Gene Silencing via Ingestion of Double-Stranded RNA in Wireworm of Agriotes Species
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Rearing of Wireworms in the Laboratory
2.2. Genomic DNA Extraction, PCR Amplification, and Sequencing of mtCOI Gene
2.3. Selection and Sequencing of Target Genes
2.4. In Vitro dsRNA Synthesis
2.5. Delivery of dsRNA by Liquid Ingestion Method
- a.
- Prior to feeding, wireworms were transferred to Petri dishes on moist filter paper and starved (without food) for 24 h in the dark at RT.
- b.
- The blue-coloured solution was freshly prepared by adding 1 mL of 0.5% sucrose solution to nuclease-free water (Invitrogen) and 8 µL of blue food colour dye in a 1.5 mL microcentrifuge tube.
- c.
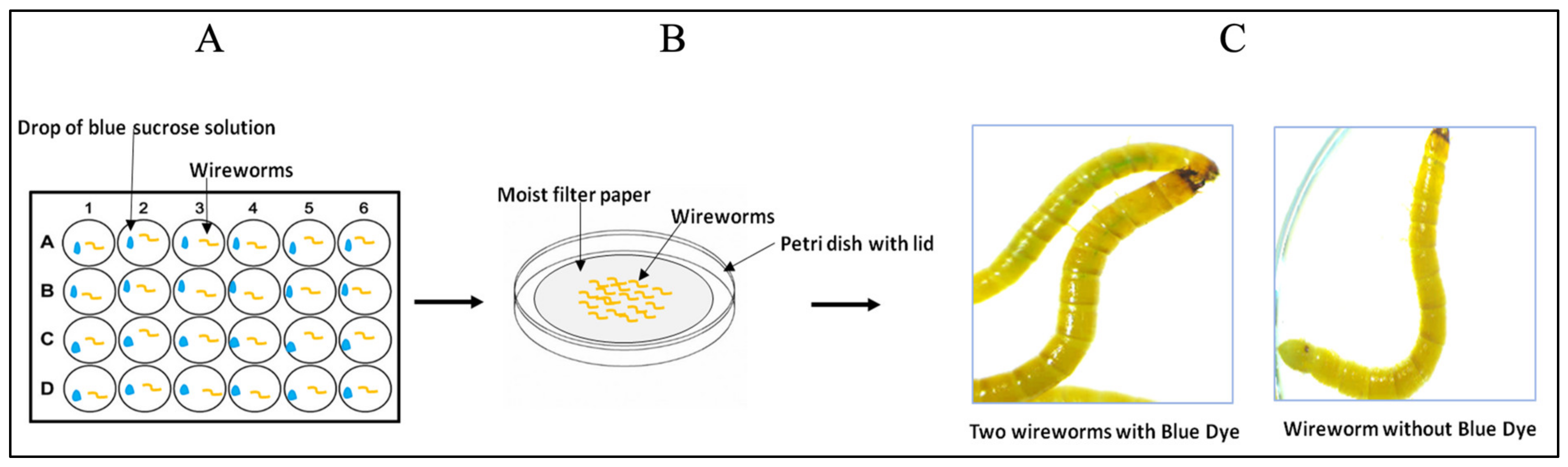
- On Day 1, one wireworm larva was placed in each well of a 24-well culture plate. Then, 4 µL droplets containing 2 µL blue-coloured sucrose solution (prepared above) and 2 µL of dsRNA (1 µg) were added, ensuring contact with the larvae mouthparts (Figure 2A). For the negative control, a droplet containing 2 µL of nuclease-free water (instead of dsRNA) and 2 µL of blue-coloured sucrose solution was added. The plate was covered and incubated for 90 min in the dark at RT.
- d.
- After the incubation, all the wireworms were transferred carefully with forceps from each well to a Petri dish (60 mm × 15 mm) (Figure 2B) and observed under a stereomicroscope (Leica, MZFLIII) to identify the ones that had ingested the blue-coloured sucrose solution (Figure 2C). The worms that showed a blue colour in their intestinal tract were only transferred to a new Petri dish (on moist filter paper), covered, and kept at RT in the dark for two days.
- e.
- On Day 3, all the wireworms were transferred again from their Petri dishes to a new 24-well culture plate, and steps “c” and “d” were repeated for the second feeding. The filter paper at the bottom of the Petri dish was made moist by misting the filter paper lightly with nuclease-free water.
- f.
- On Day 6, step “e” was repeated for the third feeding.
2.6. Gene Expression Analysis Using RT-qPCR
3. Results
3.1. Wireworm Rearing
3.2. Characterization of Agriotes spp. in Eastern Canada
3.3. Cloning and Sequencing of the Target Genes
3.4. Evaluation of the Liquid Ingestion Method
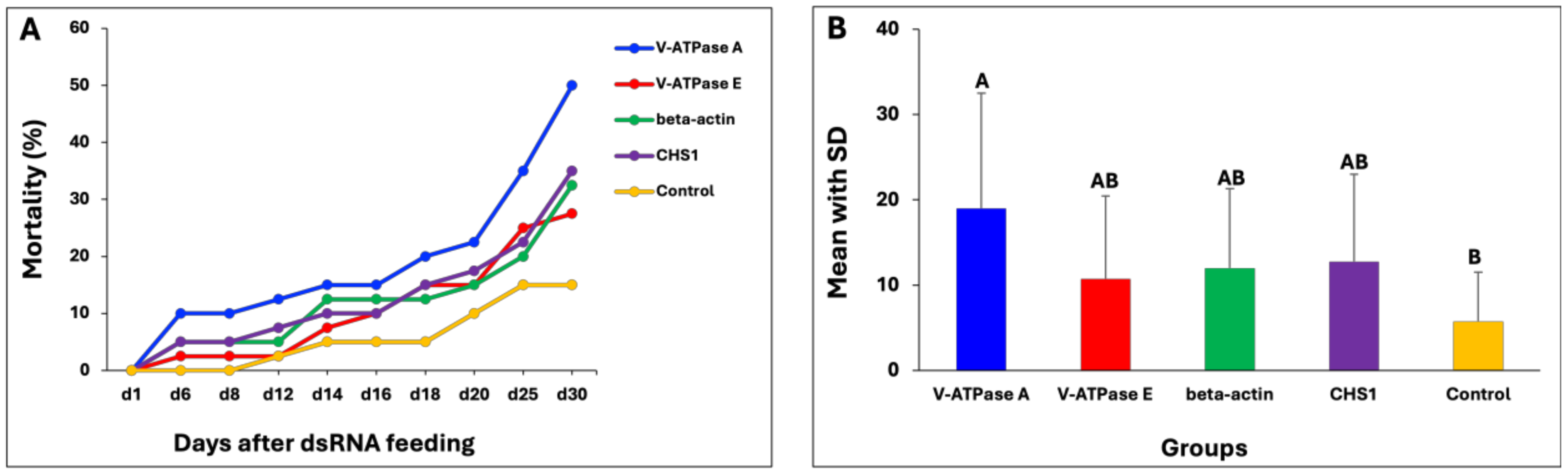
3.5. Effect of dsRNA in Wireworms
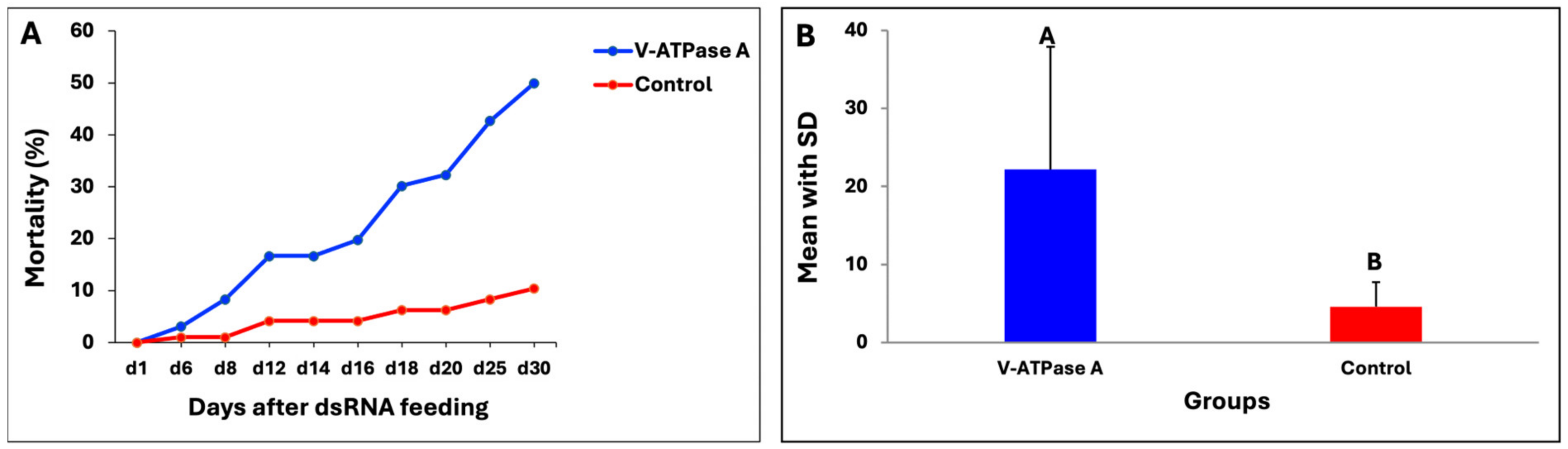
3.6. dsRNA Targeting V-ATPase A in Wireworms
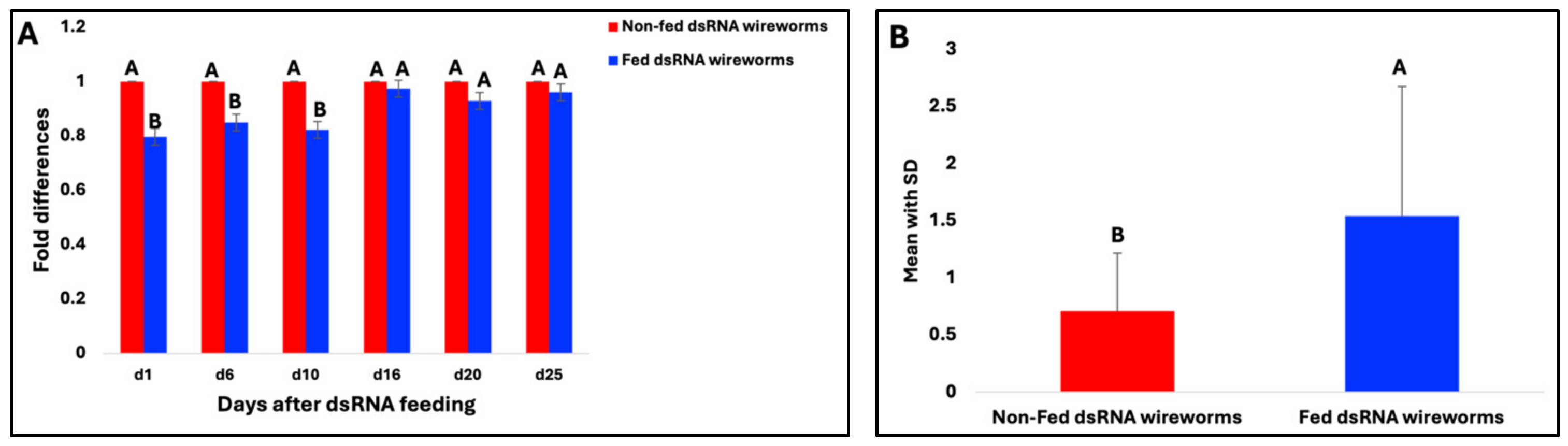
3.7. Gene Expression of V-ATPase A Target
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vernon, B.; van Herk, W. Wireworms as Pests of Potato. In Insect Pests of Potato; Elsevier: North York, ON, Canada, 2022; pp. 103–148. [Google Scholar]
- Vernon, R.S.; Tóth, M. Evaluation of Pheromones and a New Trap for Monitoring Agriotes lineatus and Agriotes obscurus in the Fraser Valley of British Columbia. J. Chem. Ecol. 2007, 33, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Vernon, R.S.; Kabaluk, T.; Behringer, A. Movement of Agriotes obscurus (Coleoptera: Elateridae) in Strawberry (Rosaceae) Plantings with Wheat (Gramineae) as a Trap Crop. Can. Entomol. 2000, 132, 231–241. [Google Scholar] [CrossRef]
- Furlan, L. The Biology of Agriotes ustulatus Schäller (Col., Elateridae). II. Larval Development, Pupation, Whole Cycle Description and Practical Implications. J. Appl. Entomol. 1998, 122, 71–78. [Google Scholar] [CrossRef]
- Furlan, L. The Biology of Agriotes ustulatus Schäller (Col., Elateridae). I. Adults and Oviposition. J. Appl. Entomol. 1996, 120, 269–274. [Google Scholar] [CrossRef]
- Traugott, M.; Schallhart, N.; Kaufmann, R.; Juen, A. The Feeding Ecology of Elaterid Larvae in Central European Arable Land: New Perspectives Based on Naturally Occurring Stable Isotopes. Soil. Biol. Biochem. 2008, 40, 342–349. [Google Scholar] [CrossRef]
- Staudacher, K.; Pitterl, P.; Furlan, L.; Cate, P.C.; Traugott, M. PCR-Based Species Identification of Agriotes Larvae. Bull. Entomol. Res. 2011, 101, 201–210. [Google Scholar] [CrossRef]
- Ellis, J.S.; Blackshaw, R.; Parker, W.; Hicks, H.; Knight, M.E. Genetic Identification of Morphologically Cryptic Agricultural Pests. Agric. For. Entomol. 2009, 11, 115–121. [Google Scholar] [CrossRef]
- Robert, S.; Vernon, A. Ground-Based Pheromone Trap for Monitoring Agriotes lineatus and A. obscurus (Coleoptera: Elateridae). J. Entomol. Soc. 2004, 101, 141–142. [Google Scholar]
- Vernon, R.; van Herk, W. Wireworm and Flea Beetle IPM in Potatoes in Canada: Implications for Managing Emergent Problems in Europe. Potato Res. 2017, 60, 269–285. [Google Scholar] [CrossRef]
- Health Canada Pest Management Regulatory Agency. Registration decision RD2020-16. Broflanilide, Cimegra, Teraxxa and Teraxxa F4. In Pest Management Regulatory Agency; Health Canada: Ottawa, ON, Canada, 2020; ISSN 1925-0878 2020. [Google Scholar]
- BASF Canada Inc. Cimegra®. Registration No. 33666. Mississauga, ON, Canada. 6 November 2020. [Google Scholar]
- Kabaluk, T. Promise versus Performance: Working toward the Use of Metarhizium anisopliae as a Biological Control for Wireworms. OBC/WPRS Bull. 2007, 30, 69–77. [Google Scholar]
- Brandl, M.A.; Schumann, M.; Przyklenk, M.; Patel, A.; Vidal, S. Wireworm Damage Reduction in Potatoes with an Attract-and-Kill Strategy Using Metarhizium brunneum. J. Pest Sci. 2017, 90, 479–493. [Google Scholar] [CrossRef]
- Noronha, C. Crop rotation as a management tool for wireworms in potatoes. IOBC/WPRS Bull. 2011, 66, 467–471. [Google Scholar]
- Jinek, M.; Doudna, J.A. A Three-Dimensional View of the Molecular Machinery of RNA Interference. Nature 2009, 457, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Whangbo, J.S.; Hunter, C.P. Environmental RNA Interference. Trends Genet. 2008, 24, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of Coleopteran Insect Pests through RNA Interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, G.; Wang-Pruski, G.; You, M. Phyllotreta striolata (Coleoptera: Chrysomelidae): Arginine Kinase Cloning and RNAi-Based Pest Control. Eur. J. Entomol. 2008, 105, 815–822. [Google Scholar] [CrossRef]
- Walshe, D.P.; Lehane, S.M.; Lehane, M.J.; Haines, L.R. Prolonged Gene Knockdown in the Tsetse Fly Glossina by Feeding Double Stranded RNA. Insect Mol. Biol. 2009, 18, 11–19. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Chandrashekar, K.; Thakur, N.; Verma, P.C.; Borgio, J.F.; Singh, P.K.; Tuli, R. RNA Interference for the Control of Whiteflies (Bemisia tabaci) by Oral Route. J. Biosci. 2011, 36, 153–161. [Google Scholar] [CrossRef]
- Burand, J.P.; Hunter, W.B. RNAi: Future in Insect Management. J. Invertebr. Pathol. 2013, 112, S68–S74. [Google Scholar] [CrossRef]
- Head, G.P.; Carroll, M.W.; Evans, S.P.; Rule, D.M.; Willse, A.R.; Clark, T.L.; Storer, N.P.; Flannagan, R.D.; Samuel, L.W.; Meinke, L.J. Evaluation of SmartStax and SmartStax PRO Maize against Western Corn Rootworm and Northern Corn Rootworm: Efficacy and Resistance Management. Pest. Manag. Sci. 2017, 73, 1883–1899. [Google Scholar] [CrossRef]
- Anderson, J.A.; Mickelson, J.; Challender, M.; Moellring, E.; Sult, T.; TeRonde, S.; Walker, C.; Wang, Y.; Maxwell, C.A. Agronomic and Compositional Assessment of Genetically Modified DP23211 Maize for Corn Rootworm Control. GM Crops Food 2020, 11, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Boeckman, C.J.; Cong, B.; Steimel, J.P.; Richtman, N.M.; Sturtz, K.; Wang, Y.; Walker, C.A.; Yin, J.; Unger, A.; et al. Characterization of DvSSJ1 Transcripts Targeting the Smooth Septate Junction (SSJ) of Western Corn Rootworm (Diabrotica virgifera virgifera). Sci. Rep. 2020, 10, 11139. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, H. The Insect V-ATPase, a Plasma Membrane Proton Pump Energizing Secondary Active Transport: Molecular Analysis of Electrogenic Potassium Transport in the Tobacco Hornworm Midgut. J. Exp. Biol. 1992, 172, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; Perzov, N.; Cohen, A.; Hagai, K.; Padler, V.; Nelson, H. The Cellular Biology of Proton-Motive Force Generation by V-ATPases. J. Exp. Biol. 2000, 203, 89–95. [Google Scholar] [CrossRef]
- Wieczorek, H.; Beyenbach, K.W.; Huss, M.; Vitavska, O. Vacuolar-Type Proton Pumps in Insect Epithelia. J. Exp. Biol. 2009, 212, 1611–1619. [Google Scholar] [CrossRef]
- Liu, X.-J.; Liang, X.-Y.; Guo, J.; Shi, X.-K.; Merzendorfer, H.; Zhu, K.Y.; Zhang, J.-Z. V-ATPase subunit A is Required for Survival and Midgut Development of Locusta migratoria. Insect Mol. Biol. 2022, 31, 60–72. [Google Scholar] [CrossRef]
- Lü, J.; Guo, M.; Chen, S.; Noland, J.E.; Guo, W.; Sang, W.; Qi, Y.; Qiu, B.; Zhang, Y.; Yang, C.; et al. Double-Stranded RNA Targeting VATPase B Reveals a Potential Target for Pest Management of Henosepilachna vigintioctopunctata. Pestic. Biochem. Physiol. 2020, 165, 104555. [Google Scholar] [CrossRef]
- Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Specht, C.A.; Tomoyasu, Y.; Lorenzen, M.D.; Kanost, M.; Beeman, R.W. The Tribolium Chitin Synthase Genes TcCHS1 and TcCHS2 Are Specialized for Synthesis of Epidermal Cuticle and Midgut Peritrophic Matrix. Insect Mol. Biol. 2005, 14, 453–463. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin Structure and Function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef]
- Mao, Y.-B.; Cai, W.-J.; Wang, J.-W.; Hong, G.-J.; Tao, X.-Y.; Wang, L.-J.; Huang, Y.-P.; Chen, X.-Y. Silencing a Cotton Bollworm P450 Monooxygenase Gene by Plant-Mediated RNAi Impairs Larval Tolerance of Gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Camargo, R.A.; Barbosa, G.O.; Possignolo, I.P.; Peres, L.E.P.; Lam, E.; Lima, J.E.; Figueira, A.; Marques-Souza, H. RNA Interference as a Gene Silencing Tool to Control Tuta absoluta in Tomato (Solanum lycopersicum). PeerJ 2016, 4, e2673. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.M.A.; Diab, M.R.; Abdelsattar, M.; Khalil, S.M.S. Characterization and RNAi-Mediated Knockdown of Chitin Synthase A in the Potato Tuber Moth, Phthorimaea operculella. Sci. Rep. 2017, 7, 9502. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Jiang, Y.-D.; An, X.; Yang, L.; Shang, F.; Niu, J.; Wang, J.-J. Effects of RNAi-Based Silencing of Chitin Synthase Gene on Moulting and Fecundity in Pea Aphids (Acyrthosiphon pisum). Sci. Rep. 2019, 9, 3694. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, J.; Palli, R.; Ferguson, J.; Palli, S.R. Ingested RNA Interference for Managing the Populations of the Colorado Potato Beetle, Leptinotarsa decemlineata. Pest. Manag. Sci. 2011, 67, 175–182. [Google Scholar] [CrossRef]
- Gu, L.; Knipple, D.C. Recent Advances in RNA Interference Research in Insects: Implications for Future Insect Pest Management Strategies. Crop Prot. 2013, 45, 36–40. [Google Scholar] [CrossRef]
- Scott, J.G.; Michel, K.; Bartholomay, L.C.; Siegfried, B.D.; Hunter, W.B.; Smagghe, G.; Zhu, K.Y.; Douglas, A.E. Towards the Elements of Successful Insect RNAi. J. Insect Physiol. 2013, 59, 1212–1221. [Google Scholar] [CrossRef]
- Koch, A.; Kogel, K.-H. New Wind in the Sails: Improving the Agronomic Value of Crop Plants through RNAi-Mediated Gene Silencing. Plant Biotechnol. J. 2014, 12, 821–831. [Google Scholar] [CrossRef]
- Hughes, A. Wireworm Management, Integrated Pest Management, PEI Department of Agriculture and Forestry. 2014. Available online: http://www.gov.pe.ca/photos/original/af_fact_wiremgm.pdf (accessed on 10 April 2017).
- Möller, E.M.; Bahnweg, G.; Sandermann, H.; Geiger, H.H. A Simple and Efficient Protocol for Isolation of High Molecular Weight DNA from Filamentous Fungi, Fruit Bodies, and Infected Plant Tissues. Nucleic Acids Res. 1992, 20, 6115–6116. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Vélez, A.M.; Khajuria, C.; Wang, H.; Narva, K.E.; Siegfried, B.D. Knockdown of RNA Interference Pathway Genes in Western Corn Rootworms (Diabrotica virgifera virgifera Le Conte) Demonstrates a Possible Mechanism of Resistance to Lethal dsRNA. PLoS ONE 2016, 11, e0157520. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xu, W.; Fu, K.; Guo, W.; Kim, D.S.; Zhang, J. Positional Effects of Double-Stranded RNAs Targeting β-Actin Gene Affect RNA Interference Efficiency in Colorado Potato Beetle. Pestic. Biochem. Physiol. 2022, 184, 105121. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.; Gillis-Madden, R. Wireworm Monitoring and Control. Available online: https://www.perennia.ca/wp-content/uploads/2020/05/WIREWORM-Fact-Sheet-final.pdf (accessed on 24 May 2021).
- Catton, H.; van Herk, W.; Saguez, J.; Svendsen, E. Guide to Pest Wireworms in Canadian Prairie Field Crop Production; Agriculture and Agri-Food Canada: Lethbridge, AB, Canada, 2021. [Google Scholar]
- Glen, R. Contributions to a Knowledge of the Larval Elateridae (Coleoptera): No. 3; Agriotes Esch. and Dalopius Esch. Can. Entomol. 1944, 76, 73–87. [Google Scholar] [CrossRef]
- Joshi, J.; Wang-Pruski, G. De Novo Transcriptome Assembly and Differential Gene Expression Analysis in Different Developmental Stages of Agriotes sputator (Click Beetle). Sci. Rep. 2024, 14, 24451. [Google Scholar] [CrossRef]
- Parker, W.E.; Howard, J.J. The Biology and Management of Wireworms (Agriotes spp.) on Potato with Particular Reference to the U.K. Agric. For. Entomol. 2001, 3, 85–98. [Google Scholar] [CrossRef]
- Bayer Group. Wireworms in Eastern Canada. Available online: https://www.cropscience.bayer.ca/en/articles/2021/wireworms-in-eastern-canada (accessed on 4 December 2024).
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA Uptake in Insects and Potential of RNAi for Pest Control: A Review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef]
- Hannon, G.J. RNA Interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef]
- Gordon, K.H.J.; Waterhouse, P.M. RNAi for Insect-Proof Plants. Nat. Biotechnol. 2007, 25, 1231–1232. [Google Scholar] [CrossRef]
- Price, D.R.G.; Gatehouse, J.A. RNAi-Mediated Crop Protection against Insects. Trends Biotechnol. 2008, 26, 393–400. [Google Scholar] [CrossRef]
- Kennerdell, J.R.; Carthew, R.W. Use of dsRNA-Mediated Genetic Interference to Demonstrate That Frizzled and Frizzled 2 Act in the Wingless Pathway. Cell 1998, 95, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Holtzman, S.; Kaufman, T.; Denell, R. Characterization of the Tribolium Deformed Ortholog and Its Ability to Directly Regulate Deformed Target Genes in the Rescue of a Drosophila Deformed Null Mutant. Dev. Genes Evol. 1999, 209, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.L.; Kaufman, T.C. RNAi Analysis of Deformed, Proboscipedia and Sex Combs Reduced in the Milkweed Bug Oncopeltus fasciatus: Novel Roles for Hox Genes in the Hemipteran Head. Development 2000, 127, 3683–3694. [Google Scholar] [CrossRef] [PubMed]
- Dzitoyeva, S.; Dimitrijevic, N.; Manev, H. Intra-Abdominal Injection of Double-Stranded RNA into Anesthetized Adult Drosophila Triggers RNA Interference in the Central Nervous System. Mol. Psychiatry 2001, 6, 665–670. [Google Scholar] [CrossRef]
- Turner, C.T.; Davy, M.W.; MacDiarmid, R.M.; Plummer, K.M.; Birch, N.P.; Newcomb, R.D. RNA Interference in the Light Brown Apple Moth, Epiphyas postvittana (Walker) Induced by Double-Stranded RNA Feeding. Insect Mol. Biol. 2006, 15, 383–391. [Google Scholar] [CrossRef]
- Bautista, M.A.M.; Miyata, T.; Miura, K.; Tanaka, T. RNA Interference-Mediated Knockdown of a Cytochrome P450, CYP6BG1, from the Diamondback Moth, Plutella xylostella, Reduces Larval Resistance to Permethrin. Insect Biochem. Mol. Biol. 2009, 39, 38–46. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Rieske, L.K.; Duan, J.J.; Mogilicherla, K.; Palli, S.R. Development of RNAi Method for Screening Candidate Genes to Control Emerald Ash Borer, Agrilus planipennis. Sci. Rep. 2017, 7, 7379. [Google Scholar] [CrossRef]
- Maori, E.; Paldi, N.; Shafir, S.; Kalev, H.; Tsur, E.; Glick, E.; Sela, I. IAPV, a Bee-Affecting Virus Associated with Colony Collapse Disorder Can Be Silenced by dsRNA Ingestion. Insect Mol. Biol. 2009, 18, 55–60. [Google Scholar] [CrossRef]
- Hunter, W.; Ellis, J.; vanEngelsdorp, D.; Hayes, J.; Westervelt, D.; Glick, E.; Williams, M.; Sela, I.; Maori, E.; Pettis, J.; et al. Large-Scale Field Application of RNAi Technology Reducing Israeli Acute Paralysis Virus Disease in Honey Bees (Apis mellifera, Hymenoptera: Apidae). PLoS Pathog. 2010, 6, e1001160. [Google Scholar] [CrossRef]
- Tabara, H.; Grishok, A.; Mello, C.C. RNAi in C. Elegans: Soaking in the Genome Sequence. Science 1998, 282, 430–431. [Google Scholar] [CrossRef]
- Maeda, I.; Kohara, Y.; Yamamoto, M.; Sugimoto, A. Large-Scale Analysis of Gene Function in Caenorhabditis elegans by High-Throughput RNAi. Curr. Biol. 2001, 11, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Christiaens, O.; Liu, J.; Niu, J.; Cappelle, K.; Caccia, S.; Huvenne, H.; Smagghe, G. Delivery of dsRNA for RNAi in Insects: An Overview and Future Directions. Insect Sci. 2013, 20, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.C.; Worby, C.A.; Simonson-Leff, N.; Muda, M.; Maehama, T.; Hemmings, B.A.; Dixon, J.E. Use of Double-Stranded RNA Interference in Drosophila Cell Lines to Dissect Signal Transduction Pathways. Proc. Natl. Acad. Sci. USA 2000, 97, 6499–6503. [Google Scholar] [CrossRef]
- Jain, R.G.; Robinson, K.E.; Asgari, S.; Mitter, N. Current Scenario of RNAi-based Hemipteran Control. Pest Manag. Sci. 2021, 77, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Terenius, O.; Papanicolaou, A.; Garbutt, J.S.; Eleftherianos, I.; Huvenne, H.; Kanginakudru, S.; Albrechtsen, M.; An, C.; Aymeric, J.-L.; Barthel, A.; et al. RNA Interference in Lepidoptera: An Overview of Successful and Unsuccessful Studies and Implications for Experimental Design. J. Insect Physiol. 2011, 57, 231–245. [Google Scholar] [CrossRef]
- Garbutt, J.S.; Bellés, X.; Richards, E.H.; Reynolds, S.E. Persistence of Double-Stranded RNA in Insect Hemolymph as a Potential Determiner of RNA Interference Success: Evidence from Manduca Sexta and Blattella Germanica. J. Insect Physiol. 2013, 59, 171–178. [Google Scholar] [CrossRef]
- Shukla, J.N.; Kalsi, M.; Sethi, A.; Narva, K.E.; Fishilevich, E.; Singh, S.; Mogilicherla, K.; Palli, S.R. Reduced Stability and Intracellular Transport of dsRNA Contribute to Poor RNAi Response in Lepidopteran Insects. RNA Biol. 2016, 13, 656–669. [Google Scholar] [CrossRef]
- Yoon, J.-S.; Gurusamy, D.; Palli, S.R. Accumulation of dsRNA in Endosomes Contributes to Inefficient RNA Interference in the Fall Armyworm, Spodoptera frugiperda. Insect Biochem. Mol. Biol. 2017, 90, 53–60. [Google Scholar] [CrossRef]
- Cooper, A.M.; Silver, K.; Zhang, J.; Park, Y.; Zhu, K.Y. Molecular Mechanisms Influencing Efficiency of RNA Interference in Insects. Pest Manag. Sci. 2019, 75, 18–28. [Google Scholar] [CrossRef]
- Xu, L.; Xu, S.; Sun, L.; Zhang, Y.; Luo, J.; Bock, R.; Zhang, J. Synergistic Action of the Gut Microbiota in Environmental RNA Interference in a Leaf Beetle. Microbiome 2021, 9, 98. [Google Scholar] [CrossRef]
- Zotti, M.; dos Santos, E.A.; Cagliari, D.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNA Interference Technology in Crop Protection against Arthropod Pests, Pathogens and Nematodes. Pest Manag. Sci. 2018, 74, 1239–1250. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Tang, X. RNAi-Based Pest Control: Production, Application and the Fate of dsRNA. Front. Bioeng. Biotechnol. 2022, 10, 1080576. [Google Scholar] [CrossRef]


| Gene Name | PCR Primer Sequences (5′–3′) | Annealing Tm (°C) | Product Length |
|---|---|---|---|
| PCR amplification and cloning | |||
| V-ATPase subunit A | F: RCGNGGNAAYGARATGTCNG R: GCCATCATNGANACRTTRTA | 57 | 207 nt Own design |
| V-ATPase subunit E | F: ATCATGGARTACTAYGARAARAARGAG R: GTTGCGWCCGAASAGMGCVTTWCGGATCTSSGG | 55 | 501 nt [18] |
| CHS1 | F: TTYGARTAYGCNATHGGNCAYTGG R: CCANCKRTCYTCNCCYTGRTCRTAYTG | 62 | 192 nt [31] |
| beta-actin | F: TCNATHATGAARTGYGAYGT R: CNCCDATCCANACNGARTAY | 51 | 187 nt Own design |
| In vitro dsRNA synthesis | |||
| V-ATPase subunit A | F: TAATACGACTCACTATAGGRCGNGGNAAYGARATGTCNG R: TAATACGACTCACTATAGGGCCATCATNGANACRTTRTA | ||
| V-ATPase subunit E | F: TAATACGACTCACTATAGGATCATGGARTACTAYGARAARAARGAG R: TAATACGACTCACTATAGGGTTGCGWCCGAASAGMGCVTTWCGGATCTSSGG | ||
| CHS1 | F: TAATACGACTCACTATAGGTTYGARTAYGCNATHGGNCAYTGG R: TAATACGACTCACTATAGGCCANCKRTCYTCNCCYTGRTCRTAYTG | ||
| beta-actin | F: TAATACGACTCACTATAGGTCNATHATGAARTGYGAYGT R: TAATACGACTCACTATAGGCNCCDATCCANACNGARTA | ||
| RT-qPCR | |||
| V-ATPase subunit A | F: CGAGCTCTCGGTGGAAATC R: AAATGGAAGCTTCACGAGCA | 65 | 107 nt Own design |
| beta-actin | F: CGCCAACACTGTACTCTCTGG R: CGATGATCTTGATCTTGATGG | 65 | 110 nt Own design |
| Target Genes | BLASTX Hits (Identity in %) with Reported Species |
|---|---|
| V-ATPase subunit A | 97%: Tribolium castenum (XP_976188.1) 97%: Diabrotica virgifera virgifera (XP_050506367.1) 95%: Dendroctonus ponderosae (XP_048525241.1) |
| V-ATPase subunit E | 79%: Agrilus planipennis (XP_018320654.1) 78%: Photinus pyralis (XP_031347368.1) 70%: Tribolium castenum (XP_970621.1) |
| CHS1 | 96%: Helicoverpa zea (AAG09738.1) 96%: Anabrus simple (WED299771) 95%: Phenacoccus solenopsis (AIE17035.1) |
| beta-actin | 100%: Agasicles hygrophila (ALP48321.1) 100%: Maruca vitrata (QYY49471.1) 100%: Rhipicephalus microplus (AAS09968.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, J.; Coffin, R.; Barrett, R.; Wang-Pruski, G. Gene Silencing via Ingestion of Double-Stranded RNA in Wireworm of Agriotes Species. Insects 2024, 15, 983. https://doi.org/10.3390/insects15120983
Joshi J, Coffin R, Barrett R, Wang-Pruski G. Gene Silencing via Ingestion of Double-Stranded RNA in Wireworm of Agriotes Species. Insects. 2024; 15(12):983. https://doi.org/10.3390/insects15120983
Chicago/Turabian StyleJoshi, Jyoti, Robert Coffin, Ryan Barrett, and Gefu Wang-Pruski. 2024. "Gene Silencing via Ingestion of Double-Stranded RNA in Wireworm of Agriotes Species" Insects 15, no. 12: 983. https://doi.org/10.3390/insects15120983
APA StyleJoshi, J., Coffin, R., Barrett, R., & Wang-Pruski, G. (2024). Gene Silencing via Ingestion of Double-Stranded RNA in Wireworm of Agriotes Species. Insects, 15(12), 983. https://doi.org/10.3390/insects15120983






