Simple Summary
Drosophila suzukii, a major pest in cherry orchards, shows distinct oviposition preferences influenced by cherry cultivar traits such as the color, firmness, and sugar content. In this study, D. suzukii laid the highest number of eggs on fruits of the “Hongdeng” and “Burlat” cherry cultivars, and the number of eggs laid on fruits of other cultivars significantly increased with the fruit maturity. These preferences correlated with the fruit characteristics, as darker-colored cherries consistently received more eggs. The developmental parameters of D. suzukii varied among the cultivars, with “Hongdeng” cherries supporting the highest pupation rate and survival rate. The analysis further showed that darker color parameters (lightness (L*) and chromaticity (b*)) were negatively correlated with the oviposition, while the CIRG (color index for red grapes) values were positively associated with increased egg-laying and pupation rates. The findings confirmed the common notion that “rotting fruit attracts flies”, and D. suzukii is no exception. The results also demonstrate that the fruit color plays a role in the host selection process of D. suzukii, providing valuable insights for developing targeted pest management strategies in cherry production.
Abstract
Drosophila suzukii is a major pest of sweet cherries. In this study, we evaluated its oviposition preferences across six cherry cultivars and assessed the effects of the fruit traits on its growth and development. Significant differences in the color, firmness, and sugar content were observed among the cultivars and ripeness stages. The highest oviposition rates were recorded for the “Hongdeng” (HD) and “Burlat” (BLT) cultivars in both the non-choice (HD: 31.9 ± 2.0 eggs/cherry; BLT: 31.3 ± 1.9 eggs/cherry) and choice (HD: 32.4 ± 3.2 eggs/cherry; BLT: 27.6 ± 1.9 eggs/cherry) tests, largely influenced by the fruit color. While the developmental parameters showed slight variation across the cultivars, significant differences were observed in the pupation rates (ranging from 0.80 to 0.89) and survival rates (ranging from 0.51 to 0.62), with both parameters being the highest for the “Hongdeng” cultivar. The correlation analysis revealed that a darker fruit color—characterized by lower lightness (L*), lower chromaticity (b*), and a higher color index for red grapes (CIRG)—positively influenced the oviposition and pupation rates. Although the other correlations were not significant, the oviposition and developmental parameters were positively correlated with sweetness and negatively correlated with firmness. These findings enhance our understanding of how cherry traits influence D. suzukii behavior, providing critical insights for pest management strategies in cherry production.
1. Introduction
Drosophila suzukii (Mastumura), commonly known as the spotted-wing drosophila, is a major fruit pest worldwide. It possesses a well-developed, serrated ovipositor, which allows it to lay eggs in unripe fruit, causing severe damage to soft-skinned stone fruits such as cherries [1,2] and leading to significant economic losses [3,4,5]. Sweet cherry (Prunus avium L.), belonging to the Rosaceae family, is one of the most popular temperate fruits and has great economic importance [6,7,8]. In recent years, China has emerged as the largest consumer of sweet cherries globally, as highlighted by the USDA’s annual report. By 2024/2025, the Chinese cherry production reached 850,000 tons, with a planting area of approximately 199,000 hectares, ranking first in the world [9]. Sweet cherries are among the key economic fruit crops in China, playing a vital role in enhancing agricultural efficiency, increasing farmers’ income, and promoting local economic development [10]. The earliest report of D. suzukii infestation in sweet cherries was in California, USA, in 2009 [11], while in China, the first occurrence was reported in 2013 in late-maturing sweet cherries in Shandong Province [12]. Currently, the pest is widespread across the major cherry-producing regions in China, severely affecting the industry’s development [13,14].
The oviposition preference of D. suzukii is influenced by various fruit characteristics, including the color [15,16,17], firmness [18,19], nutrients [19], and ripeness [20]. Studies have shown that D. suzukii can assess the suitability of different fruits as hosts based on the color reflection of the fruit [16]. Field experiments have revealed that D. suzukii tends to lay eggs on grapes with softer skins, and indoor tests on six types of berries from vineyards also confirmed this preference [18]. Additionally, although fruit nutrients, such as acids, can affect the development of the larvae, they do not have a significant impact on the hatching of adults [19]. Drosophila suzukii is particularly attracted to overripe strawberries, and its attraction increases as the fruit ripens [20]. Further research has shown that D. suzukii exhibits significant differences in its oviposition preferences among different cherry cultivars, favoring those with higher glycogen contents, lower pectin contents, and softer flesh [21].
China’s sweet cherry industry boasts remarkable genetic diversity, with over 90 cultivars currently grown, including both internationally renowned and domestically bred varieties. Cultivars such as “Tieton” (USA), “Burlat” (France), and “Rainier” (USA) are widely cultivated and highly regarded for their quality. Domestically bred cultivars have also gained prominence in recent decades. For example, “Hongdeng”, developed by the Institute of Pomology at the Dalian Academy of Agricultural Sciences (DAAS), was the first-generation main cultivar in China and played a pivotal role in the early development of the country’s cherry industry. Additionally, “Caihong”, bred by the Institute of Pomology and Forestry at the Beijing Academy of Agriculture and Forestry Sciences (BAAFS), is another widely cultivated variety. More recently, “Xiangquan No. 1”, also developed by the BAAFS, became the first self-fertile sweet cherry cultivar released in China, marking a significant breakthrough in breeding for self-fertility [22].
In practical production, cherry orchards often grow multiple cultivars, which show differences in their physiological and phenological characteristics [23]. The relationship between these differences among cultivars and the oviposition preference, growth, and development of D. suzukii require further investigation. Therefore, we hypothesize that D. suzukii exhibits differential oviposition preferences among different cherry cultivars, favoring more mature and higher-quality fruits, which may provide better conditions for the growth and development of its offspring. To test this hypothesis, we selected six major cherry cultivars with a gradient of physiological characteristics. In this study, we aimed to (1) measure the physiological traits of different cherry cultivars, including the fruit color, firmness, and sugar content; (2) evaluate the oviposition preferences of D. suzukii across these cherry cultivars; and (3) investigate the effects of different cherry cultivars on the growth, development, and emergence of D. suzukii and analyze the correlations among the oviposition preference, developmental parameters, and physiological characteristics of cherry fruits. We further aimed to elucidate the mechanisms underlying the D. suzukii oviposition behavior, providing a theoretical foundation for breeding cherry cultivars with pest resistance, and offering scientific guidance for integrated pest management in cherry production to effectively address the threat posed by D. suzukii.
2. Materials and Methods
2.1. Insect Colony
The experimental insect population was a laboratory strain of D. suzukii, reared since 2022, whose diet formulation was based on the recipe described by Liu et al. [24], with a minor modification: the replacement of 2 mL of the vitamin mixture with 2 g of insect vitamin, specifically the Vanderzant vitamin mixture for insects (catalog No. V1007-100G; Sigma-Aldrich, St. Louis, MO, USA). The diet mainly consisted of wheat bran, sucrose, brewer’s yeast powder, preservatives, and vitamins. Rearing and experimental conditions included maintaining at 24 ± 1 °C, a photoperiod of L:D = 16 h:8 h, and 70 ± 5% relative humidity. Adults aged 1–5 days post-eclosion from the same cohort were selected for the subsequent oviposition preference trials.
2.2. Test Fruits
The test cherry fruits were collected from a cherry orchard in Tongzhou District, Beijing (116°42′ E, 39°41′ N). Based on differences in the fruit color and ripeness time, six cherry cultivars were selected: “Hongdeng” (HD), “Tieton” (TT), “Burlat” (BLT), “Xiangquan No.1” (XQ1), “Rainier” (RN), and “Caihong” (CH). Among them, HD, TT, and BLT are early-ripening cultivars, while XQ1, RN, and CH ripen later. From 16 May to 12 June 2024, fruits were collected weekly for a total of five harvests. Due to the differences in the ripeness periods, the number of collection operations varied among the cultivars, and the collection times were categorized into the ripe and overripe stages (see Table S1). For each cultivar, fruits were randomly harvested from two rows, with approximately 15 trees per row, ensuring an adequate number of fruits for the experiments. After collection, the fruits were brought to the laboratory, where those with uniform size, clean surfaces, and no signs of pest damage or mechanical injury were selected for the physiological measurements and subsequent experiments. All cherry cultivars were cultivated in the same orchard, with trees approximately 10 years old. Standard agricultural practices were followed, and no insecticides were used before or during fruit maturation. Lime sulfur was applied before bud break in early spring, and mancozeb was sprayed during full bloom.
2.3. Measurement of Cherry Fruit Color, Firmness, and Sugar Content
2.3.1. Fruit Color
The color of the cherry fruits was measured by using an LS173 colorimeter (Linshang Technology Co., Ltd., Shenzhen, China). To ensure consistency and minimize measurement error, all measurements were conducted by the same operator. For each cherry cultivar, 30 fruits with uniform ripeness were selected, and color measurements were taken at three points on the fruit: the equator, the top, and the bottom. At each ach position, measurements were taken three times, and the average value was used as the color parameter for the fruit (lightness (L*) and chromaticity (a* and b*) values). L* represents lightness, ranging from 0 (black) to 100 (white). a* indicates the red–green axis, with positive values toward red and negative values toward green. b* denotes the yellow–blue axis, with positive values toward yellow and negative values toward blue [25,26]. From these, the color saturation [C* = (a2 + b2)1/2], hue angle [h° = arctan(b*/a*)] [27], and color index of red grapes (CIRG) [CIRG = (180 h°)/(L* + C*)] [28,29] were calculated. The fruit color was classified based on the CIRG value as follows: CIRG < 2, yellow-green; 2 < CIRG < 4, pink; 4 < CIRG < 5, red; 5 < CIRG < 6, dark red; CIRG > 6, blue-black [30].
2.3.2. Fruit Firmness
The fruit firmness was measured by using a GY-4 analog fruit firmness tester (Aidebao Instrument Co., Ltd., Leqing, China). To ensure data accuracy, all measurements were conducted by the same operator. An 8 mm diameter probe was used with a penetration depth of 5 mm, and the maximum value recorded was taken as the firmness of the fruit. For each cultivar, 30 fruits of uniform size were selected, and the firmness was measured at the top, middle, and bottom of the fruit. The hardest area among the three was chosen as the firmness value for that fruit.
2.3.3. Fruit Sugar Content
The sugar content of the cherry fruits was measured by using a PAL-1 refractometer (ATAGO Scientific Instruments Co., Ltd., Guangzhou, China). To minimize human error, all measurements were conducted by the same operator. For each cultivar, 30 fruits of uniform size were selected, and the flesh from the equatorial region of each fruit was blended into a homogenate. The juice was then filtered through gauze, and a thin layer of the juice was evenly applied to the PAL-1 refractometer’s prism for measurement. Each fruit’s sugar content was measured three times, and the average value was recorded as the sugar content of the fruit. The unit for sugar content data is °Brix. °Brix, or °Bx, is a unit used to measure the soluble solid content in a solution, primarily for assessing the sugar contents in fruits, juices, and products such as wine. One degree Brix is 1 g of sucrose in 100 g of solution (1°Brix = 1% sugar) [31].
2.4. Oviposition Preference Test of Drosophila suzukii on Fruits of Different Cherry Cultivars
2.4.1. Non-Choice Oviposition
Six whole cherry fruits of different cultivars were individually placed into custom-made insect rearing boxes (volume of 450 mL; top diameter of 11 cm; bottom diameter of 8.5 cm; height of 7 cm; catalog No. 12189879065; Labahua, Xiamen, China). A hole was made in the center of each box lid and covered with 120-mesh gauze to ensure ventilation. Ten adult D. suzukii flies, aged 1 to 5 days (sex ratio of 1:1), were introduced into each box for mating and oviposition. The boxes were kept in an artificial climate chamber (model RXZ-328A; Ningbo Jiangnan Instrument Factory, Ningbo, China) under controlled conditions: a temperature of 24 ± 1 °C, a photoperiod of L:D = 16 h:8 h, and a relative humidity of 70 ± 5%. After 24 h, the adult flies were removed, and each fruit was examined with a stereomicroscope (SZ51; Olympus Corporation, Tokyo, Japan) to assess the oviposition. The number of eggs laid on each fruit was recorded. Each cherry cultivar had eight replicates.
2.4.2. Choice Oviposition
The choice oviposition test was conducted only during the overripe stage, following a similar procedure to that of the non-choice oviposition test. Cherry fruits of different cultivars were placed in custom-made insect rearing boxes with their lids kept open. The number of cultivars used depended on the fruit collection availability on the day (see Table S1). The rearing boxes were then placed inside the insect rearing cages (dimensions: 20 cm × 20 cm × 20 cm; AIPU INSTRUMENT; Hangzhou, China), where 40–60 adult D. suzukii flies (sex ratio of 1:1) were introduced. The cages were kept in an artificial climate chamber under the same conditions as those of the non-choice oviposition test. After 24 h, the adult flies were removed, and the number of eggs on each fruit was counted under a stereomicroscope. Each cultivar had eight replicates, and the fruits from different cultivars were randomly positioned within the cage.
2.5. Measurement of Growth and Development Parameters of Drosophila suzukii on Different Cherry Cultivars
2.5.1. Development Periods
In the overripe stage of the cherries, fully mature and pest-free fruits from different cultivars were selected for the experiment. The cherry fruits were placed in a D. suzukii adult rearing cage (dimensions: 35 cm × 35 cm × 35 cm; AIPU INSTRUMENT; Hangzhou, China), and after 24 h, the fruits were removed to ensure that the adult flies had sufficient time to lay eggs. The hatching of larvae was observed and recorded daily, and newly hatched larvae were individually transferred into a 24-well cell culture plate (REF 3524, Corining Incorporated, Kennebunk, ME, USA), where they were fed with the flesh of the same cherry cultivar. The egg-to-larva period (Egg–Larva Period) and the pupal developmental period (Pupal Period) were recorded. Four replicate groups were set up for each cherry cultivar, with 24 larvae in each group. The experiment was conducted in an artificial climate chamber under the same environmental conditions as previously described.
2.5.2. Other Growth and Development Parameters
The six cherry cultivars were placed in separate D. suzukii rearing cages (dimensions: 35 cm × 35 cm × 35 cm; AIPU INSTRUMENT; Hangzhou, China) for 24 h to allow for oviposition. Afterward, the fruits were removed, and the number of eggs laid on the fruits of each cultivar was counted to ensure that each had approximately 150 eggs. Any excess eggs were carefully removed by using sterilized insect pins. Four replicate experiments were conducted for each cultivar. The cherry fruits (approximately four fruits per cultivar) were then individually placed in insect rearing boxes and kept in an artificial climate chamber under the same conditions as previously described. The numbers of larvae hatched, pupae formed, and emerged adults and the sex ratio of the adults were recorded daily. Based on these observations, the following parameters were calculated: hatching rate = number of larvae hatched/total number of eggs; pupation rate = number of pupae/total number of larvae; eclosion rate = number of emerged adults/total number of pupae; survival rate = number of emerged adults/total number of eggs; female ratio = number of female adults/total number of adults.
2.6. Data Analysis
All data analyses were performed with R 4.4.0 software. First, a generalized linear model (GLM) was used to analyze the effects of the cultivar, ripeness stage, and their interaction on the physiological parameters of the cherries, with t-tests conducted to compare the ripe and overripe stages for the same parameter. The same approach was applied to the non-choice oviposition data, while the choice oviposition data, which did not meet the assumption of the homogeneity of variance, were analyzed by using non-parametric tests. For the developmental parameters of D. suzukii, the normality and homogeneity of variance were first tested; if the assumptions were met, then a one-way ANOVA followed by Tukey’s HSD post hoc tests were used; otherwise, Kruskal–Wallis tests and Dunn’s test were applied. Pearson correlation coefficients were calculated by using the cor() function to assess the linear relationships between the variables, and a correlation matrix was generated. Bar plots and linear regression plots were created by using the ggplot2 package.
3. Results
3.1. Physiological Parameters of Different Cherry Cultivars
The physiological parameters of the cherry fruits, including the color (L*, a*, and b*), firmness, and sweetness, showed significant differences between the cultivars and across the different ripeness stages (ripe and overripe stages) (p < 0.001; see Table S2). The color parameters (L*, a*, and b*), which were used to calculate the color index for red grapes (CIRG), generally had higher average values in the ripe stage than in the overripe stage, with the exception of a* in XQ1 and CH and b* in RN (see Tables S3 and S4). As the fruit matured, the cherry fruit’s color (CIRG) deepened, and the firmness decreased. Among the cultivars, BLT consistently had the highest CIRG value and the lowest firmness. In terms of the sugar content, with the exception of XQ1 having a higher value in the ripe stage, the other cultivars all showed higher sugar contents in the overripe stage. Notably, HD and BLT exhibited the highest sugar contents throughout the entire period (see Tables S3 and S4 and Figure 1).
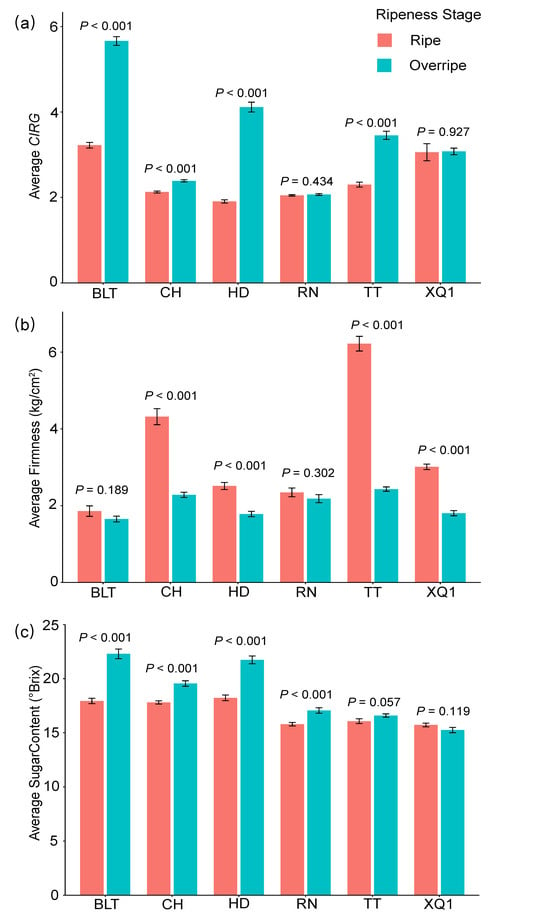
Figure 1.
CIRG (color index of red grapes), firmness, and sugar content in ripe and overripe stages of different cherry cultivars. (a) CIRG, (b) firmness, (c) sugar content. The p-values indicated in the figure represent the significance of the differences between the different ripeness stages. p < 0.05 indicates a significant difference, while p ≥ 0.05 indicates no significant difference. The bar values in the column chart represent standard errors (SDs).
3.2. Oviposition of Drosophila suzukii on Different Cherry Cultivars
3.2.1. Non-Choice Oviposition
In the non-choice oviposition experiment, D. suzukii exhibited significant differences in its oviposition on fruits of different cherry cultivars (F5, 162 = 39.45, p < 0.001) and in different ripeness stages (F1, 161 = 32.29, p < 0.001). Specifically, there was no significant difference in the oviposition on the HD and BLT cultivar fruits between the ripe and overripe stages, whereas for the other cultivars, the oviposition was significantly higher during the overripe stage than during the ripe stage (see Table S5, Figure 2). In the ripe stage, the highest average oviposition was observed on the HD cherries, with an average of 32.0 ± 4.8 eggs per cherry, followed by BLT, TT, XQ1, RN, and CH, with CH having the lowest average oviposition at 4.1 ± 0.6 eggs per cherry. In the overripe stage, the HD cherries again had the highest average oviposition at 31.9 ± 2.0 eggs per cherry, followed by BLT, XQ1, TT, RN, and CH, where CH had the lowest average oviposition at 12.1 ± 1.3 eggs per cherry (see Table S5, Figure 2). The data are presented as average values ± standard errors (SDs). Throughout the entire period, the oviposition was consistently the highest on the HD and BLT cultivar fruits, while it was the lowest on the RN and CH cultivar fruits (see Figure 2).
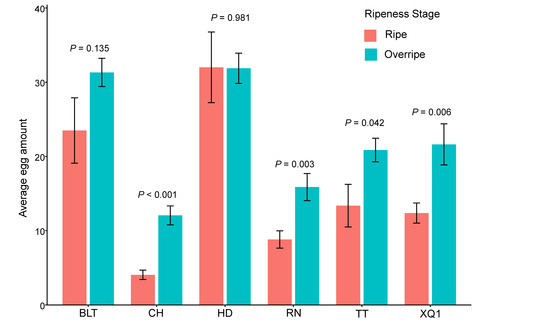
Figure 2.
The non-choice oviposition of Drosophila suzukii on fruits of different cherry cultivars and at different ripeness stages. The p-values indicated in the figure represent the significance of the differences between the different maturity stages. p < 0.05 indicates a significant difference, while p ≥ 0.05 indicates no significant difference. The bar values in the column chart represent standard errors (SDs).
3.2.2. Choice Oviposition
The choice oviposition of D. suzukii on fruits of different cherry cultivars during the overripe stage was compared, and the results show significant differences among the cultivars (χ2 = 29.10, df = 5, p < 0.001). The average number of eggs laid, from the highest to the lowest, was as follows: HD (32.4 ± 3.2 eggs), BLT (27.6 ± 1.9 eggs), XQ1 (22.44 ± 3.212 eggs), TT (22.4 ± 1.6 eggs), CH (15.3 ± 2.2 eggs), and RN (14.5 ± 2.3 eggs). The average oviposition on the HD and BLT fruits was significantly higher than that on the CH and RN fruits (see Figure 3).
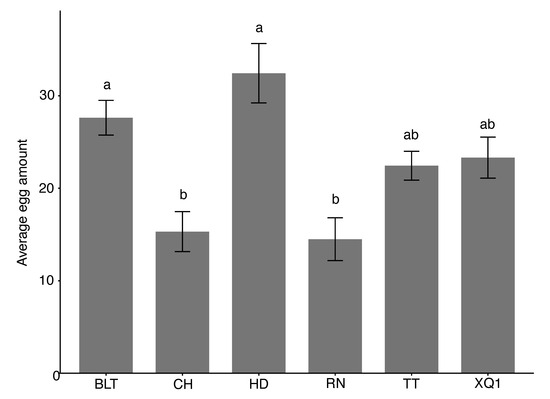
Figure 3.
The choice oviposition of Drosophila suzukii on fruits of different cherry cultivars during the overripe stage. Different lowercase letters above the bars indicate significant differences, while the same letters indicate no significant differences. The bar values in the column chart represent standard errors (SDs).
3.3. Growth and Development Parameters of Drosophila suzukii on Fruits of Different Cherry Cultivars
For assessing the developmental time of D. suzukii, the Egg–Larva Period and Pupal Period were recorded. There were significant differences in the Egg–Larva Period among the different cherry cultivars (χ2 = 41.07, df = 5, p < 0.001), with the average development duration ranging from 4.82 to 4.98 d. The order from the longest to the shortest was HD > TT > XQ1 > BLT > CH > RN. The average Pupal Period ranged from 4.70 to 4.88 d, with RN having the longest duration and XQ1 the shortest. Additionally, the hatching, pupation, eclosion, and survival rates were calculated. Both the pupation rate (F5, 42 = 2.743, p = 0.031) and survival rate (F5, 42 = 2.658, p = 0.036) showed significant differences among the cultivars. The ranking of the pupation rate from the highest to the lowest was HD > BLT > TT > XQ1 > RN > CH, while for the survival rate, the order was HD > BLT > XQ1 > TT > CH > RN. The average hatching rates and eclosion rates ranged from 0.83 to 0.89 and from 0.74 to 0.81, respectively (see Table 1). The average female ratio ranged from 0.58 to 0.67, with HD and BLT showing lower values, both below 0.60 (see Table 1).

Table 1.
Growth and developmental parameters of Drosophila suzukii on different cherry cultivars.
3.4. Correlation Analysis of Choice Oviposition, Developmental Parameters, and Fruit Physiological Parameters
The choice oviposition showed a significant negative correlation with the color parameters L* (r = −0.85, p = 0.030) and b* (r = −0.89, p = 0.019), and a marginally significant positive correlation with the CIRG (r = 0.80, p = 0.055) (see Table 2, Figure 4). The choice oviposition was negatively correlated with the fruit firmness and positively correlated with the sugar content; however, these correlations were not significant (see Table 2).

Table 2.
The correlations of choice oviposition, the developmental parameters of Drosophila suzukii, and the cherry physiological parameters.
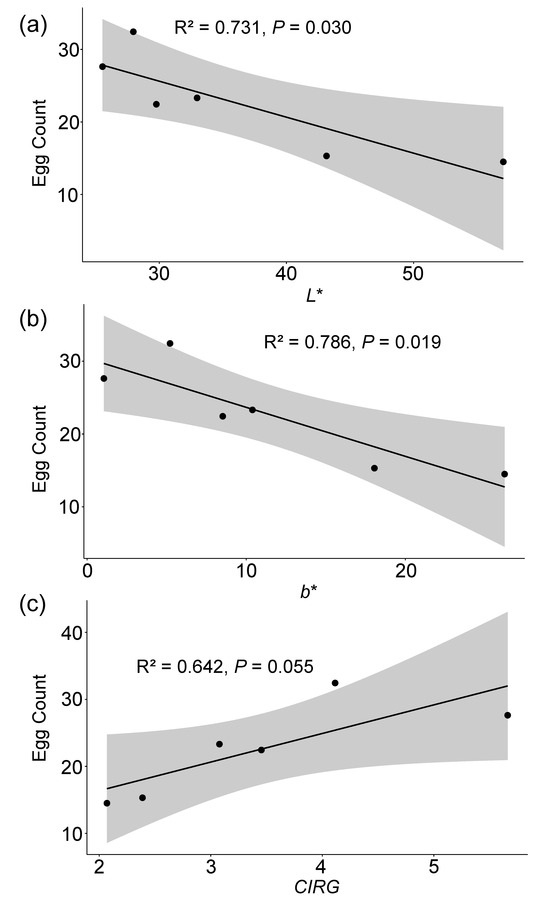
Figure 4.
Scatter plots of choice oviposition of Drosophila suzukii vs. cherry physiological parameters (L*, b*, and CIRG) with regression line and confidence interval. (a) Egg count vs. L*, (b) egg count vs. b*, and (c) egg count vs. CIRG. L* represents lightness, b* indicates the yellow–blue axis, and CIRG denotes the color index of red grapes. R2 represents the proportion of variance explained by the model in the regression analysis. p-values indicate the significance levels of the correlations.
The Egg–Larva Period also exhibited a significant negative correlation with the L* (r = −0.88, p = 0.020), and the hatching rate was also significantly negatively correlated with the L* (r = −0.82, p = 0.047). Additionally, the pupation rate was significantly negatively correlated with the b (r = −0.82, p = 0.044) and positively correlated with the CIRG (r = 0.84, p = 0.035) (see Table 2, Figure 5). The Egg–Larva Period was negatively correlated with the firmness and positively correlated with the sugar content; the Pupal Period showed positive correlations with both the firmness and sugar content. The female ratio was positively correlated with the firmness and negatively correlated with the sugar content. Additionally, the hatching, pupation, and eclosion rates were all negatively correlated with the firmness and positively correlated with the sugar content. However, none of these correlations was significant (see Table 2).
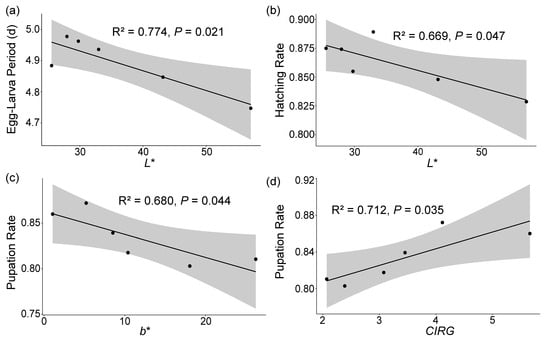
Figure 5.
The scatter plot of significant correlations between the developmental parameters of Drosophila suzukii and the cherry physiological parameters with the regression line and confidence interval. (a) Egg–Larva Period vs. L*, (b) hatching rate vs. L*, (c) pupation rate vs. b*, (d) pupation rate vs. CIRG. L* represents lightness, b* indicates the yellow–blue axis, and CIRG denotes the color index of red grapes. R2 represents the proportion of variance explained by the model in the regression analysis. p-values indicate the significance levels of the correlations.
4. Discussion
Overall, as the fruit ripened, the cherry fruit color (CIRG) deepened, the firmness decreased, and the sugar content increased. Notably, fruits of the “Hongdeng” and “Burlat” cultivars exhibited higher color intensity and sugar content, along with lower firmness. In both the non-choice and choice oviposition tests, D. suzukii demonstrated a preference for these two cultivars. The development of D. suzukii was significantly influenced by the cherry cultivar, with notable differences observed in the Egg–Larva Period and pupation rate. Drosophila suzukii preferred to oviposit on fruits with lower firmness and higher sugar content, where its development was also enhanced. Particularly, the choice oviposition and certain developmental parameters were significantly correlated with the fruit color (L*, b*, and CIRG). These findings further confirm the importance of the fruit firmness and sugar content in the D. suzukii oviposition choice and provide direct evidence of the impact of the fruit color and ripeness on D. suzukii’s oviposition preference and fitness.
The findings indicated that D. suzukii preferred to oviposit on darker-colored fruits, where its growth and development were also more favorable. Previous research has shown that darker colors, such as black, red, and green, have an attractive effect on D. suzukii [17]. In this study, the cultivars with higher oviposition egg counts, including Burlat, Hongdeng, and Tieton, all had red fruits that turned deep red in the overripe stage. However, other studies have indicated that the contrast between fruit and the leaf color, rather than the fruit color alone, might play a more crucial role in orientation and attraction in D. suzukii [15,32].
The fruit firmness and sugar content influence the oviposition preferences of D. suzukii. Although, in this study, the choice oviposition showed no significant correlation with the fruit firmness or sugar content, a negative correlation with the firmness and a positive correlation with the sugar content were still observed as trends. This aligns with previous studies that found that D. suzukii prefers to oviposit on softer-skinned grapes or berries [18,19,33]. Furthermore, as the °Brix level increases, more eggs are laid by D. suzukii [34]. The oviposition preference of D. suzukii on intact grapes of different cultivars was positively correlated with the glycogen content [35], and similarly, the glycogen content in cherry cultivars was significantly positively correlated with both choice and non-choice oviposition on cherry slices [21].
The primary fruit growth and development factor impacting the growth and development of D. suzukii is color. Specifically, darker and deeper fruit colors are associated with higher pupation and hatching rates in D. suzukii. We found that fruits with deeper red hues and lower firmness tended to have higher sugar contents. The fruit’s nutritional components, especially the sugar content, affect its suitability for D. suzukii. In this study, D. suzukii was more likely to complete its full developmental cycle successfully in fruits with higher °Brix levels. As the °Brix levels in blackberries, blueberries, cherries, raspberries, and strawberries increase, more D. suzukii emerge as adults [34]. Similarly, there is a significant positive correlation between the °Brix in tart cherries (Prunus cerasus L.) and the number of larvae and adults per gram of fruit [36], consistent with our findings. Other research has shown that higher sugar contents in fruits not only shortens the developmental time of D. suzukii but also significantly increases the average wing length in adults [19]. However, in our study, while the developmental duration tended to increase with higher °Brix levels, the average developmental periods showed little variation among the cultivars. The egg-to-larva stage ranged from 4.82 to 4.98 d, and the pupal stage ranged from 4.70 to 4.88 d. Further refinement in future studies should focus on more detailed developmental stages to better elucidate the specific effects of fruit physiological traits on growth and development.
As fruit ripens, it becomes softer, darker in color, and sweeter, which makes it more attractive for D. suzukii oviposition. Similarly, it has also been found that D. suzukii prefers feeding on decaying fruits and tends to lay eggs more readily on ripe fruits [37]. These results provide theoretical support for the commonly held notion that “overripe fruits are more attractive to fruit flies”.
The oviposition choice of D. suzukii does not necessarily correlate directly with the fitness of its offspring. In this study, the choice oviposition of D. suzukii showed a significant positive correlation with the pupation rate (r = 0.93, p = 0.007) and the survival rate (r = 0.97, p = 0.002), with no notable correlation with the other developmental parameters. Research has shown that D. suzukii prefers to lay eggs on a wild fruit, bird cherry (Prunus padus L.), although the adult emergence rates are lower and the development is slower on this fruit, indicating that D. suzukii may not effectively select oviposition sites that are more beneficial for offspring development [23]. D. suzukii has a wide range of host fruits; in one study, its oviposition preferences on blueberry, lingonberry (Vaccinium vitis-idaea L.), chokecherry (Prunus virginiana L.), and sea buckthorn (Hippophae rhamnoides L.) were compared. Despite severe infestation in blueberry orchards, blueberries were the least preferred fruit in the experiment. This research highlights the opportunistic nature of D. suzukii’s oviposition behavior and underscores the critical impact of the fruit phenological stage on the fruit susceptibility [24].
Overall, adult D. suzukii showed a clear oviposition preference for the cherry cultivars “Burlat” and “Hongdeng,” displaying higher fitness levels on these two cultivars. Although this study sheds light on the interaction mechanisms between D. suzukii and different cherry cultivars, several questions remain for further exploration. For instance, the release of volatile compounds by different cherry cultivars and their specific effects on D. suzukii behavior are not yet fully understood. Future studies could delve deeper into these mechanisms to provide stronger scientific support for integrated pest management in the cherry industry. Additionally, considering the potential impacts of ecological factors and climate change on the D. suzukii behavior and cherry physiological traits, these factors should also be incorporated into future research to enhance the effectiveness of D. suzukii control strategies.
5. Conclusions
In conclusion, we compared the physiological traits of the color, sugar content, and firmness among six major cherry cultivars in China and found that D. suzukii preferred to oviposit on fruits with darker color, higher sugar contents, and lower firmness. Although the growth and development of D. suzukii showed limited variation across the cultivars, significant differences were observed in the egg-to-larvae developmental duration and pupation rates. Moreover, a strong correlation was identified between the cherry color and D. suzukii oviposition, as well as certain developmental parameters. These findings elucidate the mechanisms underlying D. suzukii’s selection of different cherry cultivars and provide a theoretical basis for developing targeted control strategies and related products.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects15120984/s1, Table S1: Ripeness stage classification for different cherry cultivars; Table S2: Generalized Linear Model (GLM) analysis of cherry fruit cultivars, ripeness stages, and fruit physiological parameters; Table S3: Physiological data of different cherry cultivars at different ripeness stages; Table S4: Results of t-test comparing differences in fruit physiological parameters between ripeness stages; Table S5: Non-choice oviposition of Drosophila suzukii on different cherry cultivars and ripeness stages, and t-test analysis results.
Author Contributions
Conceptualization, S.W., Y.W. and F.Y.; methodology, F.Y. and S.W.; validation, Z.W., G.Q. and S.W.; formal analysis, F.Y.; investigation, F.Y., H.S., J.X., J.H. and J.W.; resources, J.W., Z.W., Y.W. and G.Q.; data curation, F.Y. and H.S.; writing—original draft preparation, F.Y.; writing—review and editing, H.S., S.W. and Z.W.; visualization, F.Y.; supervision, S.W. and Y.W.; project administration, F.Y. and S.W.; funding acquisition, F.Y. and S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Youth Research Fund of the Beijing Academy of Agriculture and Forestry Sciences (QNJJ202216), the Special Project for Scientific and Technological Innovation Capacity Building of the Beijing Academy of Agriculture and Forestry Sciences (KJCX20240403), and the Young Elite Scientists Sponsorship Program by the BAST (BYESS2023474).
Data Availability Statement
The data presented in this study are available within the article and its Supplementary Materials. For additional information or specific requests, please contact the corresponding author.
Acknowledgments
We sincerely thank Bing Liu from the Institute of Plant Protection, the Chinese Academy of Agricultural Sciences, for providing valuable suggestions on the manuscript, Yu Gao for her assistance with the figure preparation, and the Institute of Forestry and Pomology, the Beijing Academy of Agriculture and Forestry Sciences, for their long-term support of this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Rota-Stabelli, O.; Blaxter, M.; Anfora, G. Drosophila suzukii. Curr. Biol. 2013, 23, R8–R9. [Google Scholar] [CrossRef]
- Knapp, L.; Mazzi, D.; Finger, R. The economic impact of Drosophila suzukii: Perceived costs and revenue losses of Swiss cherry, plum and grape growers. Pest Manag. Scie. 2021, 77, 978–1000. [Google Scholar] [CrossRef]
- Mazzi, D.; Bravin, E.; Meraner, M.; Finger, R.; Kuske, S. Economic impact of the introduction and establishment of Drosophila suzukii on sweet cherry production in Switzerland. Insects 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- De Ros, G.; Grassi, A.; Pantezzi, T. Recent trends in the economic impact of Drosophila suzukii. In Drosophila suzukii Management; Garcia, F.R.M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 11–27. ISBN 978-3-030-62691-4. [Google Scholar]
- Zhao, X.; Yan, M.; Ding, Y.; Huo, Y.; Yuan, Z. Characterization and comparative analysis of the complete chloroplast genome sequence from Prunus avium “Summit”. PeerJ 2019, 7, e8210. [Google Scholar] [CrossRef]
- Ceccarelli, D.; Antonucci, F.; Costa, C.; Talento, C.; Ciccoritti, R. An artificial class modelling approach to identify the most largely diffused cultivars of sweet cherry (Prunus avium L.) in Italy. Food Chem. 2020, 333, 127515. [Google Scholar] [CrossRef]
- Kappel, F.; Granger, A.; Hrotkó, K.; Schuster, M. Cherry. In Fruit Breeding; Badenes, M.L., Byrne, D.H., Eds.; Springer US: Boston, MA, USA, 2012; pp. 459–504. ISBN 978-1-4419-0762-2. [Google Scholar]
- FAS Beijing; USDA Foreign Agriculture Service. China: Stone Fruit Annual. 2024. Available online: https://fas.usda.gov/data/china-stone-fruit-annual-8 (accessed on 4 December 2024).
- Overview of the development of the cherry industry in China. In Proceedings of the 9th International Cherry Symposium, Beijing, China, 21–25 May 2023.
- Beers, E.H.; Van Steenwyk, R.A.; Shearer, P.W.; Coates, W.W.; Grant, J.A. Developing Drosophila suzukii Management Programs for Sweet Cherry in the Western United States. Pest Manag. Sci. 2011, 67, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, X.; Wang, J.; Zhu, D. Occurrence, damage, and control strategies of spotted wing drosophila on sweet cherry, blueberry, and other fruit Trees. Deciduous Fruits 2014, 46, 1–3. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, K.; Yan, G.; Guo, X.; Wang, J.; Zhang, X.; Zhou, Y. Research review on spotted wing drosophila (Drosophila suzukii). J. Fruit Sci. 2014, 31, 717–721+750. (In Chinese) [Google Scholar] [CrossRef]
- Yang, F.; Wang, Z.; Sun, A.; Yu, G.; Wang, G. Occurrence dynamics of spotted wing drosophila and comparison of different bait solutions in a cherry orchard in Beijing. China Fruits 2022, 6, 66–70. (In Chinese) [Google Scholar] [CrossRef]
- Little, C.M.; Chapman, T.W.; Hillier, N.K. Effect of color and contrast of highbush blueberries to host-finding behavior by Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2018, 47, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Little, C.M.; Dixon, P.L.; Chapman, T.W.; Hillier, N.K. Role of fruit characters and colour on host selection of boreal fruits and berries by Drosophila suzukii (Diptera: Drosophilidae). Can. Entomol. 2020, 152, 546–562. [Google Scholar] [CrossRef]
- Bolton, L.G.; Piñero, J.C.; Barrett, B.A. Olfactory cues from host- and non-host plant odor influence the behavioral responses of adult Drosophila suzukii (Diptera: Drosophilidae) to visual cues. Environ. Entomol. 2021, 50, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Entling, W.; Anslinger, S.; Jarausch, B.; Michl, G.; Hoffmann, C. Berry Skin resistance explains oviposition preferences of Drosophila suzukii at the level of grape cultivars and single berries. J. Pest Sci. 2019, 92, 477–484. [Google Scholar] [CrossRef]
- Fragnière, A.-L.; Bacher, S.; Kehrli, P. Identifying candidate host plants for trap cropping against Drosophila suzukii in vineyards. J. Pest Sci. 2024, 97, 1975–1991. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, Y.; Fang, Q.; Wang, F.; Ye, G. Tropism behavior analysis of two drosophila species to fruits and volatiles of strawberry at different mature stage. Chin. J. Biol. Control. 2023, 39, 813–823. (In Chinese) [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Ma, N.; Chen, H.; Zhai, Y.; Dang, H.; Yu, Y. Oviposition selection of Drosophila suzukii (Diptera: Drosophilidae) on four cherry cultivars and its relationship to the physiological characteristics of cherry fruits. Acta Entomologica Sinica 2017, 60, 328–334. (In Chinese) [Google Scholar] [CrossRef]
- Yan, G.; Zhang, K. Major Cherry Varieties in China, 1st ed.; China Agriculture Press: Beijing, China, 2022; ISBN 978-7-109-29285-7. (In Chinese) [Google Scholar]
- Zhang, X.; Jiu, S.; Xu, Y.; Lv, Z.; Liu, R.; Wang, S.; Zhang, C. Evaluation and study on fruit traits of sweet cherry cultivars (strains). J. Plant Genet. Resour. 2024, 25, 1803–1814. (In Chinese) [Google Scholar] [CrossRef]
- Liu, B.; Ren, L.; Zhan, G.; Li, B.; Chen, N.; Wang, X. An Artificial diet and rearing method for mass rearing of spotted wing drosophila. China Patent Application Number CN201910882663.3, 18 September 2019. [Google Scholar]
- CIE. Colorimetry—Part 4: CIE 1976 Lab Colour Space; CIE: Vienna, Austria, 1976. [Google Scholar]
- Markovic, I.; Ilic, J.; Markovic, D.; Simonovic, V.; Kosanic, N. Color measurement of food products using CIE L* a* b* and RGB color space. J. Hyg. Eng. Des. 2020, 7, 50–53. [Google Scholar]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Tech. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Wu, L.; Wang, F.; Sha, R.; Li, X.; Yu, K.; Feng, J. The Effect of N and KH2PO4 on skin color, sugars, and organic acids of “Flame Seedless” grape. Agronomy 2023, 13, 902. [Google Scholar] [CrossRef]
- Carreño, J.; Martínez, A.; Almela, L.; Fernández-López, J.A. Proposal of an index for the objective evaluation of the colour of red table grapes. Food Res. Int. 1995, 28, 373–377. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Almela, L.; Muñoz, J.A.; Hidalgo, V.; Carreño, J. Dependence between colour and individual anthocyanin content in ripening grapes. Food Res. Int. 1998, 31, 667–672. [Google Scholar] [CrossRef]
- Jaywant, S.A.; Singh, H.; Arif, K.M. Sensors and instruments for Brix measurement: A review. Sensors 2022, 22, 2290. [Google Scholar] [CrossRef]
- Little, C.M.; Rizzato, A.R.; Charbonneau, L.; Chapman, T.; Hillier, N.K. Color preference of the spotted wing drosophila, Drosophila suzukii. Sci. Rep. 2019, 9, 16051. [Google Scholar] [CrossRef]
- Kinjo, H.; Kunimi, Y.; Ban, T.; Nakai, M. Oviposition efficacy of Drosophila suzukii (Diptera: Drosophilidae) on different cultivars of blueberry. J. Econ. Entom. 2013, 106, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Bruck, D.J.; Curry, H.; Edwards, D.; Haviland, D.R.; Van Steenwyk, R.A.; Yorgey, B.M. The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag. Sci. 2011, 67, 1358–1367. [Google Scholar] [CrossRef]
- Hu, Q.; Gao, H. Oviposition selection of Drosophila suzukii (Diptera: Drosophilidae) on grape cultivars and the relationship to physiological characteristics of grape fruits. North. Hortic. 2021, 2, 42–46. (In Chinese) [Google Scholar]
- Kamiyama, M.T.; Guédot, C. Varietal and developmental susceptibility of tart cherry (Rosales: Rosaceae) to Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entom. 2019, 112, 1789–1797. [Google Scholar] [CrossRef]
- Cai, P.; Song, Y.; Yi, C.; Zhang, Q.; Xia, H.; Lin, J.; Zhang, H.; Yang, J.; Ji, Q.; Chen, J. Potential host fruits for Drosophila suzukii: Olfactory and oviposition preferences and suitability for development. Entomol. Exp. Appl. 2019, 167, 880–890. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).