Effects of Various Nectar and Pollen Plants on the Survival, Reproduction, and Predation of Neoseiulus bicaudus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Cultures
2.2. Mite Cultures
2.3. Effect of Different Nectar and Pollen Plants on Adult Longevity and Fecundity of Neoseiulus bicaudus
2.4. Effect of Pollen from Nectar and Pollen Plants on the Predation Ability of Neoseiulus bicaudus
2.5. Statistical Analysis
3. Results
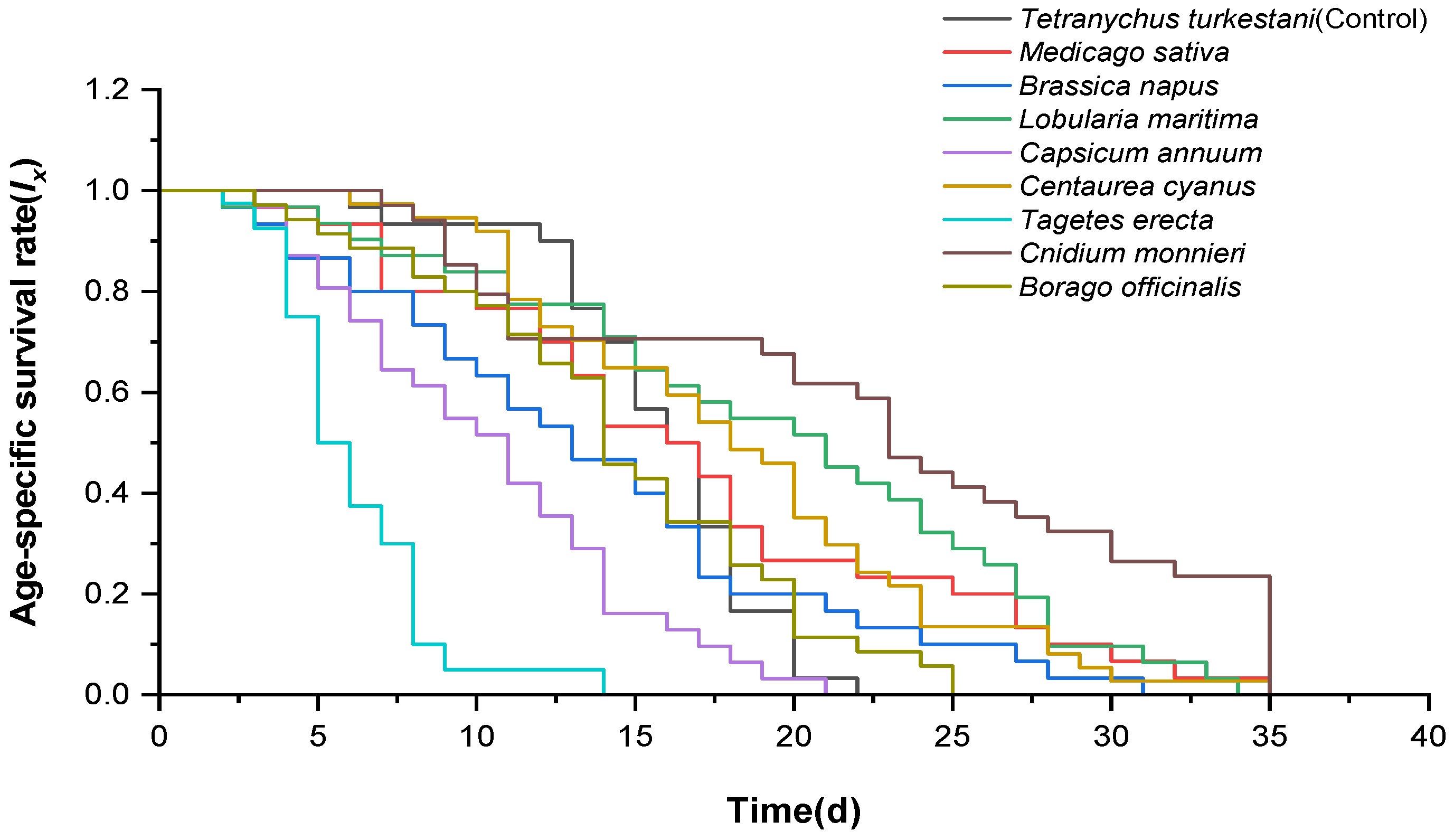
3.1. Effect of Different Nectar and Pollen Plants on Adult Longevity of Neoseiulus bicaudus
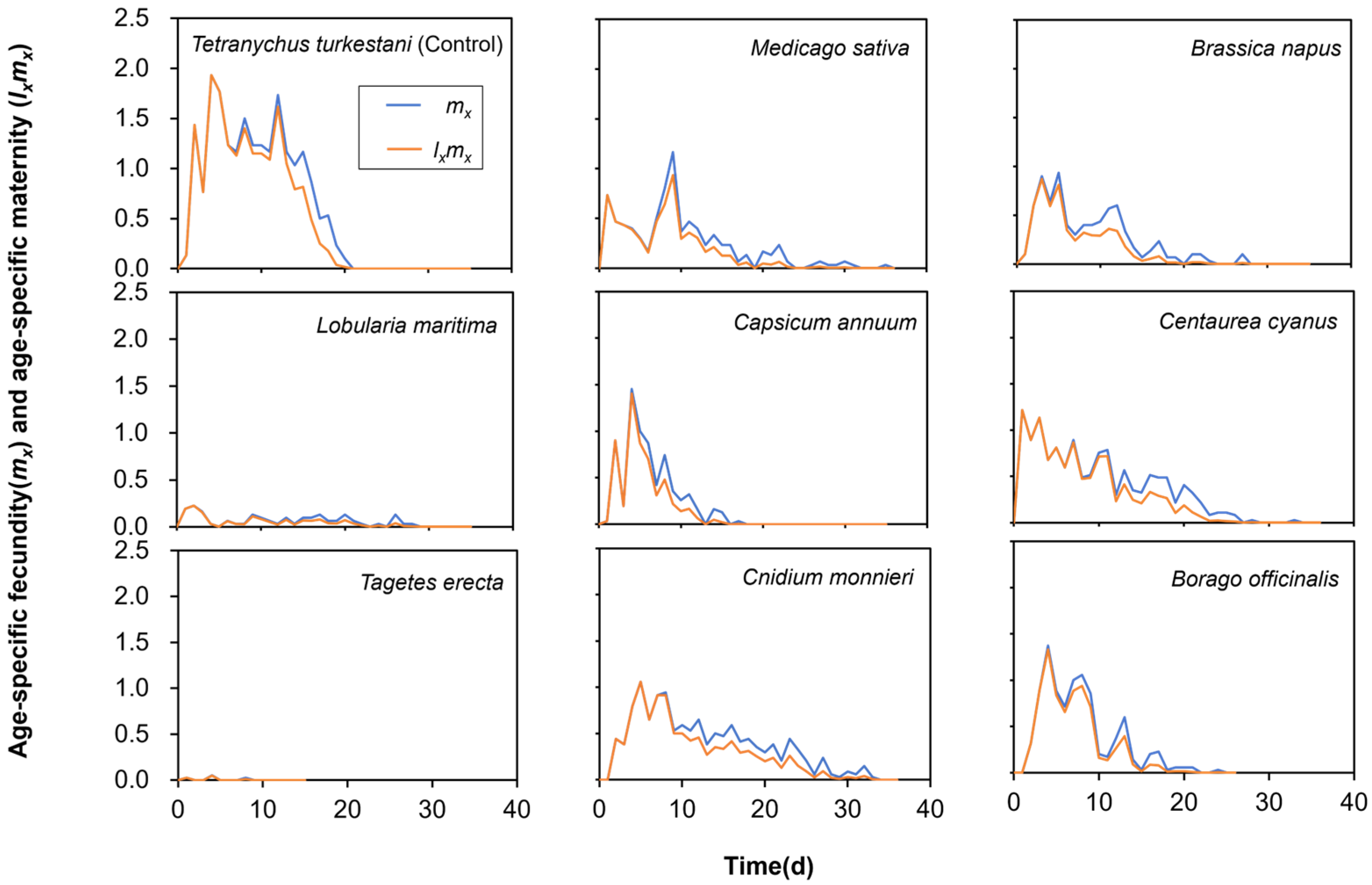
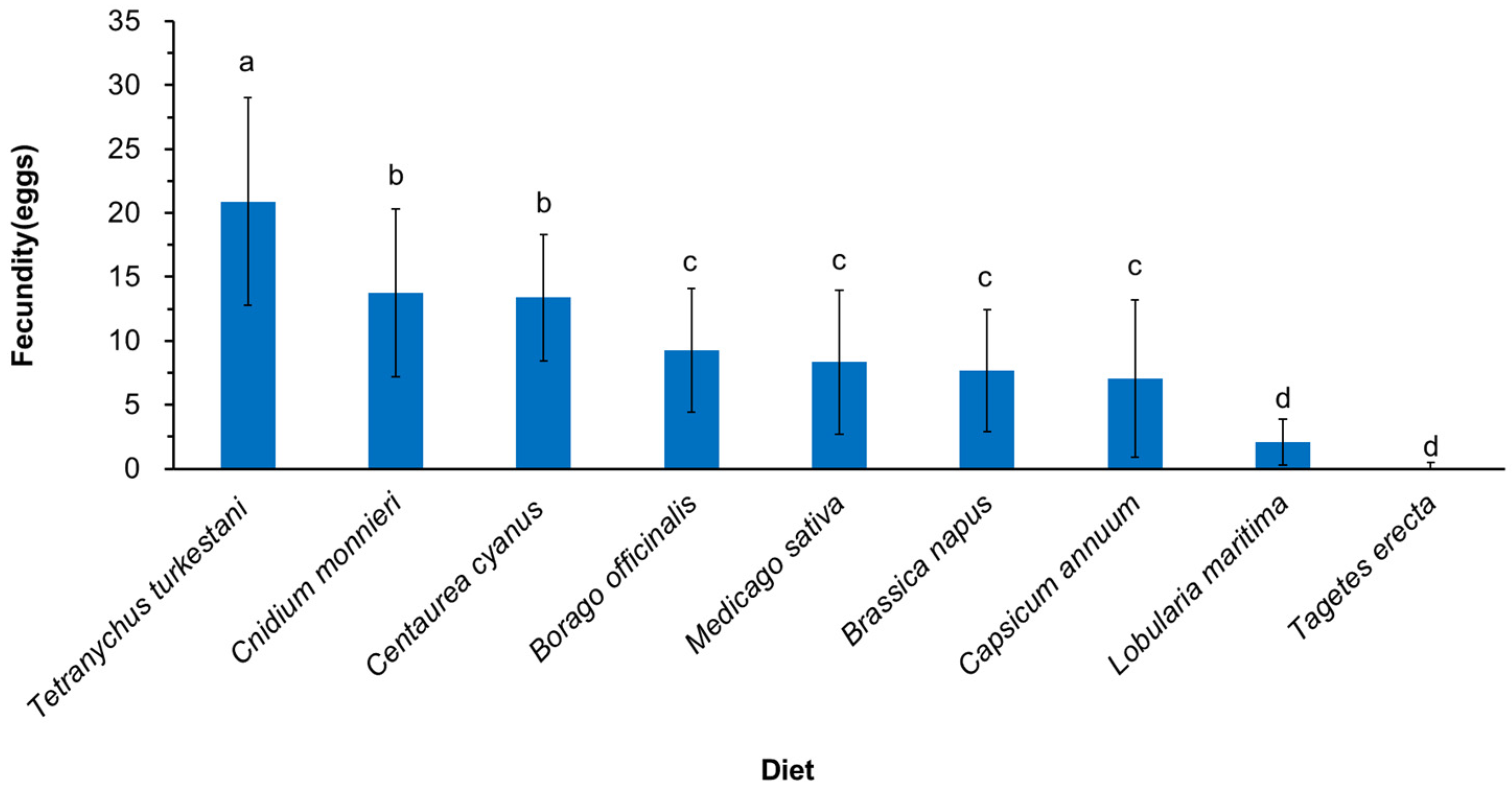
3.2. Effect of Different Nectar and Pollen Plants on Fecundity of Neoseiulus bicaudus
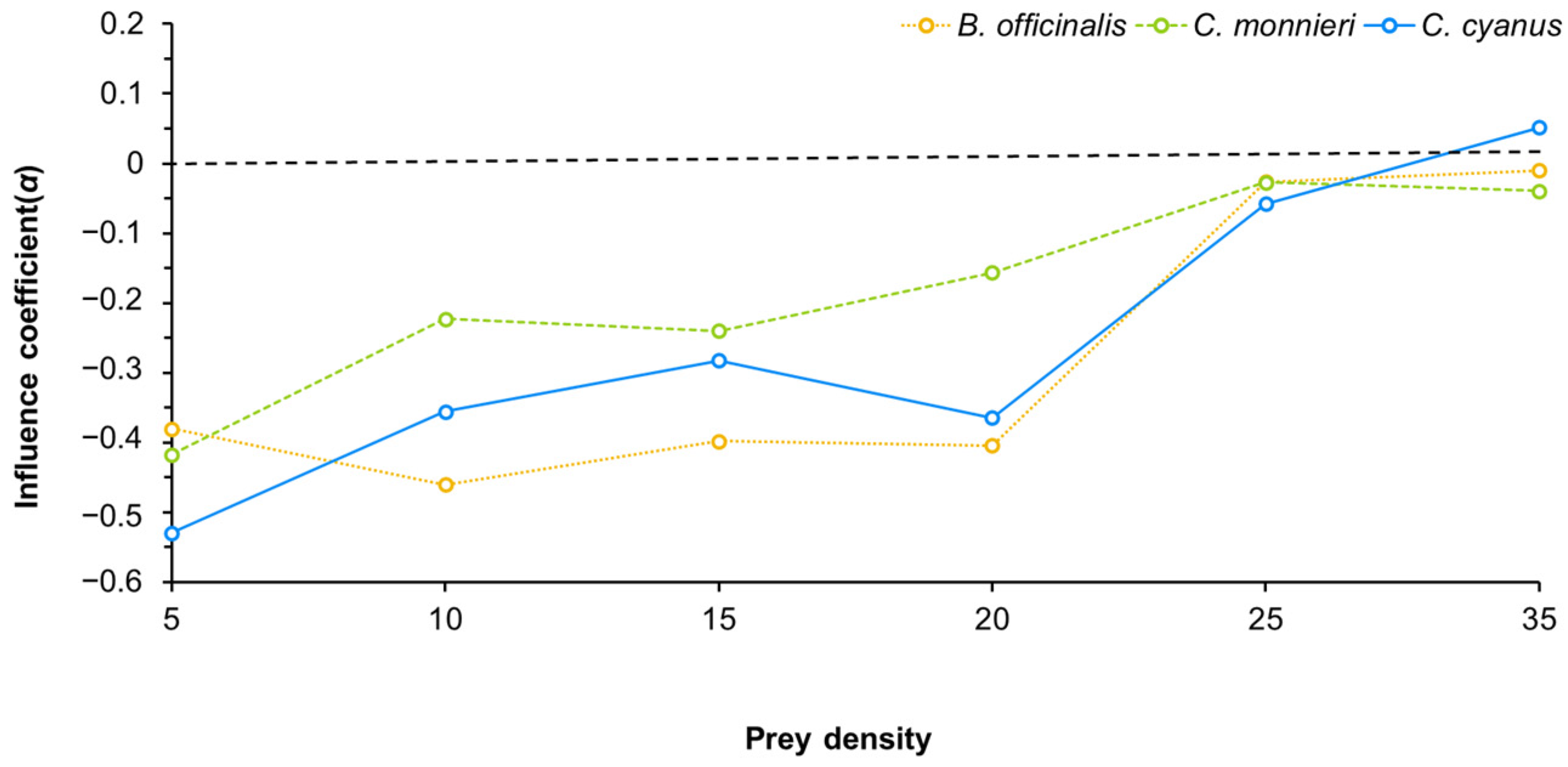
3.3. Effect of Pollen from Nectar and Pollen Plants on the Predation Ability of Neoseiulus bicaudus
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moretti, E.; Jones, C.; Schmidt-Jeffris, R.J.E.; Acarology, A. Alternative food sources for Amblydromella caudiglans (Phytoseiidae) and effects on predation. Exp. Appl. Acarol. 2023, 89, 29–44. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Ramakers, P.J.B.S. Manipulation of phytoseiid thrips predators in the absence of thrips. Bull. SROP 1990, 13, 169–172. [Google Scholar]
- Zhao, J.; Guo, X.; Tan, X.; Desneux, N.; Zappala, L.; Zhang, F.; Wang, S. Using Calendula officinalis as a floral resource to enhance aphid and thrips suppression by the flower bug Orius sauteri (Hemiptera: Anthocoridae). Pest Manag. Sci. 2017, 73, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Shimales, T.; Mendesil, E.; Zewdie, B.; Ayalew, B.; Hylander, K.; Tack, A.J.M. Management intensity affects insect pests and natural pest control on Arabica coffee in its native range. J. Appl. Ecol. 2023, 60, 911–922. [Google Scholar] [CrossRef]
- Zhu, P.; Liang, R.; Qin, Y.; Xu, H.; Zou, Y.; Johnson, A.C.; Zhang, F.; Gurr, G.M.; Lu, Z. Extrafloras and floral nectar promote biocontrol services provided by parasitoid wasps to rice crops. Entomol. Gen. 2023, 43, 971–979. [Google Scholar] [CrossRef]
- Yang, Q.-F.; Ouyang, F.; Men, X.-Y.; Ge, F. Functional plants: Current uses and future research. Chin. J. Appl. Entomol. 2020, 57, 41–48. [Google Scholar]
- Stoner, K.; Nurse, A.; Koethe, R.; Hatala, M.; Lehmann, D. Where Does Honey Bee (Apis mellifera L.) Pollen Come from? A Study of Pollen Collected from Colonies at Ornamental Plant Nurseries. Insects 2022, 13, 744. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, P.C.J.; Tanigoshi, L.K. The contribution of extrafloral nectar to survival and reproduction of the predatory mite Iphiseius degenerans on Ricinus communis. Exp. Appl. Acarol. 1999, 23, 281–296. [Google Scholar] [CrossRef]
- Parolin, P.; Bresch, C.; Poncet, C.; Suay-Cortez, R.; Van Oudenhove, L. Testing basil as banker plant in IPM greenhouse tomato crops. Int. J. Pest Manag. 2015, 61, 235–242. [Google Scholar] [CrossRef]
- Russell, M. A meta-analysis of physiological and behavioral responses of parasitoid wasps to flowers of individual plant species. Biol. Control 2015, 82, 96–103. [Google Scholar] [CrossRef]
- Zhu, P.; Zheng, X.; Xie, G.; Chen, G.; Lu, Z.; Gurr, G. Relevance of the ecological traits of parasitoid wasps and nectariferous plants for conservation biological control: A hybrid meta-analysis. Pest Manag. Sci. 2020, 76, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.L.; Gontijo, L.M. Alyssum flowers promote biological control of collard pests. Biocontrol 2017, 62, 185–196. [Google Scholar] [CrossRef]
- Pinheiro, L.A.; Torres, L.; Raimundo, J.; Santos, S.A.P. Effect of floral resources on longevity and nutrient levels of Episyrphus balteatus (Diptera: Syrphidae). Biol. Control 2013, 67, 178–185. [Google Scholar] [CrossRef]
- van Rijn, P.C.J.; Wackers, F.L. Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. J. Appl. Ecol. 2016, 53, 925–933. [Google Scholar] [CrossRef]
- Schuldiner-Harpaz, T.; Coll, M. Estimating the effect of plant-provided food supplements on pest consumption by omnivorous predators: Lessons from two coccinellid beetles. Pest Manag. Sci. 2017, 73, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Eggs, B.; Sanders, D. Herbivory in spiders: The importance of pollen for orb-weavers. PLoS ONE 2013, 8, e82637. [Google Scholar] [CrossRef]
- Hatt, S.; Osawa, N. The role of Perilla frutescens flowers on fitness traits of the ladybird beetle Harmonia axyridis. Biocontrol 2019, 64, 381–390. [Google Scholar] [CrossRef]
- He, X.; Sigsgaard, L. A Floral Diet Increases the Longevity of the Coccinellid Adalia bipunctata but Does Not Allow Molting or Reproduction. Front. Ecol. Evol. 2019, 7, 6. [Google Scholar] [CrossRef]
- Zhu, P.; Lu, Z.; Heong, K.; Chen, G.; Zheng, X.; Xu, H.; Yang, Y.; Nicol, H.I.; Gurr, G.M. Selection of Nectar Plants for Use in Ecological Engineering to Promote Biological Control of Rice Pests by the Predatory Bug, Cyrtorhinus lividipennis, (Heteroptera: Miridae). PLoS ONE 2014, 9, e108669. [Google Scholar] [CrossRef]
- Ansari-Shiri, H.; Fathipour, Y.; Hajiqanbar, H.; Riahi, E.; Riddick, E.W. Quality control of the predatory mite Amblyseius swirskii during long-term rearing on almond Prunus amygdalus pollen. Arthropod-Plant Interact. 2022, 16, 645–655. [Google Scholar] [CrossRef]
- Li, D.-Y.; Zhou, D.; Zhi, J.-R.; Yue, W.-B.; Li, S.-X. Effects of Different Parts of the Rose Flower on the Development, Fecundity, and Life Parameters of Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Insects 2023, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Avery, P.B.; Kumar, V.; Xiao, Y.; Powell, C.A.; McKenzie, C.L.; Osborne, L.S. Selecting an ornamental pepper banker plant for Amblyseius swirskii in floriculture crops. Arthropod-Plant Interact. 2014, 8, 49–56. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat Management to Suppress Pest Populations: Progress and Prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Xiao, Y.; McKenzie, C.L.; Osborne, L.S. Early establishment of the phytoseiid mite Amblyseius swirskii (Acari: Phytoseiidae) on pepper seedlings in a Predator-in-First approach. Exp. Appl. Acarol. 2015, 65, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Eini, N.; Jafari, S.; Fathipour, Y.; Zalucki, M.P. How pollen grains of 23 plant species affect performance of the predatory mite Neoseiulus californicus. Biocontrol 2022, 67, 173–187. [Google Scholar] [CrossRef]
- Goleva, I.; Zebitz, C.P.W. Suitability of different pollen as alternative food for the predatory mite Amblyseius swirskii (Acari, Phytoseiidae). Exp. Appl. Acarol. 2013, 61, 259–283. [Google Scholar] [CrossRef] [PubMed]
- Riahi, E.; Fathipour, Y.; Talebi, A.A.; Mehrabadi, M. Pollen quality and predator viability: Life table of Typhlodromus bagdasarjani on seven different plant pollens and two-spotted spider mite. Syst. Appl. Acarol. 2016, 21, 1399–1412. [Google Scholar] [CrossRef]
- Asali Fayaz, B.; Khanjani, M.; Uckermann, E. Description of immature stages and re-description of female and male of Neoseiulus bicaudus(Wainstein, 1962) (Acari: Phytoseiidae) from West of Iran. Acta Phytopathol. Entomol. Hung. 2011, 46, 329–338. [Google Scholar] [CrossRef]
- Su, J.; Liu, M.; Fu, Z.-S.; Zhu, A.-D.; Zhang, J.-P. Effects of alternative and natural prey on body size, locomotion and dispersal of Neoseiulus bicaudus (Acari: Phytoseiidae). Syst. Appl. Acarol. 2019, 24, 1579–1591. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Jiang, X.; Zhang, J.; Xu, X. Re-description of Neoseiulus bicaudus (Acari: Phytoseiidae) newly recorded from Xinjiang, China. Syst. Appl. Acarol. 2015, 20, 455–461. [Google Scholar] [CrossRef]
- Su, J.; Dong, F.; Liu, S.-M.; Lu, Y.-H.; Zhang, J.-P. Productivity of Neoseiulus bicaudus (Acari: Phytoseiidae) Reared on Natural Prey, Alternative Prey, and Artificial Diet. J. Econ. Entomol. 2019, 112, 2604–2613. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xia, L.; Wu, Y. Life Histories and Functional Responses of Two Predatory Mites Feeding on the Stored-Grain Pest Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae). Insects 2023, 14, 478. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Guo, D.-D.; Jiang, J.-Y.-Q.; Zhang, Y.-J.; Zhang, J.-P. Effects of host plant species on the development and reproduction of Neoseiulus bicaudus (Phytoseiidae) feeding on Tetranychus turkestani (Tetranychidae). Syst. Appl. Acarol. 2016, 21, 647–656. [Google Scholar] [CrossRef]
- Dong, F.; Fu, Z.-S.; Wang, J.-Y.; Su, J.; Chen, J.; Lu, Y.-H.; Zhang, J.-P. Evaluation of Control Effect of Releasing Neoseiulus bicaudus Wainstein on the Tetranychus turkestani(Ugarov et Nikolskii). Xinjiang Agric. Sci. 2019, 56, 1–12. [Google Scholar]
- Pan, M.-Z.; Liu, T.-X. Banker-plant system for biological control of pests in greenhouse-grown crops. Chin. J. Appl. Entomol. 2019, 56, 917–926. [Google Scholar]
- Ding, R.-F.; Wang, X.-L.; Xu, Y.; Li, H.-B.; Wang, F.; Wang, D.; Sun, S.-L. The Effect of Honey Plants on Arthropod Community in Apricot and Wheat Intercropping Orchard. Chin. J. Appl. Entomol. 2008, 45, 960–963. [Google Scholar]
- Zhang, X.; Ouyang, F.; Su, J.; Li, Z.; Yuan, Y.; Sun, Y.; Sarkar, S.C.; Xiao, Y.; Ge, F. Intercropping flowering plants facilitate conservation, movement and biocontrol performance of predators in insecticide-free apple orchard. Agric. Ecosyst. Environ. 2022, 340, 108–157. [Google Scholar] [CrossRef]
- Guillemin, J.-P.; Alrustom, B.; Darmency, H. Estimated effects of cornflower presence on winter wheat. Biol. Agric. Hortic. 2022, 38, 113–123. [Google Scholar] [CrossRef]
- Thom, M.D.; Eberle, C.A.; Forcella, F.; Gesch, R.; Weyers, S. Specialty oilseed crops provide an abundant source of pollen for pollinators and beneficial insects. J. Appl. Entomol. 2018, 142, 211–222. [Google Scholar] [CrossRef]
- Hoarau, C.; Campbell, H.; Prince, G.; Chandler, D.; Pope, T. Biological control agents against the cabbage stem flea beetle in oilseed rape crops. Biol. Control 2022, 167, 104–844. [Google Scholar] [CrossRef]
- Tiwari, S.; Sharma, S.; Wratten, S.D. Flowering alyssum (Lobularia maritima) promote arthropod diversity and biological control of Myzus persicae. J. Asia-Pac. Entomol. 2020, 23, 634–640. [Google Scholar] [CrossRef]
- Barloggio, G.; Tamm, L.; Nagel, P.; Luka, H. Selective flowers to attract and enhance Telenomus laeviceps (Hymenoptera: Scelionidae): A released biocontrol agent of Mamestra brassicae (Lepidoptera: Noctuidae). Bull. Entomol. Res. 2019, 109, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; McKenzie, C.L.; Avery, P.B.; Osborne, L.S. Suitability of Ornamental Pepper Cultivars as Banker Plants for the Establishment of Predatory Mite Amblyseius swirskii in Controlled Production. Sustainability 2020, 12, 8031. [Google Scholar] [CrossRef]
- Pemberton, R.W. Plant Resource Use and Pattern of Usage by the Naturalized Orchid Bee (Euglossa dilemma: Hymenoptera: Apidae) in Florida. Insects 2023, 14, 909. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tang, S.; Fang, C.; Mu, K.; Su, J.; Zhang, J. Presence of nontarget prey, Tetranychus truncatus, affected the predation by Neoseiulus bicaudus on Tetranychus turkestani. J. Econ. Entomol. 2023, 116, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Ning, S.; Zhang, W.; Sun, Y.; Feng, J. Development of insect life tables: Comparison of two demographic methods of Delia antiqua (Diptera: Anthomyiidae) on different hosts. Sci. Rep. 2017, 7, 4821. [Google Scholar] [CrossRef]
- Pritchard, D.W.; Paterson, R.A.; Bovy, H.C.; Barrios-O’Neill, D. FRAIR: An R package for fitting and comparing consumer functional responses. Methods Ecol. Evol. 2017, 8, 1528–1534. [Google Scholar] [CrossRef]
- Rogers, D. Random search and insect population models. J. Anim. Ecol. 1972, 66, 369–383. [Google Scholar] [CrossRef]
- Longstaff, B.C. The functional response of a predatory mite and the nature of the attack rate. Aust. J. Ecol. 1981, 151–158. [Google Scholar] [CrossRef]
- Yan, Y.-J.; Wu, Z.-F. Predation on Nilaparvata lugens by Erigonidium graminicolum and their simulation models. J. Fujian Agric. Coll. 1989, 3, 289–294. [Google Scholar]
- Manly, B.J.B. A model for certain types of selection experiments. Biometrics 1974, 30, 281–294. [Google Scholar] [CrossRef]
- Yang, Q.-F.; Ouyang, F.; Men, X.-Y.; Ge, F. Discovery and utilization of a functional plant, rich in the natural enemies of insect pests, in northern China. Chin. J. Appl. Entomol. 2018, 55, 942–947. [Google Scholar]
- Pumarino, L.; Alomar, O. The role of omnivory in the conservation of predators: Orius majusculus (Heteroptera: Anthocoridae) on sweet alyssum. Biol. Control 2012, 62, 24–28. [Google Scholar] [CrossRef]
- Ebrahimifar, J.; Shishehbor, P.; Rasekh, A.; Riddick, E.W. Effect of factitious diets on development and reproduction of the ladybird beetle Stethorus gilvifrons, a predator of tetranychid mites. Biocontrol 2020, 65, 703–711. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; He, L.; Wu, K. Population Fitness of Eupeodes corollae Fabricius (Diptera: Syrphidae) Feeding on Different Species of Aphids. Insects 2022, 13, 494. [Google Scholar] [CrossRef]
- Sigsgaard, L.; Betzer, C.; Naulin, C.; Eilenberg, J.; Enkegaard, A.; Kristensen, K. The effect of floral resources on parasitoid and host longevity: Prospects for conservation biological control in strawberries. J. Insect Sci. 2013, 13, 104. [Google Scholar] [CrossRef]
- Jakubowska, M.; Dobosz, R.; Zawada, D.; Kowalska, J. A review of crop protection methods against the twospotted spider mite—Tetranychus urticae Koch (Acari: Tetranychidae)—With special reference to alternative methods. Agriculture 2022, 12, 898. [Google Scholar] [CrossRef]
- Schoeller, E.N.; McKenzie, C.L.; Osborne, L.S. Chilli thrips rose management using an Amblyseius swirskii or Amblydromalus limonicus (Acari: Phytoseiidae) pepper banker plant. J. Appl. Entomol. 2022, 146, 1281–1292. [Google Scholar] [CrossRef]
- De Clercq, P. Plants in the rearing of arthropod predators and parasitoids: Benefits, constraints, and alternatives. Curr. Opin. Insect Sci. 2023, 61, 101139. [Google Scholar] [CrossRef]
- Holling, C.S. Some characteristics of simple types of predation and parasitism1. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Thao, N.T.P.; Thuy, N.T.; Quyen, H.L. Effects of Different Diets on Biological Characteristics of Predatory Mite Amblyseius Eharai (Acari: Phytoseiidae). Insects 2023, 14, 519. [Google Scholar] [CrossRef]
- Wu, P.; He, J.; Dong, H.; Zhang, R. Functional Response and Intraspecific Competition of Three Ladybird Species Feeding on Aphids on Goji Berry Plants in Laboratory and Semi-Field Conditions. Insects 2023, 14, 853. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, J.; Tian, Y.; Chen, G.; Zhang, X. Effect of Short-Term High-Temperature Stimuli on the Functional Response of Trichopria drosophilae (Matsumura). Insects 2023, 14, 748. [Google Scholar] [CrossRef] [PubMed]
- Samaras, K.; Pappas, M.L.; Pekas, A.; Waeckers, F.; Broufas, G.D. Benefits of a balanced diet? Mixing prey with pollen is advantageous for the phytoseiid predator Amblydromalus limonicus. Biol. Control 2021, 155, 104531. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, R.; Zhang, Q.; Ji, D.; Zhou, X.; Jin, L. Functional Response and Control Potential of Orius sauteri (Hemiptera: Anthocoridae) on Tea Thrips (Dendrothrips minowai Priesner). Insects 2021, 12, 1132. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M. Comparative life history characteristics of the mite predator Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) on mite and pollen diets. Int. J. Pest Manag. 2016, 62, 140–148. [Google Scholar] [CrossRef]


| Oviposition | Adult Longevity | ||||||
|---|---|---|---|---|---|---|---|
| Sources | df | F | p | df | F | p | |
| Nectar and pollen plants | Family | 5 | 18.048 | <0.001 | 5 | 8.500 | <0.001 |
| Species | 7 | 35.475 | <0.001 | 7 | 19.727 | <0.001 | |
| Treatment | Prey Density (Individuals/Chamber) | ||||||
|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 25 | 35 | 40 | |
| T. turkestani (Control) | 3.9 ± 1.04 Ca | 5.9 ± 1.58 BCa | 7.2 ± 1.99 ABa | 8.3 ± 1.68 ABa | 8.3 ± 2.10 ABa | 8.5 ± 2.06 Aa | - |
| T. turkestani + B. officinalis | 2.4 ± 1.50 Cb | 3.2 ± 1.47 BCc | 4.3 ± 2.29 BCb | 4.9 ± 1.08 Bb | 8.1 ± 3.35 Aa | 8.4 ± 2.25 Aa | - |
| T. turkestani + C. cyanus | 1.8 ± 1.57 Cb | 3.8 ± 0.60 BCbc | 5.2 ± 1.07 Bb | 5.3 ± 1.81 Bb | 7.8 ± 2.41 Aa | 8.9 ± 2.51 Aa | 8.6 ± 2.09 A |
| T. turkestani + C. monnieri | 2.3 ± 0.96 Db | 4.6 ± 1.50 Cb | 5.5 ± 1.50 BCb | 7.0 ± 1.86 ABa | 8.1 ± 2.40 Aa | 8.2 ± 2.11 Aa | - |
| Treatment | Maximum Likelihood Estimate | Attack Rate | Handing Time | Predation Efficacy | Maximum Consumtion | ||
|---|---|---|---|---|---|---|---|
| P1 ± SE | z | p | a ± SE | Th ± SE | (a/Th) | (1/Th) | |
| T. turkestani (Control) | −0.0637 ± 0.0072 | −8.901 | <0.001 | 2.41 ± 0.53 a | 0.10 ± 0.01 a | 24.00 | 9.90 |
| T. turkestani + B. officinalis | −0.0184 ± 0.0066 | −2.795 | <0.001 | 0.51 ± 0.09 b | 0.06 ± 0.02 b | 9.18 | 17.86 |
| T. turkestani + C. cyanus | −0.0222 ± 0.0046 | −4.786 | <0.001 | 0.58 ± 0.09 b | 0.06 ± 0.018 b | 9.44 | 16.39 |
| T. turkestani + C. monnieri | −0.0351 ± 0.0063 | −5.527 | <0.001 | 0.84 ± 0.14 b | 0.08 ± 0.018 a | 11.08 | 13.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Lipeizhong, W.; Liang, X.; Cai, Z.; Liu, W.; Dou, J.; Lu, Y.; Zhang, J.; Wang, S.; Su, J. Effects of Various Nectar and Pollen Plants on the Survival, Reproduction, and Predation of Neoseiulus bicaudus. Insects 2024, 15, 190. https://doi.org/10.3390/insects15030190
Han Y, Lipeizhong W, Liang X, Cai Z, Liu W, Dou J, Lu Y, Zhang J, Wang S, Su J. Effects of Various Nectar and Pollen Plants on the Survival, Reproduction, and Predation of Neoseiulus bicaudus. Insects. 2024; 15(3):190. https://doi.org/10.3390/insects15030190
Chicago/Turabian StyleHan, Yue, Wurigemu Lipeizhong, Xinqi Liang, Zhiping Cai, Weiru Liu, Jifei Dou, Yanhui Lu, Jianping Zhang, Shaoshan Wang, and Jie Su. 2024. "Effects of Various Nectar and Pollen Plants on the Survival, Reproduction, and Predation of Neoseiulus bicaudus" Insects 15, no. 3: 190. https://doi.org/10.3390/insects15030190
APA StyleHan, Y., Lipeizhong, W., Liang, X., Cai, Z., Liu, W., Dou, J., Lu, Y., Zhang, J., Wang, S., & Su, J. (2024). Effects of Various Nectar and Pollen Plants on the Survival, Reproduction, and Predation of Neoseiulus bicaudus. Insects, 15(3), 190. https://doi.org/10.3390/insects15030190







