D.W.; Woller, D.A.; Sword, G.A.; Song, H. Development of RNAi methods for the mormon cricket, Anabrus simplex (Orthoptera: Tettigoniidae). Insects 2022, 13, 739. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kovall, R.A.; Gebelein, B.; Sprinzak, D.; Kopan, R. The canonical notch signaling pathway: Structural and biochemical insights into shape, sugar, and force. Dev. Cell 2017, 41, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.H.; Lin, W.L.; Long, Y.L.; Yang, Y.K.; Zhang, H.; Wu, K.M.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Choi, S.H.; Hu, T.C.; Tiyanont, K.; Habets, R.; Groot, A.J.; Vooijs, M.; Aster, J.C.; Chopra, R.; Fryer, C.; et al. Insights into autoregulation of notch3 from structural and functional studies of its negative regulatory region. Structure 2015, 23, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.H.; Song, H.F.; Abbas, M.; Wang, Y.L.; Li, T.; Ma, E.B.; Cooper, A.M.W.; Silver, K.; Zhu, K.Y.; Zhang, J.Z. A dsRNA-degrading nuclease (dsRNase2) limits RNAi efficiency in the Asian corn borer (Ostrinia furnacalis). Insect Sci. 2021, 28, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Kebede, M.; Fite, T. RNA interference (RNAi) applications to the management of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae): Its current trends and future prospects. Front. Mol. Biosci. 2022, 9, 944774. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, S.; Ulrich, J.; Baden, C.U.; Buer, B.; Maiwald, F.; Lueke, B.; Geibel, S.; Bucher, G.; Nauen, R. The mustard leaf beetle, Phaedon cochleariae, as a screening model for exogenous RNAi-based control of Coleopteran pests. Pestic. Biochem. Physiol. 2021, 176, 104870. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.L.; Cao, J.N.; He, Y.Q.; Yang, S.; Zhang, J. Assessment on effects of transplastomic potato plants expressing Colorado potato beetle β-Actin double-stranded RNAs for three non-target pests. Pestic. Biochem. Physiol. 2021, 178, 104909. [Google Scholar] [CrossRef]
- Vélez, A.M.; Fishilevich, E.; Rangasamy, M.; Khajuria, C.; McCaskill, D.G.; Pereira, A.E.; Gandra, P.; Frey, M.L.F.; Worden, S.E.; Whitlock, S.L.; et al. Control of western corn rootworm via RNAi traits in maize: Lethal and sublethal effects of Sec23 dsRNA. Pest Manag. Sci. 2020, 76, 1500–1512. [Google Scholar] [CrossRef]
- Xu, L.T.; Xu, S.J.; Sun, L.W.; Zhang, Y.Q.; Luo, J.; Bock, R.; Zhang, J. Synergistic action of the gut microbiota in environmental RNA interference in a leaf beetle. Microbiome 2021, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.B.; Xiao, S.; Wang, Y.F.; Hua, H.X. Notch is an alternative splicing gene in brown planthopper, Nilaparvata lugens. Arch. Insect Biochem. Physiol. 2022, 110, e21894. [Google Scholar] [CrossRef] [PubMed]
- Long, G.Y.; Wang, Z.; Chen, N.N.; Zeng, Q.H.; Jin, D.C.; Yang, H.; Zhou, C.; Yang, X.B. Functional analysis of Notch gene in the white-backed planthopper Sogatella furcifera (Hemiptera: Delphacidae). J. Asia-Pac. Entomol. 2023, 26, 102130. [Google Scholar] [CrossRef]
- Tajiri, R.; Hirano, A.; Kaibara, Y.Y.; Tezuka, D.; Chen, Z.Y.; Kojima, T. Notch signaling generates the “cut here line” on the cuticle of the puparium in Drosophila melanogaster. iScience 2023, 26, 107279. [Google Scholar] [CrossRef] [PubMed]
- Baonza, A.; Garcia-Bellido, A. Notch signaling directly controls cell proliferation in the Drosophila wing disc. Proc. Natl. Acad. Sci. USA 2000, 97, 2609–2614. [Google Scholar] [CrossRef] [PubMed]
- Horne-Badovinac, S.; Bilder, D. Mass transit: Epithelial morphogenesis in the Drosophila egg chamber. Dev. Dyn. 2005, 232, 559–574. [Google Scholar] [CrossRef] [PubMed]
- López-Schier, H.; St Johnston, D. Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 2001, 15, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Angelini, D.R.; Kikuchi, M.; Jockusch, E.L. Genetic patterning in the adult capitate antenna of the beetle Tribolium castaneum. Dev. Biol. 2009, 327, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Hrycaj, S.; Mihajlovic, M.; Mahfooz, N.; Couso, J.P.; Popadic, A. RNAi analysis of nubbin embryonic functions in a hemimetabolous insect, Oncopeltus fasciatus. Evol. Dev. 2008, 10, 705–716. [Google Scholar] [CrossRef]
- Ando, T.; Fujiwara, H.; Kojima, T. The pivotal role of aristaless in development and evolution of diverse antennal morphologies in moths and butterflies. BMC Evol. Biol. 2018, 18, 8. [Google Scholar] [CrossRef]
- Mpho, M.; Seabrook, W.D. Functions of antennae and palpi in the mating behaviour of the Colorado potato beetle Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Bull. Entomol. Res. 2003, 93, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Elgar, M.A.; Zhang, D.; Wang, Q.K.; Wittwer, B.; Pham, H.T.; Johnson, T.L.; Freelance, C.B.; Coquilleau, M. Insect Antennal Morphology: The Evolution of Diverse Solutions to Odorant Perception. Yale J. Biol. Med. 2018, 91, 457–469. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Tan, Y.; Wen, X.; Deng, W.; Yu, J.; Li, M.; Meng, F.; Wang, X.; Zhu, D. The Expression and Function of Notch Involved in Ovarian Development and Fecundity in Basilepta melanopus. Insects 2024, 15, 292. https://doi.org/10.3390/insects15040292
Xie Y, Tan Y, Wen X, Deng W, Yu J, Li M, Meng F, Wang X, Zhu D. The Expression and Function of Notch Involved in Ovarian Development and Fecundity in Basilepta melanopus. Insects. 2024; 15(4):292. https://doi.org/10.3390/insects15040292
Chicago/Turabian StyleXie, Yifei, Yifan Tan, Xuanye Wen, Wan Deng, Jinxiu Yu, Mi Li, Fanhui Meng, Xiudan Wang, and Daohong Zhu. 2024. "The Expression and Function of Notch Involved in Ovarian Development and Fecundity in Basilepta melanopus" Insects 15, no. 4: 292. https://doi.org/10.3390/insects15040292
APA StyleXie, Y., Tan, Y., Wen, X., Deng, W., Yu, J., Li, M., Meng, F., Wang, X., & Zhu, D. (2024). The Expression and Function of Notch Involved in Ovarian Development and Fecundity in Basilepta melanopus. Insects, 15(4), 292. https://doi.org/10.3390/insects15040292






