Research on the Reproduction of Trichogramma chilonis Based on Samia cynthia ricini Eggs: Temperature, Functional Response and Proportional Effect
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Sources and Feeding
2.2. Experimental Design
2.2.1. Developmental Temperature
2.2.2. Developmental Duration
2.2.3. Functional Response
2.2.4. Wasp-to-Egg Ratios
2.3. Statistical Analysis
3. Results
3.1. Effects of Developmental Temperature on the Longevity and Oviposition of S. c. ricini
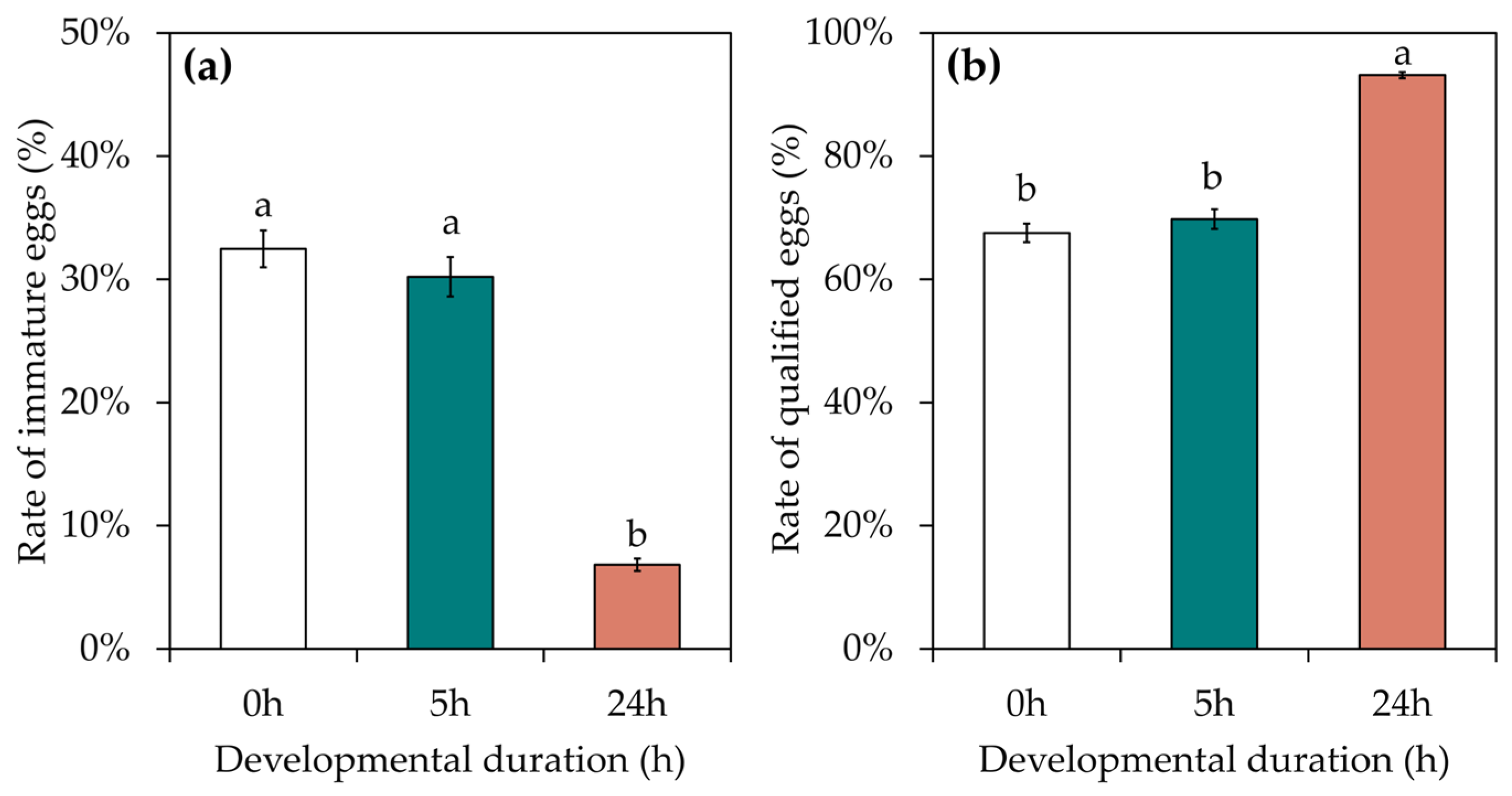
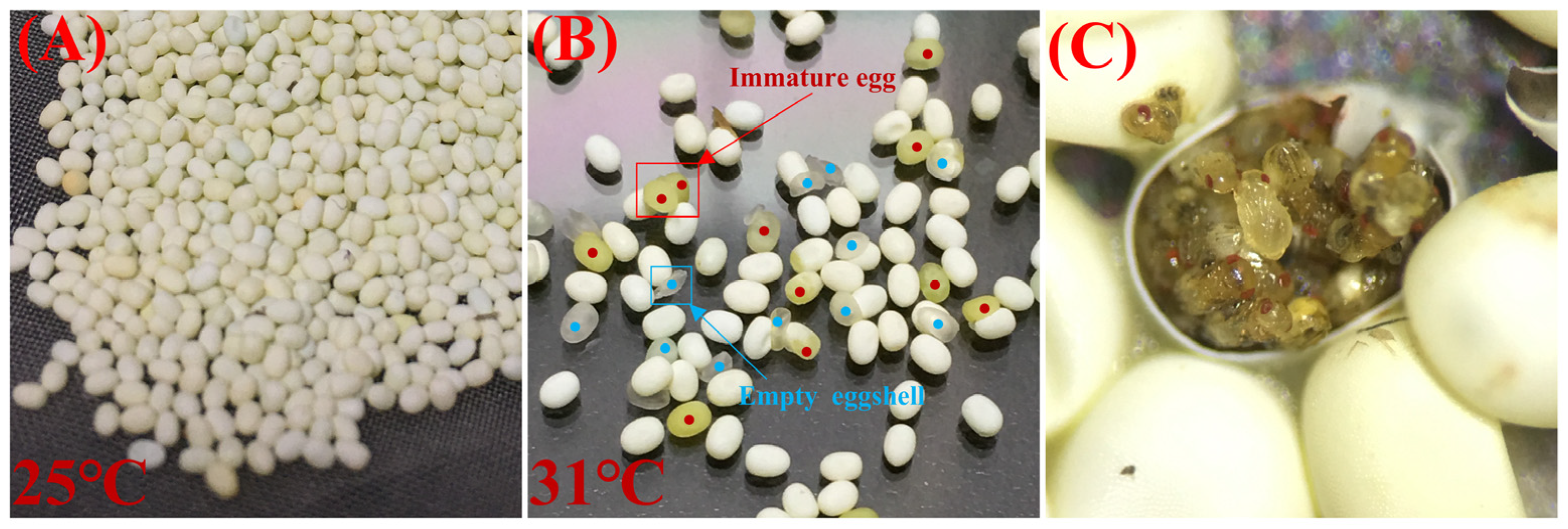
3.2. Effects of Developmental Duration on the Quality of S. c. ricini Eggs
3.3. Functional Response of T. chilonis to S. c. ricini Eggs
3.3.1. Population Growth of T. chilonis
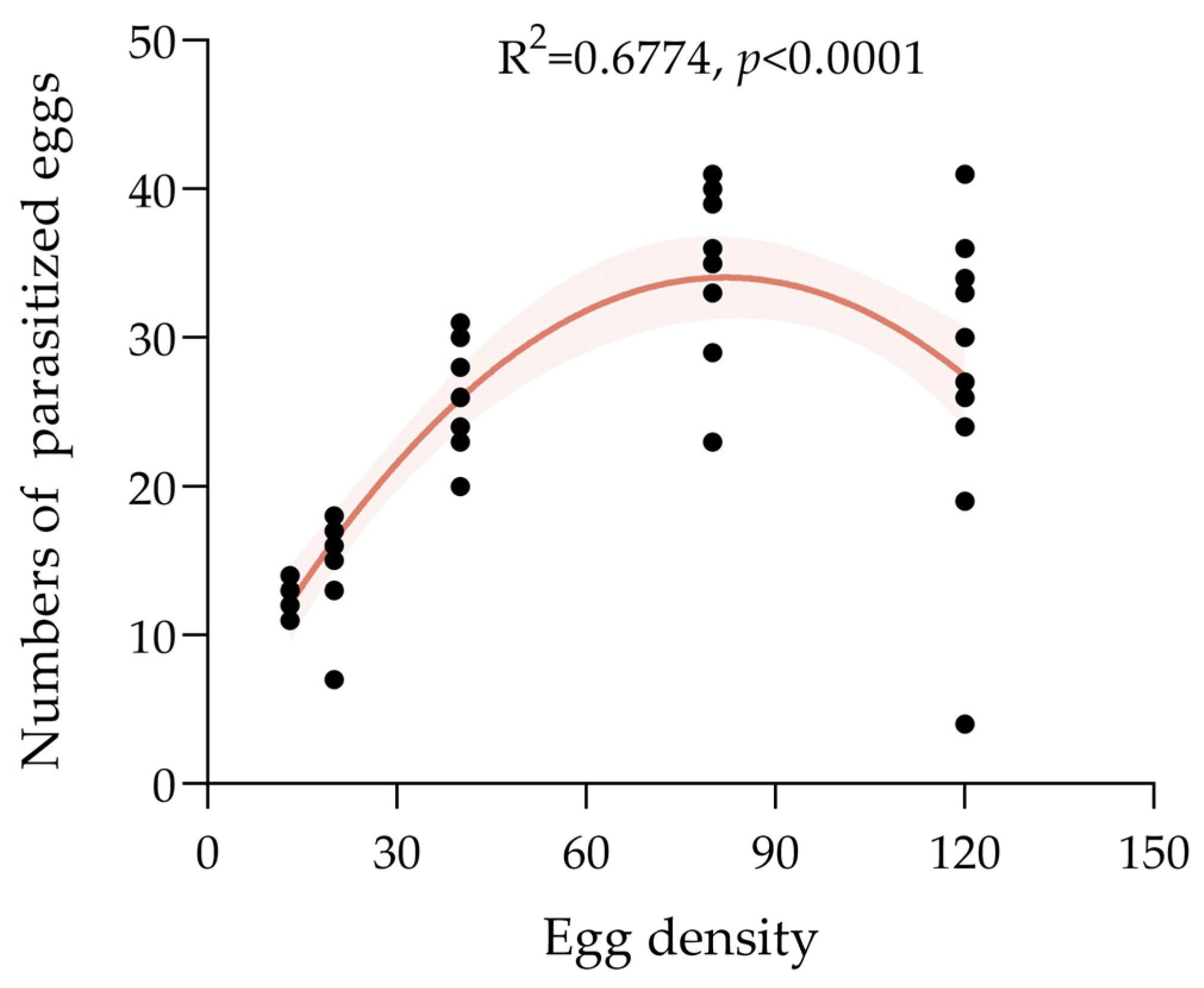
3.3.2. Parasitic capacity of T. chilonis
3.4. Effects of the Wasp–Egg Ratio on the Reproduction of T. chilonis Using S. c. ricini Eggs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Liu, Y.; Shi, M.; Huang, J.; Chen, X. Parasitoid wasps as effective biological control agents. J. Integr. Agric. 2019, 18, 705–715. [Google Scholar] [CrossRef]
- Jalali, S.K.; Mohanraj, P.; Lakshmi, B.L. Chapter 5—Trichogrammatids. In Ecofriendly Pest Management for Food Security; Omkar, Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 139–181. [Google Scholar]
- Wang, Z.; He, K.; Zhang, F.; Lu, X.; Babendreier, D. Mass rearing and release of Trichogramma for biological control of insect pests of corn in China. Biol. Control 2014, 68, 136–144. [Google Scholar] [CrossRef]
- Kerima, O.Z.; Niranjana, P.; Vinay Kumar, B.S.; Ramachandrappa, R.; Puttappa, S.; Lalitha, Y.; Jalali, S.K.; Ballal, C.R.; Thulasiram, H.V. De novo transcriptome analysis of the egg parasitoid Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae): A biological control agent. Gene Rep. 2018, 13, 115–129. [Google Scholar] [CrossRef]
- Yang, L.; Li, F.; Lü, X.; Xing, B.; Pan, X.; Shi, X.; Li, J.; Wu, S. Performance of three Trichogramma species as biocontrol agents on Spodoptera frugiperda eggs. J. Appl. Entomol. 2022, 146, 1019–1027. [Google Scholar] [CrossRef]
- Zang, L.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status, and perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef]
- Qin, Z.; Li, D.; Luo, Y.; Cuthbertson, A.G.S. Impact of different Trichogramma chilonis populations from sugarcane fields on parasitism of Corcyra cephalonica. J. Appl. Entomol. 2023, 147, 819–824. [Google Scholar] [CrossRef]
- Parra, J.R.P. Mass rearing of egg parasitoids for biological control programs. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma; Consoli, F.L., Parra, J.R.P., Zucchi, R.A., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 267–292. [Google Scholar]
- Zhang, J.; Ruan, C.; Zang, L.S.; Shao, X.; Shi, S. Technological lmprovements for mass production of Trichogramma and current status of their applications for biological control on agricultura pests in china. Chin. J. Biol. Control 2015, 31, 638–646. [Google Scholar] [CrossRef]
- Chandrawanshi, P.; Aherkar, S.; Shendage; Thakare, V. Effect of magnetic field and different diets on the biological parameters of rice moth, Corcyra cephalonica (Stainton). J. Entomol. Zool. Stud. 2018, 6, 74–76. [Google Scholar]
- Huang, S.; Zang, L.; Ruan, C. Parasitization Ecology, Mass Production, and Applications of Trichogramma; Science Press: Beijing, China, 2013. [Google Scholar]
- Nagaraja, H. Mass production of Trichogrammatid parasitoids. In Biological Control of Insect Pests Using Egg Parasitoids; Sithanantham, S., Ballal, C.R., Jalali, S.K., Bakthavatsalam, N., Eds.; Springer: New Delhi, India, 2013; pp. 175–189. [Google Scholar]
- Jia, N.; Huang, Y.; Li, Q.; Li, Y. Studies on reproducing Trichogramma by using Sitotroga cerealella olivier eggs. J. Jilin Agric. Univ. 2002, 24, 58–60. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Xue, J.-Z.; Tariq, T.; Li, T.-H.; Qian, H.-Y.; Cui, W.-H.; Tian, H.; Monticelli, L.S.; Desneux, N.; Zang, L.-S. Parasitism and suitability of Trichogramma chilonis on large eggs of Two factitious hosts: Samia cynthia ricini and Antheraea pernyi. Insects 2024, 15, 2. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Zang, L.; Du, W.; Hou, Y.; Ruan, C.; Desneux, N. Advantages of diapause in Trichogramma dendrolimi mass production on eggs of the Chinese silkworm, Antheraea pernyi. Pest Manag. Sci. 2018, 74, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dun, W.; Ruan, C.; Zang, L.; Peng, H.; Sun, G. Effect of different artificial diets on growth, development and fecundity of Corcyra cephalonice. J. Jilin Agric. Univ. 2012, 34, 603–606+611. [Google Scholar] [CrossRef]
- Wang, L. The biological characteristics and the technique of artificial feeding on Sitotroga cerealella. Heilongjiang Agric. Sci. 2007, 53–55. [Google Scholar]
- Ma, D.; Zhang, J.; Chen, L.; Guo, Y. Study on breeding and making use of rice moth in Xinjiang. J. Xinjiang Agric. Univ. 2001, 24, 25–28. [Google Scholar]
- Pu, Z.; Deng, D.; Liu, Z.; Hong, F.; Mo, Y. On the rearing of Trichogramma evanescens Westw. and its utilization for the control of sugar cane borers. Acta Entomol. Sin. 1956, 6, 1–35. [Google Scholar] [CrossRef]
- Chen, J.; Qin, R.; Wang, X.; Liu, X.; Zhang, S. Comparison of the impact of host eggs from rice meal moth Corcyra cephalonica and Chinese tussar moth Antheraea pernyi on the artificial rearing efficiency of the pine caterpillar egg parasitoid Trichogramma dendrolimi. J. Plant Prot. 2024, 51, 952–959. [Google Scholar] [CrossRef]
- Iqbal, A.; Chen, Y.; Hou, Y.; Zhang, L.; Desneux, N.; Zang, L. Factitious host species impact on the outcome of multiparasitism between egg parasitoids. J. Pest Sci. 2019, 92, 1261–1269. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Wang, X.; Zang, L. Recent advances in parasitism ecology, mass production and applications of Trichogramma in China. Plant Prot. 2023, 49, 399–409. [Google Scholar] [CrossRef]
- Iqbal, A.; Chen, Y.; Hou, Y.; Ruan, C.; Desneux, N.; Qayash Khan, M.; Zang, L.-S. Rearing Trichogramma ostriniae on the factitious host Antheraea pernyi via multiparasitism with Trichogramma chilonis facilitates enhanced biocontrol potential against Ostrinia furnacalis. Biol. Control 2021, 156, 104567. [Google Scholar] [CrossRef]
- Lin, L.; Feng, J. Preliminary study on Trichogramma in Jinan, Shandong. Insect Knowl. 1964, 63–65+57. [Google Scholar]
- Tulu, D.; Aleme, M.; Mengistu, G.; Bogale, A.; Shifa, K.; Mendesil, E. Evaluation of Castor (Ricinus communis L.) Genotypes and Their Feeding Values on Rearing Performance of Eri Silkworm (Samia cynthia ricini Boisduval) (Lepidoptera: Saturniidae) in Southwest Ethiopia. Psyche J. Entomol. 2022, 2022, 1556776. [Google Scholar] [CrossRef]
- Sangannavar, P.A.; Manjunatha, G.R.; Ahmed, S.N.; Moorthy, M.; Halagundegowda, G.R.; Yamanura, Y.; Mishra, R.K.; Sathyanarayana, K.; Sivaprasad, V. Prospects for promotion of eri culture in non-traditional areas of India—A review. J. Exp. Zool. India 2023, 26, 1341–1349. [Google Scholar] [CrossRef]
- Shifa, K.; Getu, E.; Sori, W. Rearing performance of eri-silkworm (Samia cynthia ricini Boisduval) (Lepidoptera: Saturniidae) fed with different castor (Ricinus communis L.) genotypes. J. Entomol. 2014, 11, 25–33. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, J.; Wang, G.; Wang, Z.; Jiang, Q.; Wu, S.; Tan, L.; Kuang, Y.; Chen, D.; Ceng, J.; et al. Study on the artificial diets for Samia cynthia ricini Boisduval. Guangdong Agric. Sci. 1980, 31–33. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, S.; Wang, G.; Wu, J.; Chen, D.; Ceng, J.; Liang, Y.; Tan, L. Demonstration trials of mass rearing Samia cynthia ricini Boisduval with artificial diets. Guangdong Agric. Sci. 1982, 35–36. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Gui, C.; Lu, H.; Yin, Y.; Jin, G. Study on utilization of Trichogramma ostriniae. Nat. Enemies Insect 1982, 4, 1–4. [Google Scholar]
- Shen, X.; Wang, K.; Meng, G. Field experiment of inoculative releases of Trichogramma spp. in the early season in Henan province. Chin. J. Biol. Control 1986, 2, 152–154. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, B.; Yuan, X.; Song, Z.; Liu, Z.; Li, D. Sixty years’ research on biological control of pests by Guangdong academy of agricultural sciences: Achievements and prospects. Guangdong Agric. Sci. 2020, 47, 93–102. [Google Scholar] [CrossRef]
- Hassan, S.A.; Liscsinszky, H.; Zhang, G. The Oak-silkworm egg Antheraea pernyi (Lepidoptera: Anthelidae) as a mass rearing host for parasitoids of the genus Trichogramma (Hymenoptera: Trichogrammatidae). Biocontrol Sci. Technol. 2004, 14, 269–279. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, X.; Guo, M.; Song, Z.; Yang, X. Studies on the developmental effective temperature of the castor silkworm (Philosamia cynthia ricini D) and the reproductive characteristics of the silkworms eggs. J. Sichuan For. Sci. Technol. 1995, 16, 22–28. [Google Scholar] [CrossRef]
- Harris, M.O.; Rose, S. Factors influencing the onset of egglaying in a cecidomyiid fly. Physiol. Entomol. 1991, 16, 183–190. [Google Scholar] [CrossRef]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Holling, C.S. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Farrokhi, S.; Ashouri, A.; Shirazi, J.; Allahvari, H.; Huigens, M.E. A comparative study on the functional response of Wolbachia-infected and uninfected forms of the parasitoid wasp Trichogramma brassicae. J. Insect Sci. 2010, 10, 167. [Google Scholar] [CrossRef]
- Jones, D.B.; Giles, K.L.; Berberet, R.C.; Royer, T.A.; Elliott, N.C.; Payton, M.E. Functional responses of an introduced parasitoid and an indigenous parasitoid on greenbug at four temperatures. Environ. Entomol. 2003, 32, 425–432. [Google Scholar] [CrossRef]
- Atashi, N.; Shishehbor, P.; Seraj, A.A.; Rasekh, A.; Hemmati, S.A.; Ugine, T.A. Functional and Numerical Responses of Trichogramma euproctidis (Hymenoptera: Trichogrammatidae) to Helicoverpa armigera (Lepidoptera: Noctuidae) Under Laboratory Conditions. Neotrop. Entomol. 2023, 52, 956–962. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Ji, X. Functional response of three Trichogramma (Hymenoptera: Trichogrammatidae) to parasitizing Spodoptera exigua and Plutella xylostella eggs. J. Environ. Entomol. 2023, 45, 1046–1053. [Google Scholar]
- Manohar, T.N.; Sharma, P.L.; Verma, S.C.; Sharma, K.C.; Chandel, R.S. Functional response of indigenous Trichogramma spp. to invasive tomato leafminer, Tuta absoluta (Meyrick) under laboratory conditions. Int. J. Trop. Insect Sci. 2020, 40, 101–107. [Google Scholar] [CrossRef]
- Reay-Jones, F.P.F.; Rochat, J.; Goebel, R.; Tabone, E. Functional response of Trichogramma chilonis to Galleria mellonella and Chilo sacchariphagus eggs. Entomol. Exp. Appl. 2006, 118, 229–236. [Google Scholar] [CrossRef]
- DB44/T 175-2014; Technical Specification for Mass Production and Release Application of Trichogramma chilonis. Guangdong Administration for Market Regulation (Guangdong Intellectual Property Administration): Guangzhou, China, 2014.
- Chen, K.; Wu, Y.; Shi, F.; Huang, S.; Fang, X. Analysis of the reproductive potential of Trichogramma ostriniae on Plutella xylostella. J. South China Agric. Univ. 2003, 24, 41–44. [Google Scholar]
- Huang, S.; Dai, Z.; Wu, D. The establishment and application of the experimental population life tables of Trichogramma spp. on different hosts. Acta Phytophylacica Sin. 1996, 23, 209–212. [Google Scholar]
- Xu, R. Insect Population Ecology; Beiing Normal University Press: Beiing, China, 1987; pp. 61–82. [Google Scholar]
- Yuan, X.; Wei, S.; Li, D.; Zhang, J. Lighting in dark periods reduced the fecundity of Spodoptera frugiperda and limited its population growth. Agronomy 2023, 13, 971. [Google Scholar] [CrossRef]
- Design and Analysis of Ecological Experiments; Oxford University Press: Oxford, UK, 2001.
- Pervez, A.; Omkar, O. Functional responses of coccinellid predators: An illustration of a logistic approach. J. Insect Sci. 2005, 5, 5. [Google Scholar] [CrossRef]
- Juliano, S.A. Nonlinear curve fitting: Predation and functional response curves. In Design and Analysis of Ecological Experiments; Scheiner, S.M., Gurevitch, J., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 178–196. [Google Scholar]
- Rogers, D. Random Search and Insect Population Models. J. Anim. Ecol. 1972, 41, 369–383. [Google Scholar] [CrossRef]
- Irlich, U.M.; Terblanche, J.S.; Blackburn, T.M.; Chown, S.L. Insect Rate-Temperature Relationships: Environmental Variation and the Metabolic Theory of Ecology. Am. Nat. 2009, 174, 819–835. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F.; Owayss, A.A.; Ibrahim, Y.Y.; Basuny, N.K. A review of impacts of temperature and relative humidity on various activities of honey bees. Insectes Sociaux 2017, 64, 455–463. [Google Scholar] [CrossRef]
- Ghosh, S.M.; Testa, N.D.; Shingleton, A.W. Temperature-size rule is mediated by thermal plasticity of critical size in Drosophila melanogaster. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130174. [Google Scholar] [CrossRef]
- Li, J.; Gu, D.; Wei, C. On the relationship between temperature and the duration of egg stage of Philosamia Cynthia Ricini Boisd. Acta Sci. Nat. Univ. Sunyatseni 1965, 526–529. [Google Scholar]
- Wongsorn, D.; Sirimungkararatp, S.; Saksirirat, W. Effect of temperatures on growth, yields and heat shock protein expression of Eri silkworm (Samia ricini D.). KKU Res. J. 2015, 20, 189–197. [Google Scholar] [CrossRef]
- Rebaudo, F.; Rabhi, V.-B. Modeling temperature-dependent development rate and phenology in insects: Review of major developments, challenges, and future directions. Entomol. Exp. Appl. 2018, 166, 607–617. [Google Scholar] [CrossRef]
- Barr, J.S.; Martin, L.E.; Tate, A.T.; Hillyer, J.F. Warmer environmental temperature accelerates aging in mosquitoes, decreasing longevity and worsening infection outcomes. Immun. Ageing 2024, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, A.; Mazzi, D.; Dorn, S. Stay cool, travel far: Cold-acclimated oriental fruit moth females have enhanced flight performance but lay fewer eggs. Entomol. Exp. Appl. 2014, 151, 11–18. [Google Scholar] [CrossRef]
- Ye, G.; Hu, C.; Gong, H. Effect of high temperature on ovarian growth and development in the Japanese oak silkworm, Antheraea yamamai (Lepidoptera: Saturiidae), a precious silkworm. Acta Ecol. Sin. 2000, 20, 490–494. [Google Scholar]
- Sales, K.; Vasudeva, R.; Dickinson, M.E.; Godwin, J.L.; Lumley, A.J.; Michalczyk, Ł.; Hebberecht, L.; Thomas, P.; Franco, A.; Gage, M.J.G. Experimental heatwaves compromise sperm function and cause transgenerational damage in a model insect. Nat. Commun. 2018, 9, 4771. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.-H.; Uemura, T.; Kang, S. Road to sexual maturity: Behavioral event schedule from eclosion to first mating in each sex of Drosophila melanogaster. iScience 2023, 26, 107502. [Google Scholar] [CrossRef]
- Oaten, A.; Murdoch, W.W. Functional response and stability in predator-prey systems. Am. Nat. 1975, 109, 289–298. [Google Scholar] [CrossRef]
- Solomon, M.E. The natural control of animal populations. J. Anim. Ecol. 1949, 18, 1. [Google Scholar] [CrossRef]
- Li, L.; Lu, X.; Zhang, G.; Zhou, S.; Chang, X.; Ding, Y. Study on the best proportion of female Trichogramma to host eggs and time for inoculation aiming at propagation Trichogramma japonicum with rice moth eggs. J. Northeast Agric. Sci. 2019, 44, 34–37. [Google Scholar] [CrossRef]
- Lu, X.; Li, L.; Zhang, G. Effect of inoculation times on strains of Trichogramma chilonis. J. Northeast Agric. Sci. 2004, 29, 32–34. [Google Scholar] [CrossRef]
- Li, T.; Tian, C.; Zang, L.; Hou, Y.; Ruan, C.; Yang, X.; Monticelli, L.; Desneux, N. Multiparasitism with Trichogramma dendrolimi on egg of Chinese oak silkworm, Antheraea pernyi, enhances emergence of Trichogramma ostriniae. J. Pest Sci. 2019, 92, 707–713. [Google Scholar] [CrossRef]
- Liu, H.; Fang, X.; Liu, J.; Huang, S.; Ouyang, G. Superparasitism of Trichogramma chilonis (Hymenoptera:Trichogrammatidae) by different inoculation proportion and its influences on offspring. China Plant Prot. 2017, 37, 5–10. [Google Scholar]


| Treatments (Wasp-to-Egg Ratio) | 3:1 | 2:1 | 1:1 | 1:2 | 1:3 |
|---|---|---|---|---|---|
| Number of T. chilonis | 39 | 40 | 40 | 40 | 40 |
| Number of S. c. ricini eggs | 13 | 20 | 40 | 80 | 120 |
| Temperature (°C) | Longevity (d) | Tse | Are | Rre (%) | Te |
|---|---|---|---|---|---|
| 22 | 10.50 ± 0.49 b | 144.4 ± 16.2 b | 95.2 ± 17.5 a | 35.81± 6.39 a | 239.6 ± 17.9 b |
| 25 | 12.13 ± 0.40 a | 251.4 ± 13.5 a | 27.2 ± 7.2 b | 11.89 ± 3.27 b | 273.3 ± 11.5 ab |
| 28 | 9.28 ± 0.34 c | 239.6 ± 14.9 a | 42.3 ± 10.8 b | 15.08 ± 3.47 b | 281.9 ± 12.2 a |
| 31 | 9.02 ± 0.26 c | 265.1 ± 17.5 a | 39.5 ± 9.2 b | 14.02 ± 3.24 b | 304.6 ± 14.9 a |
| Egg Density | R0 | rm | λ | T (d) | FWpf | Sex Ratio (%) | FW | TW |
|---|---|---|---|---|---|---|---|---|
| 13 | 0.475 ± 0.028 c | −0.073 ± 0.032 e | 0.930 ± 0.010 c | −9.50 ± 0.17 d | 0.485 ± 0.271 e | 97.9 ± 2.74 a | 19.0 ± 6.88 c | 19.4 ± 7.07 c |
| 20 | 1.088 ± 0.318 b | 0.008 ± 0.027 d | 1.008 ± 0.018 b | 83.51 ± 0.89 a | 1.238 ± 0.885 d | 87.8 ± 4.95 b | 43.4 ± 16.15 bc | 49.4 ± 18.11 bc |
| 40 | 1.893 ± 0.265 b | 0.063 ± 0.006 c | 1.063 ± 0.027 b | 11.07 ± 0.37 b | 2.047 ± 0.713 c | 92.4 ± 2.02 ab | 75.7 ± 12.43 b | 81.9 ± 14.40 b |
| 80 | 4.033 ± 1.893 a | 0.137 ± 0.012 b | 1.146 ± 0.008 b | 5.07 ± 0.62 c | 4.280 ± 0.830 b | 94.2 ± 2.79 ab | 161.3 ± 26.38 a | 171.2 ± 26.78 a |
| 120 | 4.670 ± 1.438 a | 0.151 ± 0.006 a | 1.163 ± 0.012 a | 4.59 ± 0.54 c | 5.160 ± 1.375 a | 90.5 ± 1.52 ab | 186.8 ± 22.73 a | 206.4 ± 26.54 a |
| Parameters | Estimate | Standard Error | χ2 | p-Value |
|---|---|---|---|---|
| P0 | 3.4196 | 0.4753 | 51.76 | <0.0001 |
| P1 | −0.1126 | 0.0267 | 17.85 | <0.0001 |
| P2 | 0.00125 | 0.000415 | 9.05 | 0.0026 |
| P3 | −0.000000528 | 0.0000001896 | 7.74 | 0.0054 |
| Functional Response Type | II-Type |
|---|---|
| Maximal parasitic (egg/d) | 2.16 |
| A | 0.1840 ± 0.0711 |
| Th (d) | 0.4628 ± 0.0502 |
| Functional equation | Na = 0.1840N/(1 + 0.4628N) |
| R2 | 0.9703 |
| Treatment | Development and Survival of T. chilonis Offspring | Reproductive Efficiency | ||||||
|---|---|---|---|---|---|---|---|---|
| eNp | ER (%) | eNfw | neNw | NAo | fNAo | fRo (%) | PC | |
| 3:1 | 1.20 ± 0.42 c | 9.39 ± 3.21 d | 19.00 ± 6.88 c | 30.24 ± 1.31 b | 19.40 ± 7.07 c | 19.00 ± 6.88 c | 98.41 ± 1.12 a | 0.4750 ± 0.1720 c |
| 2:1 | 3.10 ± 0.90 bc | 20.60 ± 5.31 c | 43.40 ± 16.15 bc | 34.55 ± 1.30 a | 49.40 ± 18.11 bc | 43.40 ± 16.15 bc | 85.64 ± 4.95 c | 1.0850 ± 0.4038 bc |
| 1:1 | 6.20 ± 1.04 b | 23.96 ± 3.96 c | 75.70 ± 12.43 b | 28.19 ± 1.19 bc | 81.90 ± 14.40 b | 75.70 ± 12.43 b | 94.33 ± 2.02 ab | 1.8925 ± 0.3107 b |
| 1:2 | 12.10 ± 1.63 a | 35.28 ± 3.87 b | 161.30 ± 26.38 a | 24.92 ± 1.28 c | 171.20 ± 26.78 a | 161.30 ± 26.38 a | 92.79 ± 2.79 ab | 4.0325 ± 0.6595 a |
| 1:3 | 12.60 ± 1.46 a | 46.87 ± 2.31 a | 186.80 ± 22.73 a | 28.87 ± 0.72 c | 206.40 ± 20.54 a | 186.80 ± 22.72 a | 91.83 ± 1.52 ab | 4.6700 ± 0.5682 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Li, D.; Deng, W. Research on the Reproduction of Trichogramma chilonis Based on Samia cynthia ricini Eggs: Temperature, Functional Response and Proportional Effect. Insects 2024, 15, 963. https://doi.org/10.3390/insects15120963
Yuan X, Li D, Deng W. Research on the Reproduction of Trichogramma chilonis Based on Samia cynthia ricini Eggs: Temperature, Functional Response and Proportional Effect. Insects. 2024; 15(12):963. https://doi.org/10.3390/insects15120963
Chicago/Turabian StyleYuan, Xi, Dunsong Li, and Weili Deng. 2024. "Research on the Reproduction of Trichogramma chilonis Based on Samia cynthia ricini Eggs: Temperature, Functional Response and Proportional Effect" Insects 15, no. 12: 963. https://doi.org/10.3390/insects15120963
APA StyleYuan, X., Li, D., & Deng, W. (2024). Research on the Reproduction of Trichogramma chilonis Based on Samia cynthia ricini Eggs: Temperature, Functional Response and Proportional Effect. Insects, 15(12), 963. https://doi.org/10.3390/insects15120963





