Simple Summary
The Oriental fruit fly, Bactrocera dorsalis (Hendel), significantly impacts agriculture in Southeast Asia and some Pacific islands as a major pest. Its widespread distribution, robust invasive ability, and detrimental effects on market access categorize it as a significant threat to numerous countries. Irradiation treatment is recognized as an effective and promising approach, considered as a potential alternative to the traditional phytosanitary treatment method, methyl bromide fumigation. However, the low-oxygen environment can influence the efficacy of irradiation treatment. The aim of this study is to investigate the impact of low-oxygen levels on the phytosanitary irradiation effects against larvae of B. dorsalis. The effects of normoxic (21% O2), hypoxic (5% O2), and super-hypoxic (0.5% O2) conditions on the development and metabolic profile of third-instar larvae of B. dorsalis were evaluated and compared at phytosanitary irradiation dose. Our research emphasizes the importance of lipid metabolism pathways and their associated metabolites in the irradiation tolerance of insects; moreover, neither hypoxic nor super-hypoxic conditions have increased the emergence rate of the evaluated fruit fly species under the current phytosanitary irradiation dose. These findings provide new insights into the mechanisms of radioprotection in insects under low-oxygen environments and advocate for international organizations and regulatory agencies to update guidelines on the application of phytosanitary irradiation under hypoxic conditions.
Abstract
X-ray irradiation and modified atmospheres (MAs) provide eco-friendly, chemical-free methods for pest management. Although a low-oxygen atmospheric treatment improves the performance of some irradiated insects, its influence on the irradiation of quarantine insects and its impacts on pest control efficacy have yet to be investigated. Based on bioassay results, this study employed direct immersion solid-phase microextraction (DI-SPME) combined with gas chromatography-mass spectrometry (GC-MS) to determine metabolic profiles of late third-instar B. dorsalis larvae under normoxia (CON, Air), hypoxia (95% N2 + 5% O2, HY), super-hypoxia (99.5% N2 + 0.5% O2, Sup-HY), irradiation-alone (116 Gy, IR-alone), hypoxia + irradiation (HY + IR) and super-hypoxia + irradiation (Sup-HY + IR). Our findings reveal that, compared to the IR-alone group, the IR treatment under HY and Sup-HY (HY + IR and Sup-HY + IR) increases the larval pupation of B. dorsalis, and weakens the delaying effect of IR on the larval developmental stage. However, these 3 groups further hinder adult emergence under the phytosanitary IR dose of 116 Gy. Moreover, all IR-treated groups, including IR-alone, HY + IR, and Sup-HY + IR, lead to insect death as a coarctate larvae or pupae. Pathway analysis identified changed metabolic pathways across treatment groups. Specifically, changes in lipid metabolism-related pathways were observed: 3 in HY vs. CON, 2 in Sup-HY vs. CON, and 5 each in IR-alone vs. CON, HY + IR vs. CON, and Sup-HY + IR vs. CON. The treatments of IR-alone, HY + IR, and Sup-HY + IR induce comparable modifications in metabolic pathways. However, in the HY + IR, and Sup-HY + IR groups, the third-instar larvae of B. dorsalis demonstrate significantly fewer changes. Our research suggests that a low-oxygen environment (HY and Sup-HY) might enhance the radiation tolerance in B. dorsalis larvae by stabilizing lipid metabolism pathways at biologically feasible levels. Additionally, our findings indicate that the current phytosanitary IR dose contributes to the effective management of B. dorsalis, without being influenced by radioprotective effects. These results hold significant importance for understanding the biological effects of radiation on B. dorsalis and for developing IR-specific regulatory guidelines under MA environments.
1. Introduction
The oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae), is a highly invasive species. It was first reported in East India in 1794 and has since widely been distributed throughout the Asia-Pacific region and much of sub-Saharan Africa. B. dorsalis is the polyphagous fruit fly with the broadest host range in the genus Bactrocera, which poses a severe threat to fruit and vegetable production [1,2]. This has led many nations and regions to list it as a quarantine pest. Both the adult and larval stages of B. dorsalis can inflict damage on fruits and vegetables, resulting in significant direct losses in agricultural yield and trade. The serious quarantine restrictions on potentially infested commodities can also lead to indirect economic losses [3]. Consequently, commodities susceptible to infestation must undergo phytosanitary treatments for disinfestation before export to regulated or quarantine areas.
Irradiation, including γ-rays, X-rays, and e-Beam, is an emerging phytosanitary treatment technique that has been effectively employed for the disinfection of fresh commodities, thereby fostering the growth of international agricultural trade [4]. Currently, it stands as the only method with a universally accepted dose to sterilize fruit flies on any host, as documented by the United States Department of Agriculture’s Animal and Plant Health Inspection Service (USDA-APHIS), with effective radiation doses ranging between 50 to 400 Gy [5,6]. Modified atmosphere packaging (MAP) is a well-proven technology for extending the shelf life of fresh commodities and preserving natural quality [7]. Therefore, the combination of MA/MAP with IR treatment is highly appealing to growers. However, this strategy poses a challenge for regulatory authorities because it is known to increase the radiotolerance of insects in a hypoxic environment [8]. Follett et al. have reported that the survival rate of the third-instar melon fly, Bactrocera cucurbitae (Cocquillett), subjected to IR treatment increases under MA conditions with oxygen levels of 1-3% [9]. Furthermore, it has been confirmed that various insects, including the cabbage looper, Trichoplusia ni (Hubner), the Caribbean fruit fly, Anastrepha Suspensa (Loew), and B. dorsalis, demonstrate an increased radiotolerance in hypoxic environments [10]. As a result, both the USDA-APHIS and International Plant Protection Convention (IPPC) regulations have imposed restrictions on the application of phytosanitary IR for fruits and vegetables under MAs.
Given the established fact that hypoxic environments enhance the radiotolerance of insects and other organisms, regulatory authorities’ concerns about using MA with phytosanitary IR without definitive evidence of its effectiveness in such conditions are understandable [11]. However, this does not automatically translate to the IR treatment being ineffective under hypoxic conditions. The primary objectives of phytosanitary IR are to prevent the development and reproduction, rather than to achieve the near-immediate mortality, of insects after treatment [12]. Viewed in this light, none of the studies mentioned above indicate the treatment’s failure under hypoxic conditions. Even if certain studies show a higher survival rate of insects within 24 h after IR in hypoxic conditions, the treatment is still considered successful if it prevents the emergence of adults or results in the sterility of the adult or F1 generation. Currently, the IPPC lacks specific quarantine guidelines for commodities in an MA subjected to phytosanitary IR. Therefore, until the efficacy of the currently recommended phytosanitary IR dose is verified under hypoxic conditions, it is wise not to adjust the IR dose in order to maintain the sensory quality of fresh commodities.
Furthermore, the mechanism of radioprotective effects on insects remains a “black box.” Generally, modern direct immersion microextraction sample preparation techniques have helped to make the analyte targets ready for sensitive treatment while subsequently revealing genuine information about molecular content [13,14]. Using the DI-SPME-GC-MS technique, this study analyzed the metabolome of third-instar B. dorsalis larvae exposed to IR in MA environments to gain molecular insights into the combined effects of HY or Sup-HY and IR. SPME is a recognized method for quick sample processing, especially in analytical biology, pharmaceuticals, and food research. When integrated with GC-MS, SPME significantly elevates the purity, consistency, and sensitivity of samples, surpassing conventional detection methodologies [15,16]. For instance, Liu et al., employed HS-SPME-GC-MS to analyze the metabolic changes in B. dorsalis larvae under different IR doses, and preliminarily provided eight potentially differential metabolites [17]. Alnajim et al. applied DI-SPME-GC-MS to extract and analyze hydrocarbons in the cuticle of both phosphine-resistant and sensitive strains of Tribolium castaneum (Herbst) and Rhyzopertha dominica (Fabricius), finding that the substantial presence of hydrocarbons could play a significant role in hindering the entry of phosphine into the bodies of resistant insects [18]. These studies suggest that SPME-GC-MS contributes to understanding potential radiobiological mechanisms and holds promise in the field of radiation detection.
2. Materials and Methods
2.1. Insect Rearing
The insects used in this study were collected from a mango orchard in the Guangxi Zhuang Autonomous Region, China, and reared in the laboratory of the Chinese Academy of Inspection and Quarantine (CAIQ). Third-instar larvae were transferred from mango fruits to a humid sterile sand for pupation, and the pupae were placed in cages (40 × 40 × 50 cm). Adult flies were fed a solid mixture of orange slices with sucrose and hydrolyzed yeast (3:1). Eggs were collected from adult females 2 weeks after emergence by allowing them to oviposit through mesh cage onto distilled water to maintain a temperature of 26 ± 1 °C. Eggs were collected in a 20-30 mL suspension containing eggs produced in 8–12 h. Larvae were reared on an artificial diet described by Vargas et al. (1984) under controlled conditions of 25 ± 2 °C, 70 ± 5% relative humidity, and a 12:12 (D:L) h photoperiod [19,20]. The emerged third-instar larvae were selected from the third to sixth generations and were utilized for all IR treatments.
2.2. Modified Atmosphere Treatments
Prior to IR treatment, MA treatments of B. dorsalis larvae were conducted using 3 L gas-tight air bags (Dalian Delin Gas Packaging Co., Ltd., Dalian, China). Each treatment involved collecting approximately 200 late third-instar larvae into a perforated plastic box. The larvae were then placed in a gas-tight bag, which was sealed through its opening. Subsequently, the exhaust valve was opened, and the bags were flushed with certified gas mixtures (95% N2 + 5% O2 and 99.5% N2 + 0.5% O2; provided by Beijing Green Oxygen Tiangang Technology Development Co., Ltd., Beijing, China) for 3 min. This procedure was repeated 3 times to purify the gas within the bags. Following this initial preparation, the gas-tight air bags containing the larvae were placed inside an incubator for approximately 12.4 h for the MA environments. For the HY + IR and Sup-HY + IR groups, gas-tight air bags containing the larvae and 1.5–1.6 L of MA were removed from the incubator after 12 h and then subjected to IR treatment. After the IR treatment, the gas-tight air bags for all groups were opened simultaneously to ensure consistent sealing time. In both the control group and the IR-alone treatment group, the larvae were placed in plastic boxes and enclosed in the gas-tight bags without requiring any additional gas flushing procedures.
2.3. Irradiation Treatments
X-ray IR treatment was conducted in the CAIQ Irradiation Processing Laboratory using the RS-2000 ProX irradiator (Rad Source-Technologies, Inc., Coral Springs, FL, USA) at room temperature. Operating parameters were established at 220 kV and 17.6 mA. Larvae, enclosed in a gas-tight air bag, were positioned in an exposure chamber with dimensions of 17 inches in width, 15 inches in depth, and 17 inches in height. A RadCal dosimeter (model 2086, RadCal Corp., Monrovia, CA, USA) equipped with a 10 × 6−0.6 ion chamber was positioned near the test insects. Following each irradiation treatment, the dosimeter recorded the cumulative dose. The irradiator stopped operation when the cumulative dose reached the minimum dose amount (116 Gy) [17,21]. The dose rate observed in this study was approximately 5.0 Gy/min, with the IR duration lasting about 23.2 min.
2.4. Develpomental Test
Following the treatments, 160 late third-instar B. dorsalis larvae were randomly sampled from each group, including the control and all treatment groups. Each larva was individually placed in a plastic box (5 cm diameter, 3 cm height) with sterile moist sand inside an environmental control chamber (KBF 720; WTC Binder, Tuttlingen, Germany). Rearing conditions were set at 25 °C, 60% RH, and a 12:12 h (L:D) photoperiod. The developmental progress of each individual was observed and recorded daily at 9:00 a.m. over a 2-week period. Figure 1 illustrates the processes adopted to achieve the aforementioned objectives [22].
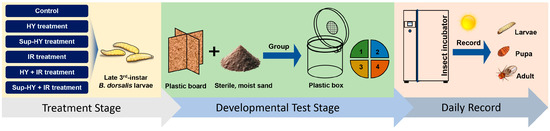
Figure 1.
Schematic diagram of the technical process for the development test of the late third-instar B. dorsalis (Diptera: Tephritidae) larvae under different treatment conditions (Control, HY, Sup-HY, IR, HY + IR, and Sup-HY + IR).
The age-stage-specific proportions represent the probability of third-instar B. dorsalis larvae growing to a specific stage and were calculated as follows.
where N is the number of individuals used at the beginning of the developmental study, and Nxj is the number of individuals growing to day x and stage j [23].
2.5. Sample Preparation and Extraction Using DI-SPME
After completing all treatment procedures, 3 late third-instar B. dorsalis larvae were immediately selected from each of the 6 experimental groups (CON, IR-alone, HY, Sup-HY, HY + IR, and Sup-HY + IR). These larvae were moved into a 2 mL microtube (Eppendorf, Germany) and promptly frozen using liquid nitrogen. They were stored in an ultra-low-temperature freezer (MDF-U73V, Sanyo Electric Co. Ltd., Osaka, Japan) at −80 °C for the subsequent experiments. These samples were intended for studying the impact of different treatment methods on the metabolites of B. dorsalis larvae. The frozen samples of the insects were ground into powder within the microtube and immediately suspended in 1.3 mL of HPLC-grade acetonitrile (≥99.9%, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). The microtubes were centrifuged at 4000 rpm for 3 min at 4 °C using a centrifuge (5417R, Eppendorf, Hamburg, Germany). The supernatant (1.3 mL) was transferred to an amber-colored chromatography vial with PTFE-coated septa (Supelco, Darmstadt, Germany). The SPME fiber, coated with 50/30 µm of Carboxen/DVB/PDMS (Sigma-Aldrich, Bellefonte, PA, USA), was introduced into the samples for extraction and conditioned at 25 ± 1 °C for 1 h. Subsequently, a GC-MS analysis was conducted on the DI-SPME, with a desorption time of 15 min. The samples were analyzed in triplicate for biological replicates.
2.6. Metabolites Analysis with GC-MS
DI-SPME samples were analyzed using an Agilent 8890 gas chromatograph (GC) coupled with an Agilent 5977B mass spectrometry detector (MSD) system in splitless mode. The HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm; Agilent J&W Scientific, Santa Clara, CA, USA) was used for separation. Helium gas (>99.999%) was employed as the carrier gas at a constant flow rate of 1.0 mL/min. The GC-MS injection temperature was maintained at 270 °C. The initial temperature was set at 60 °C and held for 2 min, then ramped at 7 °C/min to 200 °C, 5 °C/min to 300 °C, and was finally increased to 320 °C at a rate of 50 °C/min, and held for 3 min. The ion source temperature was set at 230 °C, and the MSD transfer line temperature was set at 280 °C. Electron impact ionization (70 eV) was utilized in full-scan mode (m/z 30-550), with the default settings for the other parameters. The solvent delay time was set to 5 min, and the total run time was 50.4 min. Additionally, quality control samples were first analyzed during the experimental process to assess stability.
2.7. Statistical Analysis
GC-MS data were first processed with Agilent MassHunter Qualitative Analysis Software (Version 10.0), with peak identification augmented by Kovat’s retention index (RI) method [24]. The generated three-dimensional dataset comprised the sample details, retention time (RT), and peak intensities. Univariate and multivariate analyses, as well as a pathway analysis, were performed using the R platform (Version 4.1.3) with the prcomp, MixOmics, and clusterProfiler packages. These analyses included a one-way ANOVA, principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA), heatmap analysis, and KEGG enrichment analysis. Additionally, unless otherwise specified, a significance level of 0.05 was employed for assessing differences.
3. Results
3.1. Develpment of Bactrocera dorsalis (Diptera: Tephritidae) Larvae in a Hypoxic Environment with Phytosanitary Irradiation
As shown in Figure 2(A(1)–A(3)),(B(1)–B(3)), distinct differences are observed in the curves of B. dorsalis third-instar larvae across the six different conditions. Within these curves, overlaps are observed, suggesting variations in the developmental rates among individuals. MA environments, including HY and Sup-HY, markedly delay the development of B. dorsalis larvae, extending their pupation phases (Table 1). However, the developmental postponement effects of Sup-HY and HY on the B. dorsalis third-instar larvae are nearly identical, possibly due to the reduced sensitivity of the B. dorsalis to oxygen when the concentration drops to a certain level. Under normoxic, hypoxic, and super-hypoxic conditions, IR effectively inhibits emergence. The results indicate that the current phytosanitary irradiation dose (116 Gy) fully meets the standard for B. dorsalis quarantine treatment. These three approaches sustain the B. dorsalis larvae pupation rate near 95%. Notably, the IR treatment under MA environments had slight antagonistic effects. This might be attributed to the reduced sensitivity of the larvae to IR under HY or Sup-HY, resulting in an increased pupation rate in the HY/Sup-HY + IR treatment group compared to the normoxic IR treatment group, and significantly mitigating the delaying effect of IR on the larval developmental periods, as shown in Table 1. This novel finding, not previously reported, is hypothesized to be related to metabolic changes within the larvae induced by the hypoxic environment and a potential radioprotective effect.
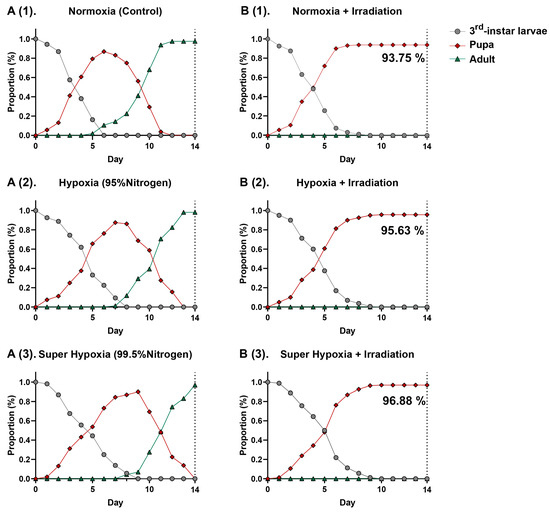
Figure 2.
Proportion of B. dorsalis (Diptera: Tephritidae) larvae in the control (A(1)), hypoxia (A(2)), super hypoxia (A(3)), irradiation (B(1)), hypoxia + irradiation (B(2)) and super-hypoxia + irradiation (B(3)) treatment groups.

Table 1.
Developmental periods (days) (mean ± SE) of the B. dorsalis (Diptera: Tephritidae) larvae in the different treatment groups.
3.2. Metabolic Profiles Analyzed via GC-MS
As illustrated in Table 2, we identified a total of 25 metabolites from 6 different groups—CON, IR-alone, HY, Sup-HY, HY + IR, and Sup-HY + IR—based on a compound matching degree of over 80% and RI. Specifically, 23 metabolites were identified in the CON group, 12 in the IR-alone group, 19 in the HY group, 16 in the Sup-HY group, 13 in the HY + IR group, and 10 in the Sup-HY + IR group. While some metabolites were ubiquitously expressed across all groups, others exhibited significant variations in specific groups. A one-way ANOVA, followed by Fisher‘s LSD post hoc analysis, revealed significant differences (p < 0.05) in the GC-MS response of 24 compounds, as depicted in Figure S1. Metabolites such as 4-methyl-heptadecane, 1-docosene, 2-pentadecanone, nonadecane, and squalene were found to be upregulated in the IR, HY, Sup-HY, HY+IR, and Sup-HY + IR groups. Conversely, metabolites like n-hexadecanoic acid, sitosterol, (E)-2-decenal, 2-dodecenal, and tetradecanal manifested a downregulation following these treatments. Notably, the majority of metabolites that were downregulated were co-clustered in the cluster analysis, suggesting a potential similarity in functions within the metabolic pathways of the B. dorsalis larvae.

Table 2.
Metabolite profile of B. dorsalis (Diptera: Tephritidae) larvae exposed to MAs under IR (116 Gy).
To visually represent the expression patterns of the metabolites across different groups, a clustering heatmap was constructed to cluster the identified metabolites based on the similarity of their content changes. In this heatmap, each cell represents the average content of a metabolite, with blue indicating downregulation, and red indicating upregulation. As shown in Figure 3, the 24 metabolites subjected to hierarchical clustering in each group (CON, IR, HY, Sup-HY, HY + IR, and Sup-HY + IR) showed different regulatory directions significantly. This provides a clear representation, revealing the variations in the metabolite patterns of the B. dorsalis larvae under different treatment conditions. To gain further insights into the metabolic responses of B. dorsalis larvae to these five treatment methods, we will further employ multivariate statistical methods for analysis.
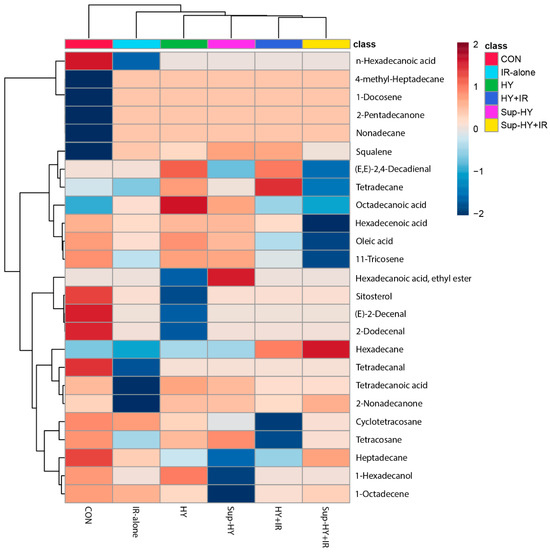
Figure 3.
Heatmap displaying the abundance changes of the metabolites significantly influenced by different treatment methods, with significance determined via one-way ANOVA at p < 0.05.
3.3. Multivariate Statistical Analysis of B. dorsalis Larvae Metabolites
An unsupervised PCA was employed to obtain a general overview of the clustering information between groups, with the grouping in a PCA score plot being based on the similarities between the metabolic profiles of the samples. As shown in Figure 4, the first principal component accounted for 20.7% of the total variance in the original data, while the second principal component explained 15.8%. On the first principal component, a distinct separation was observed between the IR-alone group and the Sup-HY + IR group. However, the 95% Hotelling’s T-squared ellipse overlapped between the CON and the HY, Sup-HY, and HY + IR groups. In comparison to the PCA, the partial least squares discriminant analysis (PLS-DA) model, being a supervised learning method, demonstrated superior discriminatory power. The score plot (Figure 5) shows that the IR-alone, HY, Sup-HY, HY + IR, and Sup-HY + IR groups were clearly separated from the control group. Meanwhile, clear separations were observed among the IR-alone, HY, Sup-HY, HY + IR, and Sup-HY + IR groups in the PLS-DA model. These findings indicate that all five treatments—IR-alone, HY, Sup-HY, HY + IR, and Sup-HY + IR—significantly altered the metabolic profiles of the B. dorsalis larvae. Consequently, we can employ statistical methodologies to further identify the key differential metabolites between the treatment groups.
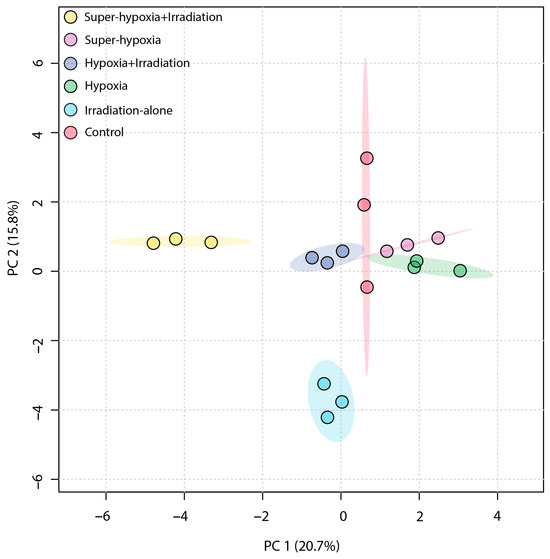
Figure 4.
PCA score plot of the metabolic profiles in the B. dorsalis (Diptera: Tephritidae) larvae exposed to different MAs under IR (116 Gy).
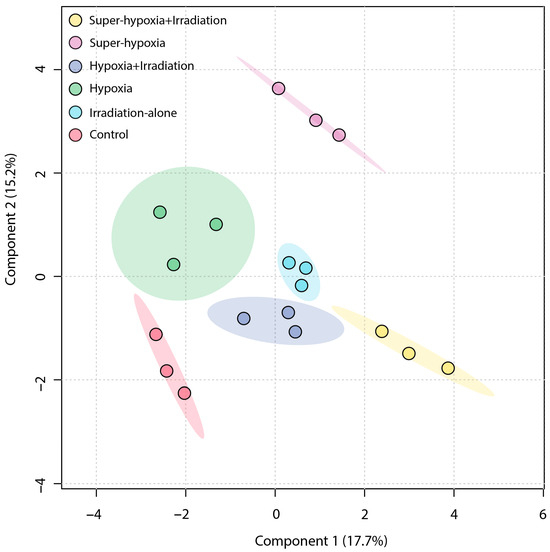
Figure 5.
PLS-DA score plot of the metabolic profiles in the B. dorsalis (Diptera: Tephritidae) larvae exposed to different MAs under IR (116 Gy).
3.4. Statistical Analysis and Differentially Regulated Metabolites
“Differential metabolites” were defined as substances that were identified among different samples but showed significant variations in their respective concentrations. For differentials among the CON vs. IR-alone, CON vs. HY, CON vs. Sup-HY, CON vs. HY + IR, and CON vs. Sup-HY + IR, separate PLS-DA analyses were conducted on their respective metabolic datasets. Metabolites that contributed to the differentiation were identified based on a variable importance in the projection (VIP) value of the PLS-DA model (threshold > 1).
Furthermore, metabolites specific to the IR-alone, HY, Sup-HY, HY + IR, and Sup-HY + IR groups were individually identified, as depicted in Figure 6A–E. By consolidating the results from the Student’s t-test (with a threshold of p < 0.05) and a VIP value greater than 1.0, we identified 18, 9, 10, 17, and 15 key differential metabolites in the B. dorsalis larvae treated with IR-alone, HY, Sup-HY, HY + IR, and Sup-HY + IR, respectively.
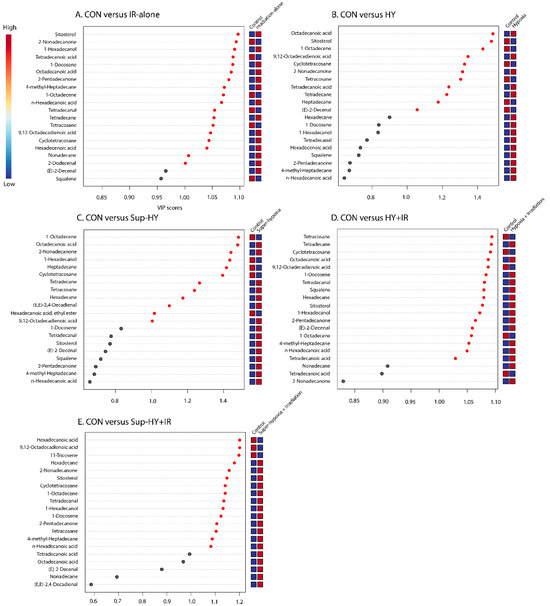
Figure 6.
A variable importance plot showing the contribution of each metabolite to the first component (ranked by VIP scores) in the CON vs. IR (A), CON vs. HY (B), CON vs. Super-HY (C), CON vs. HY + IR (D), and CON vs. Sup-HY + IR (E) groups.
Importantly, 2-dodecenal and nonadecane were unique to the IR-alone group; hexadecanoic acid, ethyl ester, and heptadecane were specific to the Sup-HY group; (E)-2-decenal and squalene were exclusive to the HY + IR group; and 11-tricosene was only detected in the Sup-HY + IR group, as illustrated in Figure 7.
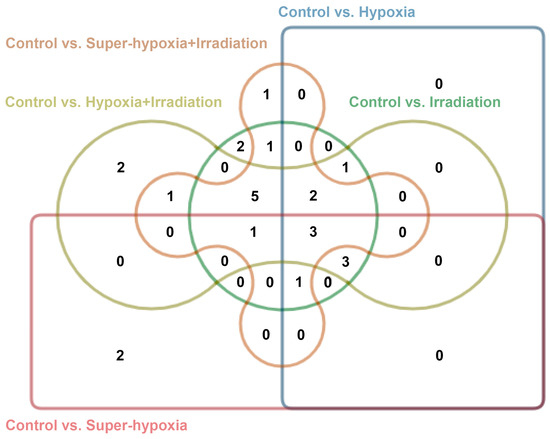
Figure 7.
Edward’s Venn diagram of the CON vs. IR, CON vs. HY, CON vs. Sup-HY, CON vs. HY + IR, and CON vs. Sup-HY + IR groups. Different colors represent 5 groups of data sets, and numbers indicate the quantity of elements in the overlapping areas.
3.5. Key Metabolic Pathway Analysis
As shown in Table 3, compared to the CON, the IR-alone, HY, Sup-HY, HY + IR, and Sup-HY + IR groups identified five, three, two, five, and five differential metabolic pathways, respectively. This suggests that the metabolic response of B. dorsalis larvae to the IR treatment is more complex than that to the MA treatment. Among these six groups, two common metabolic pathways were observed: the biosynthesis of unsaturated fatty acids and fatty acid biosynthesis.

Table 3.
Pathway analysis results from the B. dorsalis (Diptera: Tephritidae) larvae metabolomics for the CON vs. IR, CON vs. HY, CON vs. Sup-HY, CON vs. HY + IR, and CON vs. Sup-HY + IR groups.
Based on the criteria of p (>0.05) and impact value (>0.1), the key metabolic pathways for the CON vs. IR-alone, CON vs. HY, CON vs. Sup-HY, CON vs. HY + IR, and CON vs. Sup-HY + IR groups were identified as five, two, zero, five, and zero, respectively, as depicted in Figure 8. Specifically, in the CON vs. IR-alone group, the pathways of the biosynthesis of unsaturated fatty acids, fatty acid biosynthesis, fatty acid elongation, fatty acid degradation, and steroid biosynthesis were deemed critical, with corresponding impact values of 0.117, 0.153, 0.124, 0.127, and 0.137. In the CON vs. HY group, fatty acid biosynthesis and steroid biosynthesis emerged as the key pathways, with impact values of 0.109 and 0.122, respectively. For the HY + IR group, the key metabolic pathways included the biosynthesis of unsaturated fatty acids, steroid biosynthesis, fatty acid biosynthesis, fatty acid elongation, and fatty acid degradation, with impact values of 0.141, 0.164, 0.184, 0.148, and 0.152, respectively.
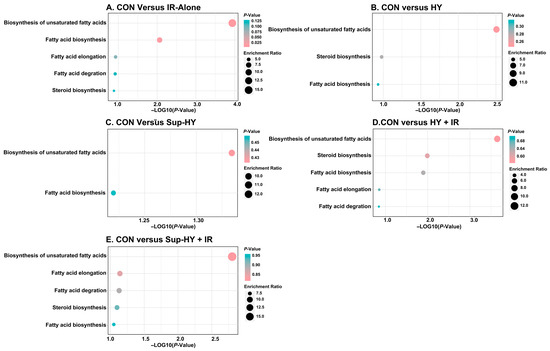
Figure 8.
Metabolome view map of the significant metabolic pathways characterized in the B. dorsalis (Diptera: Tephritidae) larvae exposed to different modified atmospheres under a phytosanitary irradiation dose. (A) CON vs. IR-alone, (B) CON vs. HY, (C) CON vs. Sup-HY, (D) CON vs. HY + IR, and (E) CON vs. Sup-HY + IR.
Although the differential metabolites in the Sup-HY and Sup-HY + IR groups were enriched in two and five metabolic pathways, respectively, their impact on these pathways was not significant. Notably, the pathways of fatty acid elongation and fatty acid degradation emerged as characteristic metabolic pathways in the B. dorsalis larvae following IR treatment (IR-alone, HY + IR, and Sup-HY + IR). Furthermore, IR treatments under both the hypoxic and super-hypoxic conditions influenced five metabolic pathways, the biosynthesis of unsaturated fatty acids, fatty acid biosynthesis, fatty acid elongation, fatty acid degradation, and steroid biosynthesis. The findings seem to correlate with the developmental results of the six groups of B. dorsalis larvae described in Section 3.1. Hence, these five metabolic pathways might play an important role in influencing the sensitivity of B. dorsalis larvae to IR.
3.6. Comparison of the Main Differential Metabolic Responses
Through a detailed analysis of the affected metabolites and their associated pathways, we aim to understand the differential metabolic responses of B. dorsalis larvae under various treatments, including IR-alone, HY, Sup-HY, HY + IR, and Sup-HY + IR.
Based on the KEGG database, we constructed a metabolic pathway network, highlighting the interconnections among the aforementioned five core metabolic pathways and their key compounds, as illustrated in Figure 9A,B. These pathways reveal the primary metabolic variations and shared responses of B. dorsalis larvae under diverse treatment conditions. Notably, the levels of tetradecanoic acid, n-hexadecanoic acid, octadecanoic acid, and oleic acid were significantly reduced in all IR-treated groups. These fatty acids are essential components of several metabolic pathways, including the biosynthesis of unsaturated fatty acids, fatty acid biosynthesis, fatty acid elongation, and fatty acid degradation. This significant decrease can be attributed to the surge in ROS production induced by IR treatment, resulting in localized mitochondrial autophagy [25]. Consequently, mitochondrial energy metabolism is inhibited, prompting the swift oxidation of fatty acids into acetyl-CoA that subsequently enters the TCA cycle for energy generation.
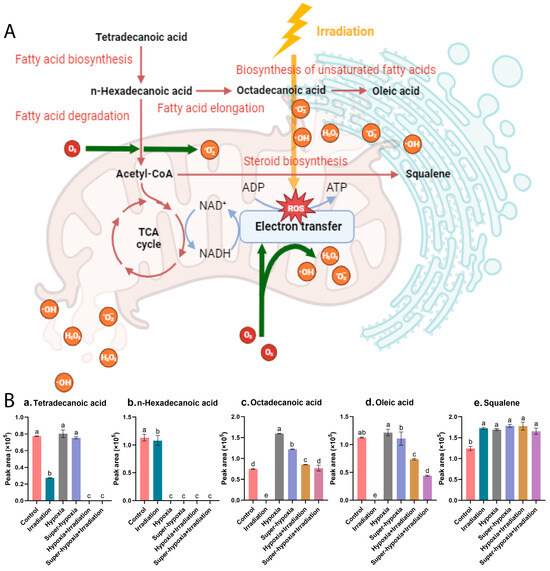
Figure 9.
(A) The integrated schematic diagram of the key metabolic pathways and metabolites in the B. dorsalis (Diptera: Tephritidae) larvae exposed to different modified atmospheres under a phytosanitary irradiation dose, and (B) the changes in peak areas of 5 key compounds ((a). tetradecanoic acid, (b). n-hexadecanoic acid, (c). octadecanoic acid, (d). oleic acid and (e). squalene) across different treatment conditions.
In both hypoxic treatment groups, HY and Sup-HY, there was a notable decrease in n-hexadecanoic acid levels and a corresponding increase in octadecanoic acid levels. This pattern suggests the activation of both the fatty acid degradation and fatty acid elongation metabolic pathways. The presence of oleic acid suggested the activation of the biosynthesis of unsaturated fatty acids pathway. However, there was no significant difference in oleic acid concentration compared to the control group. The likely cause of this observation is the activation of Hypoxia-Inducible Factor-1 (HIF-1) in both the hypoxic and super-hypoxic conditions. The activation of HIF-1 boosts the expression of antioxidative enzymes, thereby regulating ROS levels within the organism [26]. Consequently, oleic acid experiences reduced oxidative stress, diminishing the formation of lipid peroxides.
Regarding the IR treatment under the HY and Sup-HY conditions, certain fatty acid levels decreased compared to other groups, presumably because of the combined effects of low-oxygen and IR. The notable reduction in tetradecanoic acid and n-hexadecanoic acid levels indicates a gradual shift in the B. dorsalis larvae’s primary energy metabolism from carbohydrates to lipids for efficient ATP production. This metabolic shift typically indicates that the organism is exploiting its “energy reserves” to achieve a temporary stress-adaptive capacity under adverse conditions. Consequently, we hypothesize that the fatty acid metabolism might be a crucial pathway for the B. dorsalis larvae to initiate their antioxidative defense mechanism, assisting in the clearance of ROSs induced by IR and reducing their IR sensitivity. Notably, this antioxidative effect appears to amplify as the oxygen concentration decreases, as evidenced by the results in Section 3.1. This observation offers a persuasive explanation for the developmental experiments of the B. dorsalis in our study. While the precise mechanism behind such radioprotective effects remains a ‘black-box’, our findings could offer a valuable reference for subsequent research.
The observed alteration in the squalene concentration is noteworthy, given its apparent sensitivity to both low-oxygen and IR conditions. In biological systems, squalene is recognized for augmenting the activity of superoxide dismutase (SOD) [27]. Consequently, elevated squalene levels might correlate with the impact of hypoxia and irradiation on ROS concentrations in B. dorsalis larvae.
4. Discussion
Until recently, there have been only a few studies on the impacts of IR in hypoxic conditions on the reproduction and development of B. dorsalis [8,10,28]. The hypoxic environment diminishes the efficacy of IR on certain insects, leading the IPPC to withhold support for IR for the quarantine treatment of MAP commodities [12]. This study, however, demonstrates that the current phytosanitary IR dose is effective in inhibiting B. dorsalis larvae even under HY/Sup-HY conditions (Figure 2), lending support to the administrative adjustments by the USDA-APHIS revising the minimum O2 level for treatments from 18% to 10% [29]. Our research findings also provide a robust basis for the IPPC to reassess the restrictions on phytosanitary IR applications for B. dorsalis under hypoxic conditions.
IR-induced DNA damage, a significant factor causing male sterility and impacting insect growth [30,31], might suggest the activation of DNA repair genes under hypoxic conditions. However, the RNA-seq analysis conducted by Wang et al. on DNA repair-related genes in the cowpea weevil (Callosobruchus maculatus), including XRCC1, XRCC3, RAD23, and RAD51, indicates otherwise [8]. Their findings challenge the hypothesis that hypoxia aids in DNA-damage repair, suggesting that the mechanisms behind radioprotective effects in hypoxic conditions warrant further investigation. Our study first employed the DI-SPME-GC-MS technique, a validated and effective tool for metabolomic analysis, to explore this area.
Topological analysis showed that treatments like IR-alone, HY, Sup-HY, HY + IR, and Sup-HY + IR primarily exhibit changes in the lipid metabolism pathways compared to the control group, especially in unsaturated fatty acids and fatty acid biosynthesis. These pathways are crucial for maintaining biological tissue structure and function [32,33]. Interestingly, there was an overlap in the differential metabolic pathways observed across these five treatment methods, suggesting a certain correlation in the mechanisms of action of IR-alone, HY, Sup-HY, HY + IR and Sup-HY + IR on B. dorsalis larvae. This also implies that the combined impacts of IR and MAs extend beyond a mere additive “1 + 1” effect.
In this study, significant changes were observed in the free fatty acids of B. dorsalis larvae across different treatment groups. These fatty acids are not only essential for maintaining cell membrane fluidity and permeability but are also vital in anti-oxidative stress [34,35,36]. Notably, n-hexadecanoic acid was consistently downregulated across all treatment groups, indicating a marked inhibition of the fatty acid biosynthesis pathway. As depicted in Figure 9B, n-hexadecanoic acid, a key differential regulatory metabolite, holds a central position in the differential metabolic pathways of the B. dorsalis larvae subjected to IR-alone, HY, Sup-HY, HY + IR, and Sup-HY + IR treatments. N-hexadecanoic acid, a precursor to many long-chain and unsaturated fatty acids, can be transformed into significant amounts of free fatty acids like octadecanoic acid through the fatty acid elongation pathway, and their accumulation in the endoplasmic reticulum may inhibit certain protein synthesis pathways, negatively affecting insect growth and development [37,38]. Furthermore, squalene is synthesized in the endoplasmic reticulum through the steroid biosynthesis pathway and promotes biological oxidation and metabolism, thereby enhancing the defense and stress-response capabilities of insects [39]. Currently, the specific changes in the lipid metabolism within the insects following the IR-alone, HY, Sup-HY, HY + IR and Sup-HY treatments are not fully understood. Therefore, further studies are needed to comprehensively understand the roles of lipid metabolic pathways.
Research suggests that diminished metabolic activity can counteract the oxidative stress caused by IR under hypoxic conditions. As previous studies have highlighted, both IR and MA treatments impact the mitochondrial electron transport chain and the TCA cycle [40,41,42]. Thus, carbohydrates might have a limited role in the insect energy metabolism post IR and MA treatments. This finding further strengthens the conclusion that lipid metabolic pathways have a significant impact on insects after treatments such as IR-alone, HY, Sup-HY, IR + HY, and IR + Sup-HY. Furthermore, Pablo et al.’s research showed that the development of the Mediterranean fruit fly (Ceratitis capitata) is regulated by changes in oxygen consumption rates and the levels of primary energy molecules, such as lipids, glycogen, and trehalose. Yan et al. analyzed the expression patterns and functions of fatty acid synthase genes (SlFAS1 and SlFAS2) in the larvae of the tobacco cutworm (Spodotera litura), demonstrating the crucial role of fatty acid synthesis and storage in insects’ metabolism and metamorphic development [43,44]. These findings support the results observed in this study of the developmental delays in B. dorsalis larvae under hypoxic conditions, as depicted in Figure 2(A(1)–A(3)); the decreased pupation rate and hindered emergence, as shown in Figure 2(B(1)–B(3)), suggest that IR further impairs metabolic activity in insects, thereby hindering their normal development within the expected timeframe.
Based on metabolomics, this study provides a preliminary mechanistic explanation for the enhanced tolerance of B. dorsalis larvae to IR under hypoxic stress for the first time. It is well established that the metabolic rate of B. dorsalis larvae significantly decreases in hypoxic conditions [8,45]. However, under hypoxic conditions, while both the carbohydrate and lipid metabolisms are limited, cells favor the fatty acid metabolism—a more ATP-productive pathway—with the primary source of these fatty acids being the oxidation of intracellular lipid droplets [46,47,48]. In the early phases of hypoxic stress, cells quickly amass substantial ROSs, mainly from an overload of electrons in the electron transport chain due to a lack of oxygen [49,50]. Furthermore, IR not only damages DNA structures but also disrupts the electron transport chain, resulting in substantial ROS production [8,30,51]. Excessive ROSs can act as signaling molecules to enhance the activity of antioxidative enzymes, maintaining an ROS balance in organisms [52]. Key factors in the oxygen-sensing pathway, such as HIF-1 and nuclear factor erythroid-derived 2-like 2 (Nrf2), induced and upregulated by hypoxic stress, regulate the gene expression of antioxidative enzymes. This enhances the organism’s oxidative resistance, improving stress resistance during development. Specifically, HIF-1 activation inhibits the TCA cycle, increases the production of reducing molecules in organisms, and promotes lipid degradation [53]. Meanwhile, Nrf2 translocates to the cell nucleus, binding to the antioxidant response element (ARE), thereby activating the expression of antioxidative and detoxifying genes [54]. Moreover, studies have shown that hypoxic stress also can induce mitochondrial autophagy mediated by BNIP3 and FUNDC1, further alleviating oxidative damage caused by ROSs [55,56].
This study has potential limitations. We evaluated the effects of IR based on observations of naked B. dorsalis larvae, which differs from typical phytosanitary treatments on whole fruits. However, our simplified experimental design ensured each larva received a uniform and precise IR dose, allowing us to directly link metabolic changes to the irradiation treatment. Our evaluation may be conservative, and underestimate the impact of fruits on these treatments, as (1) fruits provide a complex treatment environment for insects, influenced by both biotic and abiotic factors. This increases the risk related to the fruit’s impact on metabolic changes in insects [57]; (2) Fruit tissues and structures attenuate and scatter X-rays, affecting the dose of IR subjected to insects [58,59]; (3) The efficacy of IR treatment may be influenced by the behavior of insect larvae. For example, some third-instar larvae are often found inside fruit galleries, while others, especially those nearing pupariation, seek shelter outside the fruit [60]. This study did not employ typical phytosanitary treatment programs involving whole fruits with insects, as our focus was more on the metabolic changes in insects resulting from different treatment methods. The execution of experiments requires strong stability and reproducibility; however, introducing fruits could lead to numerous extraneous variables, thereby interfering with the accurate assessment of experimental results.
In conclusion, we utilized the DI-SPME-GC-MS technique to first explore the mechanism by which hypoxic stress enhances the radiotolerance of B. dorsalis larvae from a lipid metabolism perspective. This research contributed to a deeper understanding of the radioprotection mechanisms in insects. Furthermore, our findings indicate that the B. dorsalis larvae, as a non-model insect, exhibit a conservative response pathway when subjected to an MA or IR stress. In addition, insects, as complex biological systems, have stress responses to external environmental threats that are co-regulated by multiple metabolic pathways, including those of amino acids, sugars, and lipids. Therefore, relying solely on a single metabolic pathway can hardly provide a comprehensive interpretation of these responses. Future research should focus more on the interactions between different metabolic pathways to further elucidate the radioprotection mechanisms in insects.
5. Conclusions
X-ray irradiation has a lethal effect on many insects. However, a hypoxic environment can weaken the impact of IR treatment, reducing the sensitivity of B. dorsalis larvae to IR and subsequently disrupting their normal development and metabolism. Notably, IR can induce significant changes in the lipid metabolism pathways of B. dorsalis larvae, as well as in the associated levels of free fatty acids, which are moderated in hypoxic conditions. Future research should emphasize the role of lipid metabolism pathways and their associated metabolites in evaluating the IR tolerance of insects, providing support for developing new post-harvest pest management strategies.
Furthermore, our preliminary research reveals the mechanisms by which the larvae of the B. dorsalis enhance their tolerance to IR under hypoxic conditions. These insights have practical implications for quarantine treatments. By limiting the oxygen content in the environment, the efficacy of IR treatments can be optimized, providing an important theoretical and data basis for the combined application of an MA and IR in quarantine procedures. Our findings indicate that either HY or Sup-HY environments influence the pupation rate and developmental period of the evaluated fruit fly species at the phytosanitary IR dose. Nevertheless, none of the B. dorsalis larvae treated under these conditions developed into adults. As a result, this study advocates for the updating of guidelines by international organizations and regulatory agencies regarding phytosanitary IR applications for B. dorsalis in low-oxygen environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects15030177/s1, Figure S1: Metabolites identified in B. dorsalis (Diptera: Tephritidae) larvae exposed to different modified atmospheres under phytosanitary irradiation dose. The  represented compounds that were selected based on a significant p-value threshold (<0.05), while the
represented compounds that were selected based on a significant p-value threshold (<0.05), while the  indicated non-significant compounds.
indicated non-significant compounds.
 represented compounds that were selected based on a significant p-value threshold (<0.05), while the
represented compounds that were selected based on a significant p-value threshold (<0.05), while the  indicated non-significant compounds.
indicated non-significant compounds.Author Contributions
Conceptualization, C.S., B.L. and T.L.; methodology, C.S., B.L. and T.L.; software, C.S., Q.L. and H.Z.; validation, C.S.; formal analysis, C.S.; investigation, C.S., B.L., L.L., Q.L. and H.Z.; resources, B.L., L.L., Q.L. and H.Z.; data curation, L.L., Q.L. and H.Z.; writing—original draft, C.S.; writing—review and editing, C.S., L.L., Q.L., H.Z. and T.L.; visualization, C.S.; supervision, T.L.; project administration, B.L. and T.L.; funding acquisition, B.L. and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the scientific research fund of the Chinese Academy of Inspection and Quarantine (2019JK029) and the technical support fund on the postharvest control of biological contaminants of the State Administration for Market Regulation.
Data Availability Statement
All data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Clarke, A.R.; Li, Z.H.; Qin, Y.J.; Zhao, Z.H.; Liu, L.J.; Schutze, M.K. Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) is not invasive through Asia: It’s been there all along. J. Appl. Entomol. 2019, 143, 797–801. [Google Scholar] [CrossRef]
- DeMeyer, M.; Ekesi, S. Exotic invasive fruit flies (Diptera: Tephritidae): In and out of Africa. In Fruit Fly Research and Development in Africa—Towards a Sustainable Management Strategy to Improve Horticulture; Springer: Cham, Switzerland, 2016; pp. 127–150. [Google Scholar]
- Clarke, A.R.; Measham, P.F. Competition: A Missing component of fruit fly (Diptera: Tephritidae) risk assessment and planning. Insects 2022, 13, 1065. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Rashid, A. Current activities in food irradiation as a sanitary and phytosanitary treatment in the Asia and the Pacific Region and a comparison with advanced countries. Food Control 2017, 72, 345–359. [Google Scholar] [CrossRef]
- Follett, P.A. Phytosanitary irradiation for fresh horticultural commodities: Generic treatments, current issues, and next steps. Stewart Postharvest Rev. 2014, 3, 2014. [Google Scholar]
- USDA; APHIS. Treatments for fruits and vegetables. Rules and Regulations. Fed. Regist. 2006, 71, 4451–4464. [Google Scholar]
- Rashvand, M.; Matera, A.; Altieri, G.; Genovese, F.; Fadiji, T.; Opara, U.L.; Di Renzo, G.C. Recent advances in the potential of modeling and simulation to assess the performance of modified atmosphere packaging (MAP) systems for the fresh agricultural product: Challenges and development. Trends Food Sci. Technol. 2023, 136, 48–63. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, W.; Meng, J.; Speakmon, M.; Qiu, J.; Pillai, S.; Zhu-Salzman, K. Hypoxic environment protects cowpea bruchid (Callosobruchus maculatus) from electron beam irradiation damage. Pest Manag. Sci. 2019, 75, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Follett, P.A.; Swedman, A.; Mackey, B. Effect of low-oxygen conditions created by modified atmosphere packaging on radiation tolerance in Drosophila suzukii (Diptera: Drosophilidae) in sweet cherries. J. Econ. Entomol. 2018, 111, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.S.; Hallman, G.J.; Martínez-Barrera, O.Y.; Hurtado, N.V.; Cardoso, A.A.; Parker, A.G.; Myers, S.W. Modified atmosphere does not reduce the efficacy of phytosanitary irradiation doses recommended for tephritid fruit flies. Insects 2020, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Follett, P.A.; Neven, L.G. Phytosanitary irradiation: Does modified atmosphere packaging or controlled atmosphere storage creating a low oxygen environment threaten treatment efficacy. Radiat. Phys. Chem. 2020, 173, 108874. [Google Scholar] [CrossRef]
- Hallman, G.J. Explanatory Document on International Standard for Phytosanitary Measures No. 18: Guidelines for the Use of Irradiation as a Phytosanitary Treatment: ISPM No. 18/Explanatory Document; International Plant Protection Convention (IPPC): Rome, Italy, 2006. [Google Scholar]
- Alexovič, M.; Andruch, V.; Balogh, I.S.; Šandrejová, J. A single-valve sequential injection manifold (SV-SIA) for automation of air-assisted liquid-phase microextraction: Stopped flow spectrophotometric determination of chromium (VI). Anal. Methods 2013, 5, 2497–2502. [Google Scholar] [CrossRef]
- Alexovič, M.; Horstkotte, B.; Solich, P. Automation of static and dynamic non-dispersive liquid phase microextraction. Part 1: Approaches based on extractant drop-, plug-, film-and microflow-formation. Anal. Chim. Acta 2016, 906, 22–40. [Google Scholar] [CrossRef]
- Gallo, M.; Ferranti, P. The evolution of analytical chemistry methods in foodomics. J. Chromatogr. A 2016, 1428, 3–15. [Google Scholar] [CrossRef]
- Zheng, J.; Kuang, Y.; Zhou, S.; Gong, X.; Ouyang, G. Latest improvements and expanding applications of solid-phase microextraction. Anal. Chem. 2023, 95, 218–237. [Google Scholar] [CrossRef]
- Shan, C.; Li, B.; Li, L.; Li, B.; Ren, Y.; Liu, T. Correlation between irradiation treatment and metabolite changes in Bactrocera dorsalis (Diptera: Tephritidae) Larvae using solid-phase microextraction (SPME) coupled with gas chromatography-mass spectrometry (GC-MS). Molecules 2022, 27, 4641. [Google Scholar] [CrossRef] [PubMed]
- Alnajim, I.; Agarwal, M.; Liu, T.; Li, B.; Du, X.; Ren, Y. Preliminary study on the differences in hydrocarbons between phosphine-susceptible and-resistant strains of Rhyzopertha dominica (Fabricius) and Tribolium castaneum (Herbst) using direct immersion solid-phase microextraction coupled with GC-MS. Molecules 2020, 25, 1565. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.I.; Miyashita, D.; Nishida, T. Life history and demographic parameters of three laboratory-reared tephritids (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1984, 77, 651–656. [Google Scholar] [CrossRef]
- Shan, C.; Li, B.; Li, L.; Du, X.; Ren, Y.; McKirdy, S.J.; Liu, T. Comparison of fumigation efficacy of methyl bromide alone and phosphine applied either alone or simultaneously or sequentially against Bactrocera correcta in Selenicereus undatus (red pitaya) fruit. Pest Manag. Sci. 2023, 79, 4942–4951. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, J.; Wu, M.; Jiao, X.; Wang, Z.; Liang, F.; Zhan, G. Gamma radiation as a phytosanitary treatment against larvae and pupae of Bactrocera dorsalis (Diptera: Tephritidae) in guava fruits. Food Control 2017, 72, 360–366. [Google Scholar] [CrossRef]
- Cao, K.; Lan, R.; Yang, X.; Gong, B.; Zhang, J.; Zhou, X.; Jin, L. Two-Sex Life Table Analysis of the Predator Arma chinensis (Hemiptera: Pentatomidae) and the Prediction of Its Ability to Suppress Populations of Scopula subpunctaria (Lepidoptera: Geometridae). Agriculture 2023, 13, 1254. [Google Scholar] [CrossRef]
- Pan, K.; Xin, T.; Chen, Y.; Wang, H.; Wen, K.; Liu, Y.; Xia, B. Age-Stage, Two-Sex Life Table and Functional Response of Amblyseius orientalis (Acari: Phytoseiidae) Feeding on Different Nutrient Sources. Insects 2022, 13, 983. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shan, C.; Liu, Q.; Li, B.; Liu, T. Comparative Analysis of the Metabolic Profiles of Strains of Tribolium castaneum (Herbst) Adults with Different Levels of Phosphine Resistance Based on Direct Immersion Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. Molecules 2023, 28, 7721. [Google Scholar] [CrossRef]
- Hou, J.; Han, Z.P.; Jing, Y.Y.; Yang, X.; Zhang, S.S.; Sun, K.; Wei, L.X. Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells. Cell Death Dis. 2013, 4, 844. [Google Scholar] [CrossRef] [PubMed]
- Arvold, N.D.; Guha, N.; Wang, D.; Matli, M.; Deen, D.F.; Warren, R.S.; Haas-Kogan, D.A. Hypoxia-induced radioresistance is independent of hypoxia-inducible factor-1A in vitro. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Farvin, K.H.S.; Surendraraj, A.; Anandan, R. Protective effect of squalene on mitochondrial alterations in isoprenaline-induced myocardial injury. Int. J. Biomed. Pharmacol. Sci. 2010, 4, 32–37. [Google Scholar]
- Hallman, G.J.; Hénon, Y.M.; Parker, A.G.; Blackburn, C.M. Phytosanitary irradiation: An overview. Fla. Entomol. 2016, 99, 1–13. [Google Scholar]
- USDA Treatment Manual. Available online: https://www.aphis.usda.gov/import_export/plants/manuals/ports/downloads/treatment.pdf (accessed on 1 May 2020).
- Ali, H.M.; Moustafa, H.Z.; Sayed, R.M. Alterations in Biomarkers Associated with Sterility in Pectinophora gossypiella (Saunders) Induced by Gamma Irradiation. Braz. Arch. Biol. Technol. 2018, 60, e17160634. [Google Scholar] [CrossRef]
- Vimal, N.; Angmo, N.; Sengupta, M.; Seth, R.K. Radiation Hormesis to Improve the Quality of Adult Spodoptera litura (Fabr.). Insects 2022, 13, 933. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Boguś, M. The metabolism and role of free fatty acids in key physiological processes in insects of medical, veterinary and forensic importance. PeerJ 2021, 9, e12563. [Google Scholar] [CrossRef]
- Zhu, H.; Han, M. Exploring developmental and physiological functions of fatty acid and lipid variants through worm and fly genetics. Annu. Rev. Genet. 2014, 48, 119–148. [Google Scholar] [CrossRef]
- Rawat, N.; Singla-Pareek, S.L.; Pareek, A. Membrane dynamics during individual and combined abiotic stresses in plants and tools to study the same. Physiol. Plant. 2021, 171, 653–676. [Google Scholar] [CrossRef]
- Inayat, M.; Abbas, F.; Rehman, M.H.; Mahmud, A. Physico-chemical parameters, oxidative stress, and fatty acid profile of American Pekin ducks (Anas platyrhynchos domesticus) raised under different production systems. Braz. J. Poult. Sci. 2023, 25, 1–8. [Google Scholar] [CrossRef]
- Qiu, K.; Zhao, Q.; Wang, J.; Qi, G.H.; Wu, S.G.; Zhang, H.J. Effects of pyrroloquinoline quinone on lipid metabolism and anti-oxidative capacity in a high-fat-diet metabolic dysfunction-associated fatty liver disease chick model. Int. J. Mol. Sci. 2021, 22, 1458. [Google Scholar] [CrossRef] [PubMed]
- El-Maradny, A. Identification of Fatty Acid Methyl Esters, Long Chain Aldehydes and Free Fatty Acids in a Sponge Chalinula saudensis along the coast of Jeddah, Saudi Arabia. J. Chem. Soc. Pak. 2012, 34, 673–678. [Google Scholar]
- Liu, Y.; Liu, J.; Deng, C.; Zhang, X. Graphene and graphene oxide: Two ideal choices for the enrichment and ionization of long-chain fatty acids free from matrix-assisted laser desorption/ionization matrix interference. Rapid Commun. Mass Spectrom. 2011, 25, 3223–3234. [Google Scholar] [CrossRef]
- Senbagalakshmi, P.; Muthukrishnan, S.; Jebasingh, T.; Kumar, T.S.; Rao, M.; Kumar, T.S.; Rao, M.V. Squalene, Biosynthesis and its role in production of bioactive compounds, a Proper Scientific Challenge—A Review. J. Emerg. Technol. Innov. Res. 2019, 6, 505–526. [Google Scholar]
- Atamna, H.; Mackey, J.; Dhahbi, J.M. Mitochondrial pharmacology: Electron transport chain bypass as strategies to treat mitochondrial dysfunction. Biofactors 2012, 38, 158–166. [Google Scholar] [CrossRef]
- Aretz, I.; Hardt, C.; Wittig, I.; Meierhofer, D. An impaired respiratory electron chain triggers down-regulation of the energy metabolism and de-ubiquitination of solute carrier amino acid transporters. Mol. Cell. Proteom. 2016, 15, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kitazawa, H.; Wang, X.; Sun, H. Regulation of respiratory pathway and electron transport chain in relation to senescence of postharvest white mushroom (Agaricus bisporus) under high O2/CO2 controlled atmospheres. J. Agric. Food Chem. 2017, 65, 3351–3359. [Google Scholar] [CrossRef]
- Bochicchio, P.A.; Pérez, M.M.; Quesada-Allué, L.A.; Rabossi, A. Completion of metamorphosis after adult emergence in Ceratitis capitata (Diptera: Tephritidae). Curr. Res. Insect Sci. 2021, 1, 100017. [Google Scholar] [CrossRef]
- Song, Y.; Gu, F.; Liu, Z.; Li, Z.; Wu, F.A.; Sheng, S. The key role of fatty acid synthase in lipid metabolism and metamorphic development in a destructive insect pest, Spodoptera litura (Lepidoptera: Noctuidae). Int. J. Mol. Sci. 2022, 23, 9064. [Google Scholar] [CrossRef]
- Nathaniel, T.I.; Otukonyong, E.; Abdellatif, A.; Soyinka, J.O. Effect of hypoxia on metabolic rate, core body temperature, and c-fos expression in the naked mole rat. Int. J. Dev. Neurosci. 2012, 30, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Scisciola, L.; Chianese, U.; Caponigro, V.; Basilicata, M.G.; Salviati, E.; Altucci, L.; Sommella, E. Multi-omics analysis reveals attenuation of cellular stress by empagliflozin in high glucose-treated human cardiomyocytes. J. Transl. Med. 2023, 21, 662. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.E.; Bucarey, J.L.; Espinosa, A. Muscle lipid metabolism: Role of lipid droplets and perilipins. J. Diabetes Res. 2017, 2017, 1789395. [Google Scholar] [CrossRef] [PubMed]
- Welte, M.A. Expanding roles for lipid droplets. Curr. Biol. 2015, 25, 470–481. [Google Scholar] [CrossRef]
- Clanton, T.L. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J. Appl. Physiol. 2007, 102, 2379–2388. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Szumiel, I. Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: The pivotal role of mitochondria. Int. J. Radiat. Biol. 2015, 91, 1–12. [Google Scholar] [CrossRef]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive oxygen species, antioxidants and signaling in plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Naeini, S.H.; Mavaddatiyan, L.; Kalkhoran, Z.R.; Taherkhani, S.; Talkhabi, M. Alpha-ketoglutarate as a potent regulator for lifespan and healthspan: Evidences and perspectives. Exp. Gerontol. 2023, 175, 112154. [Google Scholar] [CrossRef]
- Lv, C.; Ma, X.; Liang, C.; Chen, Y.; Qin, F.; Zhou, C.; Hou, L. The interaction of pterostilbene with Kelch-like ECH-associated protein 1 and its regulation on the nuclear factor erythroid 2-related factor 2/antioxidant response element pathway. Process Biochem. 2023, 132, 228–235. [Google Scholar] [CrossRef]
- Pan, C.; Ai, C.; Liang, L.; Zhang, B.; Li, Q.; Pu, L.; Wang, X. Sestrin2 protects against hypoxic nerve injury by regulating mitophagy through SESN2/AMPK pathway. Front. Mol. Biosci. 2023, 10, 1266243. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Liu, R.; Zhou, Y.; Zhang, Z.; Jiang, X.; Xu, J.; Hu, Z. FOXO3a-dependent up-regulation of HSP90 alleviates cisplatin-induced apoptosis by activating FUNDC1-mediated mitophagy in hypoxic osteosarcoma cells. Cell. Signal. 2023, 101, 110500. [Google Scholar] [CrossRef] [PubMed]
- Olazcuaga, L.; Baltenweck, R.; Leménager, N.; Maia-Grondard, A.; Claudel, P.; Hugueney, P.; Foucaud, J. Metabolic consequences of various fruit-based diets in a generalist insect species. eLife 2023, 12, e84370. [Google Scholar] [CrossRef] [PubMed]
- Bayer, R.K.; Cagiao, M.E.; Calleja, F.B. Structure development in amorphous starch as revealed by X-ray scattering: Influence of the network structure and water content. J. Appl. Polym. Sci. 2006, 99, 1880–1886. [Google Scholar] [CrossRef]
- Jacobs, H.; Mischenko, N.; Koch, M.H.; Eerlingen, R.C.; Delcour, J.A.; Reynaers, H. Evaluation of the impact of annealing on gelatinisation at intermediate water content of wheat and potato starches: A differential scanning calorimetry and small angle X-ray scattering study. Carbohydr. Res. 1998, 306, 1–10. [Google Scholar] [CrossRef]
- Neven, L.G. Physiological effects of physical postharvest treatments on insects. HortTechnology 2003, 13, 272–275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).