Salicylic Aldehyde and Its Potential Use in Semiochemical-Based Pest Control Strategies Against Trypophloeus binodulus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects, Traps, and Lures’ Preparation
2.2. Chemicals
2.3. Volatiles Collection
2.4. Chemical Analyses
2.5. Electroantennography
2.6. Field Trapping
2.7. Statistical Analysis
3. Results
3.1. Identification of Volatiles
3.2. Field Experiments
Captures Based on Clones and Attractants
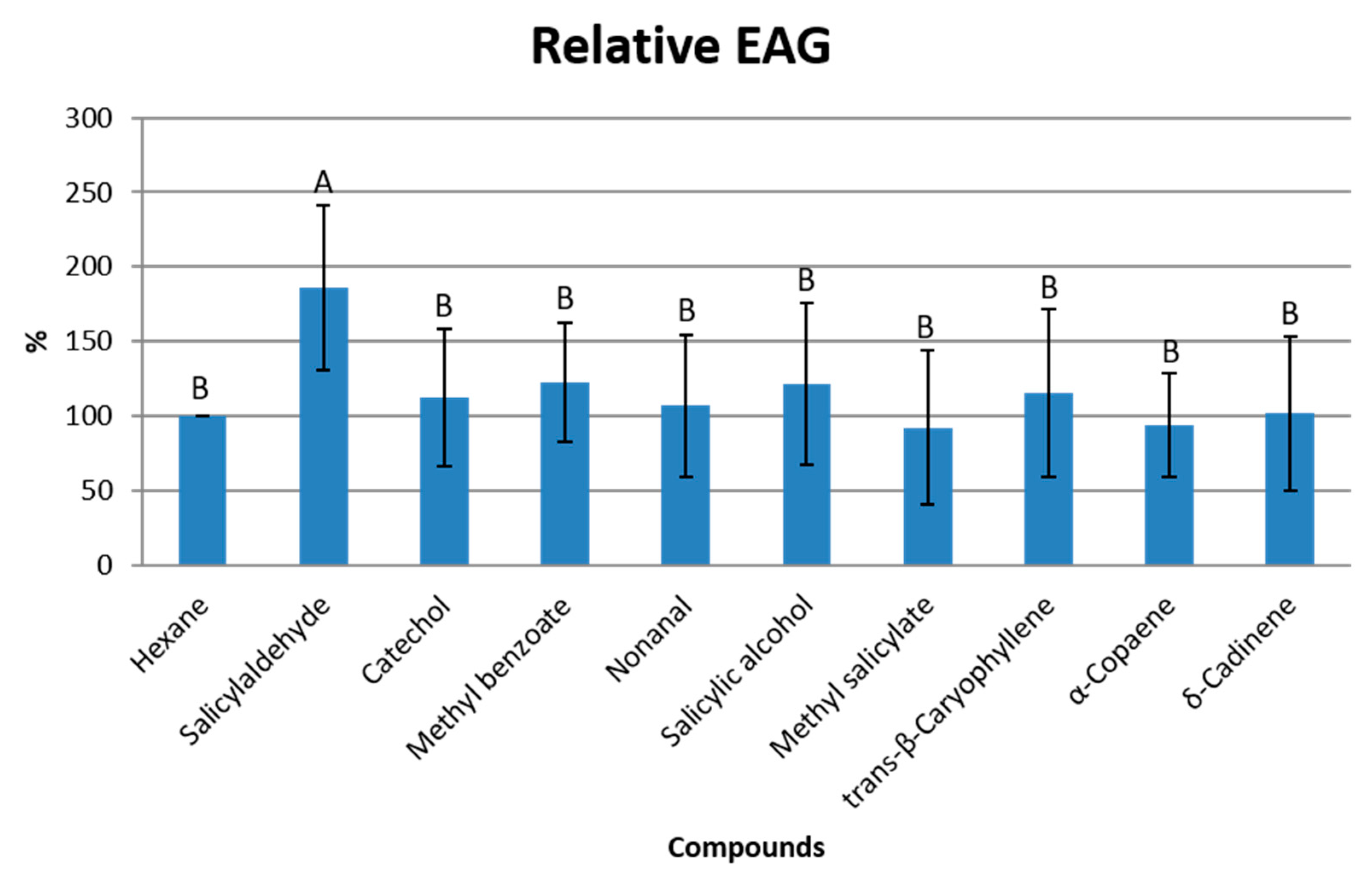
3.3. Electrophysiological Response of T. binodulus to Identified Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of Plant Volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Wood, D.L. The Role of Pheromones of Bark Beetles. Annu. Rev. Entomol. 1982, 27, 411–446. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Pickett, J.A. Perception of Plant Volatile Blends by Herbivorous Insects—Finding the Right Mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Borden, J.H. Disruption of Semiochemical-Mediated Aggregation in Bark Beetles. In Insect Pheromone Research; Cardé, R.T., Minks, A.K., Eds.; Springer: Boston, MA, USA, 1997; pp. 421–438. [Google Scholar]
- Seybold, S.J.; Bentz, B.J.; Fettig, C.J.; Lundquist, J.E.; Progar, R.A.; Gillette, N.E. Management of Western North American Bark Beetles with Semiochemicals. Annu. Rev. Entomol. 2018, 63, 407–432. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Guerrero, A. Interactions of Insect Pheromones and Plant Semiochemicals. Trends Plant Sci. 2004, 9, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Gitau, C.W.; Bashford, R.; Carnegie, A.J.; Gurr, G.M. A Review of Semiochemicals Associated with Bark Beetle (Coleoptera: Curculionidae: Scolytinae) Pests of Coniferous Trees: A Focus on Beetle Interactions with Other Pests and Their Associates. For. Ecol. Manag. 2013, 297, 1–14. [Google Scholar] [CrossRef]
- Ranger, C.M.; Schultz, P.B.; Reding, M.E.; Frank, S.D.; Palmquist, D.E. Flood Stress as a Technique to Assess Preventive Insecticide and Fungicide Treatments for Protecting Trees against Ambrosia Beetles. Insects 2016, 7, 40. [Google Scholar] [CrossRef]
- Schowalter, T.D.; Coulson, R.N.; Crossley, D.A. Role of Southern Pine Beetle and Fire in Maintenance of Structure and Function of the Southeastern Coniferous Forest. Environ. Entomol. 1981, 10, 821–825. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Erbilgin, N.; Klepzig, K.D.; Wallin, K.F. Interactions Among Conifer Terpenoids and Bark Beetles Across Multiple Levels of Scale: An Attempt to Understand Links Between Population Patterns and Physiological Processes. Recent Adv. Phytochem. 2005, 39, 79–118. [Google Scholar] [CrossRef]
- Byers, J.A. Chemical Ecology of Bark Beetles. Experientia 1989, 45, 271–283. [Google Scholar] [CrossRef]
- Andersson, M.N. Mechanisms of Odor Coding in Coniferous Bark Beetles: From Neuron to Behavior and Application. Psyche 2012, 149572. [Google Scholar] [CrossRef]
- Powers, J.S.; Sollins, P.; Harmon, M.E.; Jones, J.A. Plant-Pest Interactions in Time and Space: A Douglas-Fir Bark Beetle Outbreak as a Case Study. Landsc. Ecol. 1999, 14, 105–120. [Google Scholar] [CrossRef]
- Hulcr, J.; Mogia, M.; Isua, B.; Novotny, V. Host Specificity of Ambrosia and Bark Beetles (Col., Curculionidae: Scolytinae and Platypodinae) in a New Guinea Rainforest. Ecol. Entomol. 2007, 32, 762–772. [Google Scholar] [CrossRef]
- Fryxell, J.M. Forage Quality and Aggregation by Large Herbivores. Am. Nat. 1991, 138, 478–498. [Google Scholar] [CrossRef]
- Grosman, D.M.; Salom, S.M.; Ravlin, F.W.; Young, R.W. Geographic and Gender Differences in Semiochemicals in Emerging Adult Southern Pine Beetle (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 1997, 90, 438–446. [Google Scholar] [CrossRef]
- Seybold, S.J.; Huber, D.P.W.; Lee, J.C.; Graves, A.D.; Bohlmann, J. Pine Monoterpenes and Pine Bark Beetles: A Marriage of Convenience for Defense and Chemical Communication. Phytochem. Rev. 2006, 5, 143–178. [Google Scholar] [CrossRef]
- Chen, H.; Tang, M. Spatial and Temporal Dynamics of Bark Beetles in Chinese White Pine in Qinling Mountains of Shaanxi Province, China. Environ. Entomol. 2007, 36, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Petty, J. Bionomics of Two Aspen Bark Beetles, Trypophloeus Populi and Procryphalus Mucronatus (Coleoptera: Scolytidae). West. N. Am. Nat. 1977, 37, 105–127. [Google Scholar]
- Cao, Y.; Luo, Z.; Wang, S.; Zhang, P. Bionomics and Control of Trypophloeus Klimeschi. Entomol. Knowl. 2004, 41, 36–38. [Google Scholar]
- Gao, G.; Dai, L.; Gao, J.; Wang, J.; Chen, H. Electroantennogram, Behavioural Responses, and Field Trapping of Trypophloeus Klimeschi (Coleoptera: Curculionidae: Scolytinae) to Eight Host Volatiles. Can. Entomol. 2019, 151, 236–250. [Google Scholar] [CrossRef]
- Gao, G.; Gao, J.; Hao, C.; Dai, L.; Chen, H. Biodiversity and Activity of Gut Fungal Communities across the Life History of Trypophloeus Klimeschi (Coleoptera: Curculionidae: Scolytinae). Int. J. Mol. Sci. 2018, 19, 2010. [Google Scholar] [CrossRef] [PubMed]
- Rexrode, C.O.; Baumgras, J.E. Distribution of Gum Spots by Causal Agent in Black Cherry and Effects On Log and Tree Quality. South. J. Appl. For. 1984, 8, 22–28. [Google Scholar] [CrossRef]
- Rexrode, C.O.; Smith, H.C. Occurrence of Gum Spots in Black Cherry After Partial Harvest Cutting; US Department of Agriculture, Forest Service, North Central Research Station: St. Paul, MN, USA, 1990. [Google Scholar]
- Lombardero, M.J. Plantas Huésped y Escolítidos (Col: Scolytidae) En Galicia (Noroeste de La Península Ibérica). Boletín Sanid. Veg. 1995, 21, 357–370. [Google Scholar]
- Gao, G.; Dai, L.; Gao, J.; Wang, J.; Chen, H. Volatile Organic Compound Analysis of Host and Non-Host Poplars for Trypophloeus Klimeschi (Coleoptera: Curculionidae: Ipinae). Russ. J. Plant Physiol. 2018, 65, 916–925. [Google Scholar] [CrossRef]
- Garnica El Escarabajo Trypophloeus Sp (Bark Beetle), Un Mal Enemigo Para Los Chopos. Available online: https://www.garnica.one/blog/el-escarabajo-trypophloeus-sp-bark-beetle-un-mal.html (accessed on 29 May 2024).
- Schneider, D. Plant Recognition by Insects: A Challenge for Neuro-Ethological Research. In Insects-Plants. Proceedings of the 6th International Symposium on Insect—Plant Relationships (PAU 1986); Labyrie, V., Fabres, G., Lachaise, D., Eds.; W. Junk Publishers: Dordrecht, The Netherlands, 1987; pp. 117–123. ISBN 906193642X. [Google Scholar]
- Visser, J.H.; Fu-shun, Y. Electroantennogram Responses of the Grain Aphids Sitobion Avenae (F.) and Metopolophiurn Dirhodurn (Walk.) (Horn., Aphididae) to Plant Odour Components. J. Appl. Entomol. 1995, 119, 539–542. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publ.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Raguso, R.A.; Light, D.M. Electroantennogram Responses of Male Sphinx Perelegans Hawkmoths to Floral and “Green-Leaf Volatiles”. Entomol. Exp. Appl. 1998, 86, 287–293. [Google Scholar] [CrossRef]
- Jerković, I.; Mastelić, J. Volatile Compounds from Leaf-Buds of Populus nigra L. (Salicaceae). Phytochemistry 2003, 63, 109–113. [Google Scholar] [CrossRef]
- Kimmerer, T.W.; Kozlowski, T.T. Ethylene, Ethane, Acetaldehyde, and Ethanol Production by Plants under Stress. Plant Physiol. 1982, 69, 840–847. [Google Scholar] [CrossRef]
- Miller, D.R.; Rabaglia, R.J. Ethanol and (-)-α-Pinene: Attractant Kairomones for Bark and Ambrosia Beetles in the Southeastern US. J. Chem. Ecol. 2009, 35, 435–448. [Google Scholar] [CrossRef]
- Dodds, K.J.; Allison, J.D.; Miller, D.R.; Hanavan, R.P.; Sweeney, J. Considering Species Richness and Rarity When Selecting Optimal Survey Traps: Comparisons of Semiochemical Baited Flight Intercept Traps for Cerambycidae in Eastern North America. Agric. For. Entomol. 2015, 17, 36–47. [Google Scholar] [CrossRef]
- Bauer, J.; Vité, J.P. Host Selection by Trypodendron Lineatum. Naturwissenschaften 1975, 62, 539. [Google Scholar] [CrossRef]
- Borden, J.H.; Lindgren, B.S.; Chong, L. Ethanol and α-Pinene as Synergists for the Aggregation Pheromones of Two Gnathotrichus Species. Can. J. For. Res. 1980, 10, 290–292. [Google Scholar] [CrossRef]
- Schroeder, L.M.; Lindelöw, Å. Attraction of Scolytids and Associated Beetles by Different Absolute Amounts and Proportions of α-Pinene and Ethanol. J. Chem. Ecol. 1989, 15, 807–817. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Joseph, G. Ethanol in Ponderosa Pine as an Indicator of Physiological Injury from Fire and Its Relationship to Secondary Beetles. Can. J. For. Res. 2003, 33, 870–884. [Google Scholar] [CrossRef]
- Miller, D.R. Ethanol and (-)-α-Pinene: Attractant Kairomones for Some Large Wood-Boring Beetles in Southeastern USA. J. Chem. Ecol. 2006, 32, 779–794. [Google Scholar] [CrossRef]
- Dunn, J.P.; Potter, D.A. Synergistic Effects of Oak Volatiles with Ethanol in the Capture of Saprophagous Wood Borers. J. Entomol. Sci. 1991, 26, 425–429. [Google Scholar] [CrossRef]
- Miller, D.R.; Dodds, K.J.; Eglitis, A.; Fettig, C.J.; Hofstetter, R.W.; Langor, D.W.; Mayfield, A.E.; Munson, A.S.; Poland, T.M.; Raffa, K.F. Trap Lure Blend of Pine Volatiles and Bark Beetle Pheromones for Monochamus spp. (Coleoptera: Cerambycidae) in Pine Forests of Canada and the United States. J. Econ. Entomol. 2013, 106, 1684–1692. [Google Scholar] [CrossRef]
- Miller, D.R. Coleopteran Predators of Bark and Woodboring Beetles Attracted to Traps Baited with Ethanol and α-Pinene in Pine (Pinaceae) Forests of the Southern United States of America. Can. Entomol. 2023, 155. [Google Scholar] [CrossRef]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex Pheromones and Their Impact on Pest Management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef]
- Boeckler, G.A.; Gershenzon, J.; Unsicker, S.B. Phenolic Glycosides of the Salicaceae and Their Role as Anti-Herbivore Defenses. Phytochemistry 2011, 72, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Thieme, H.; Benecke, R. Phenolglycosides of Salicaceae. 8. Studies on the Glycoside Accumulation in Some Middle European Populus Species. Pharmazie 1971, 26, 227–231. [Google Scholar] [PubMed]
- Boeckler, G.A.; Paetz, C.; Feibicke, P.; Gershenzon, J.; Unsicker, S.B. Metabolism of Poplar Salicinoids by the Generalist Herbivore Lymantria Dispar (Lepidoptera). Insect Biochem. Mol. Biol. 2016, 78, 39–49. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L.; Głód, D.; Jesionek, A.; Łuczkiewicz, M.; Krauze-Baranowska, M. Studies on the Polyphenolic Composition and the Antioxidant Properties of the Leaves of Poplar (Populus spp.) Various Species and Hybrids. Chem. Biodivers. 2021, 18, e2100227. [Google Scholar] [CrossRef] [PubMed]
- Bentz, B.J.; Rgnire, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negron, J.F.; Seybold, S.J. Climate Change and Bark Beetles of the Western United States and Canada: Direct and Indirect Effects. Bioscience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Weed, A.S.; Ayres, M.P.; Hicke, J.A. Consequences of Climate Change for Biotic Disturbances in North American Forests. Ecol. Monogr. 2013, 83, 441–470. [Google Scholar] [CrossRef]
- Campbell, S.A.; Borden, J.H. Additive and Synergistic Integration of Multimodal Cues of Both Hosts and Non-Hosts during Host Selection by Woodboring Insects. Oikos 2009, 118, 553–563. [Google Scholar] [CrossRef]
- El-Sayed the Pherobase: Database of Pheromones and Semiochemicals. Available online: https://pherobase.com/ (accessed on 27 May 2024).
- Soto, A.; Orengo, L.; Estrela, A. Estudio de Poblaciones de Insectos Escolítidos (Coleoptera: Scolytidae) En Las Masas de Pinus Halepensis Miller Del Parque Natural Del Montgó (Alicante). Bol. San. Veg. Plagas 2002, 28, 445–456. [Google Scholar]
| Relative Abundance (%) c,d | |||||||
|---|---|---|---|---|---|---|---|
| Compound | RT a (min) | RI-5 b | Clone USA 184-411 | Clone I-214 | Clone USA 184-411 | Clone I-214 | Identification e |
| Isobutanol | 3.66 | 635 | 0.17 ± 0.07 | 0.28 ± 0.10 | n.d. | n.d. | MS; STD |
| (E)-3-Hexen-1-ol | 6.63 | 853 | n.d. | n.d. | 0.09 ± 0.03 | 0.06 ± 0.03 | MS; STD |
| (E)-2-Hexenal | 6.71 | 857 | n.d. | n.d. | 1.71 ± 0.24 | 0.29 ± 0.09 | MS; STD |
| (Z)-3-Hexen-1-ol | 6.89 | 861 | n.d. | n.d. | 2.04 ± 1.35 | 1.38 ± 0.58 | MS; STD |
| Benzaldehyde | 6.92 | 966 | 0.83 ± 0.24 | 0.37 ± 0.21 | 0.30 ± 0.20 | n.d. | MS; STD |
| Limonene | 6.97 | 1030 | 0.13 ± 0.05 | 0.28 ± 0.11 | 0.39 ± 0.09 | n.d. | MS; STD |
| 1.8-Cineole | 8.19 | 1038 | n.d | 0.09 ± 0.02 | 0.07 ± 0.02 | n.d. | MS; STD |
| Salicylaldehyde | 6.46 | 1045 | 11.38 ± 2.77 | 7.90 ± 0.34 | 62.73 ± 14.5 | 3.05 ± 0.54 | MS; STD |
| β-Ocimene | 4.12 | 1048 | 0.16 ± 0.07 | n.d. | 0.87 ± 0.19 | n.d. | MS; STD |
| p-Cresol | 4.73 | 1078 | 2.39 ± 0.90 | 0.47 ± 0.17 | 3.18 ± 1.80 | n.d. | MS; STD |
| Methyl benzoate | 5.32 | 1090 | 0.16 ± 0.10 | 0.64 ± 0.25 | 0.48 ± 0.17 | 0.57 ± 0.13 | MS; STD |
| Linalool | 5.56 | 1099 | 0.07 ± 0.03 | n.d. | 0.15 ± 0.06 | 0.22 ± 0.04 | MS; STD |
| Nonanal | 8.87 | 1108 | 1.19 ± 0.82 | 0.13 ± 0.04 | 0.64 ± 0.09 | 0.14 ± 0.05 | MS; STD |
| Catechol | 9.11 | 1199 | 0.28 ± 0.07 | 0.51 ± 0.12 | 0.16 ± 0.07 | 0.12 ± 0.06 | MS; STD |
| Salicylic alcohol | 8.82 | 1230 | 4.18 ± 2.44 | 1.50 ± 0.33 | 0.29 ± 0.13 | 0.26 ± 0.10 | MS; STD |
| Methyl salicylate | 11.24 | 1294 | 2.89 ± 0.80 | 0.16 ± 0.12 | 0.30 ± 0.06 | 0.19 ± 0.08 | MS; STD |
| Eugenol | 11.35 | 1357 | n.d. | n.d. | 3.67 ± 1.17 | 17.26 ± 3.5 | MS; STD |
| trans-β-Ionone | 11.72 | 1366 | n.d. | n.d. | 0.23 ± 0.10 | 0.09 ± 0.04 | MS; STD |
| α-Copaene | 11.89 | 1379 | 5.66 ± 3.32 | 2.11 ± 0.54 | 1.07 ± 0.35 | 0.16 ± 0.05 | MS; STD |
| β-Cubebene | 12.00 | 1390 | 0.11 ± 0.01 | n.d. | n.d. | n.d. | MS |
| α-Caryophyllene | 12.21 | 1408 | 0.92 ± 0.17 | 0.13 ± 0.04 | 0.35 ± 0.22 | n.d. | MS; STD |
| E-β-Caryophyllene | 12.84 | 1417 | 1.82 ± 0.29 | 0.18 ± 0.07 | 0.66 ± 0.28 | 1.30 ± 0.25 | MS; STD |
| α-Guaiene | 13.05 | 1441 | 0.38 ± 0.20 | n.d. | n.d. | 0.67 ± 0.15 | MS |
| trans-β-Farnesene | 13.18 | 1458 | 0.46 ± 0.18 | n.d. | 0.79 ± 0.33 | n.d. | MS; STD |
| Alloaromadendrene | 13.26 | 1453 | 1.49 ± 0.58 | n.d. | n.d. | 1.04 ± 0.43 | MS |
| β-Guaiene | 13.32 | 1491 | 0.67 ± 0.18 | n.d. | 0.38 ± 0.08 | n.d. | MS; STD |
| Valencene | 14.54 | 1494 | 0.57 ± 0.17 | 0.57 ± 0.23 | n.d. | 0.14 ± 0.05 | MS |
| E-β-Bergamotene | 13.67 | 1580 | 0.04 ± 0.02 | n.d. | 0.49 ± 0.24 | n.d. | MS |
| γ-Muurolene | 13.79 | 1479 | 0.28 ± 0.03 | n.d. | 0.37 ± 0.19 | 0.39 ± | MS |
| α-Muurolene | 14.88 | 1501 | 0.72 ± 0.27 | n.d. | n.d. | n.d. | MS |
| α-Bisabolene | 15.00 | 1503 | n.d. | n.d. | n.d. | 0.58 ± 0.22 | MS |
| (E.E)-α-Farnesene | 15.18 | 1507 | 0.11 ± 0.05 | n.d. | 0.46 ± 0.09 | 0.22 ± 0.05 | MS; STD |
| β-Bisabolene | 15.29 | 1512 | 0.26 ± 0.11 | n.d. | n.d. | n.d. | MS; STD |
| Curcumene | 15.41 | 1516 | 0.19 ± 0.03 | n.d. | n.d. | n.d. | MS |
| δ-Cadinene | 16.03 | 1527 | 3.03 ± 0.05 | 2.04 ± 0.89 | 0.29 ± 0.13 | 2.81 ± 0.55 | MS; STD |
| α-Calacorene | 17.20 | 1542 | 0.13 ± 0.02 | n.d. | 0.19 ± 0.09 | 0.27 ± 0.12 | MS |
| trans-Nerolidol | 17.65 | 1564 | n.d. | 0.05 ± 0.01 | n.d. | n.d. | MS |
| α-Caryophyllene | 18.37 | 1583 | 0.32 ± 0.20 | n.d. | n.d. | 0.62 ± 0.16 | MS |
| Guaiol | 18.52 | 1608 | 0.70 ± 0.32 | n.d. | 0.19 ± 0.13 | 0.24 ± 0.13 | MS; STD |
| γ-Eudesmol | 18.66 | 1621 | 0.46 ± 0.10 | n.d. | n.d | n.d | MS |
| δ-Eudesmol | 18.71 | 1631 | 2.69 ± 0.94 | n.d. | 2.56 ± 1.68 | 0.29 ± 0.22 | MS |
| Cubenol | 19.00 | 1639 | n.d. | n.d. | n.d. | 3.81 ± 1.61 | MS |
| β-Eudesmol | 19.15 | 1653 | 2.69 ± 0.41 | n.d | n.d | 1.80 ± 0.76 | MS |
| α-Cadinol | 19.48 | 1652 | 0.71 ± 0.28 | n.d. | 0.27 ± 0.09 | 0.86 ± 0.36 | MS |
| α-Muurolol | 20.11 | 1653 | 0.33 ± 0.05 | n.d. | n.d. | n.d. | MS |
| Tridecanoic acid | 22.13 | 1670 | 2.09 ± 0.96 | n.d. | n.d. | n.d. | MS |
| α-Bisabolol | 22.40 | 1675 | 1.33 ± 0.35 | n.d. | n.d. | n.d. | MS |
| Methyl linoleate | 30.37 | 1716 | n.d | 0.77 ± 0.09 | n.d. | 2.25 ± 0.57 | MS |
| Hexadecanoic acid | 34.50 | 1968 | 2.35 ± 1.00 | 7.46 ± 2.73 | 1.12 ± 0.30 | 6.77 ± 0.35 | MS; STD |
| Hexatriacontane | 39.88 | 2095 | n.d | 26.09 ± 7.03 | n.d. | n.d. | MS |
| Linoleic acid | 40.03 | 2127 | 2.89 ± 1.45 | 0.30 ± 0.10 | n.d | 0.40 ± 0.20 | MS; STD |
| Oleic acid | 40.62 | 2136 | 0.35 ± 0.27 | 0.31 ±0.14 | n.d. | 0.19 ± 0.02 | MS; STD |
| Octadecanoic acid | 42.12 | 2175 | 2.15 ± 0.38 | 1.26 ±0.39 | n.d. | 0.73 ± 0.10 | MS; STD |
| Poplar Plantations | Attractants | T. binodulus Captures | ||||||
|---|---|---|---|---|---|---|---|---|
| Clone I-214 | Clone USA 184-411 | F | df | p | ||||
| Villasabariego | Ethanol | 4.32 ± 0.80 b 1 | 7.16 ± 1.20 b | 3.887 | (1.36) | 0.050 | ||
| Ethanol + MB | 2.47 ± 0.64 b | 4.21 ± 1.00 b | 2.106 | (1.36) | 0.155 | |||
| Ethanol + SA | 10.37 ± 2.03 a | 25.37 ± 5.20 a | 7.196 | (1.36) | 0.011 | |||
| F | 9.848 | F | 13.320 | |||||
| df | (2.54) | df | (2.54) | |||||
| p | <0.001 | p | <0.001 | |||||
| Villoria de Órbigo | Ethanol | 5.00 ± 1.34 a | 6.89 ± 1.87 b | 0.673 | (1.36) | 0.417 | ||
| Ethanol + MB | 2.16 ± 0.36 a | 9.68 ± 2.42 b | 9.008 | (1.36) | 0.005 | |||
| Ethanol + SA | 2.37 ± 0.62 a | 34.16 ± 7.02 a | 20.320 | (1.36) | <0.001 | |||
| F | 2.894 | F | 11.488 | |||||
| df | (2.54) | df | (2.54) | |||||
| p | 0.064 | p | <0.001 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, A.; Ruiz-Martos, L.; Bruno, A.; Vega-Valdés, C.; Díez-Presa, E.; Delgado-Salán, L.; Mínguez-Bermejo, D.; Casquero, P.A.; Rodríguez-González, Á. Salicylic Aldehyde and Its Potential Use in Semiochemical-Based Pest Control Strategies Against Trypophloeus binodulus. Insects 2024, 15, 964. https://doi.org/10.3390/insects15120964
Ortiz A, Ruiz-Martos L, Bruno A, Vega-Valdés C, Díez-Presa E, Delgado-Salán L, Mínguez-Bermejo D, Casquero PA, Rodríguez-González Á. Salicylic Aldehyde and Its Potential Use in Semiochemical-Based Pest Control Strategies Against Trypophloeus binodulus. Insects. 2024; 15(12):964. https://doi.org/10.3390/insects15120964
Chicago/Turabian StyleOrtiz, Antonio, Lucía Ruiz-Martos, Andy Bruno, Carmen Vega-Valdés, Eva Díez-Presa, Lucía Delgado-Salán, Dana Mínguez-Bermejo, Pedro A. Casquero, and Álvaro Rodríguez-González. 2024. "Salicylic Aldehyde and Its Potential Use in Semiochemical-Based Pest Control Strategies Against Trypophloeus binodulus" Insects 15, no. 12: 964. https://doi.org/10.3390/insects15120964
APA StyleOrtiz, A., Ruiz-Martos, L., Bruno, A., Vega-Valdés, C., Díez-Presa, E., Delgado-Salán, L., Mínguez-Bermejo, D., Casquero, P. A., & Rodríguez-González, Á. (2024). Salicylic Aldehyde and Its Potential Use in Semiochemical-Based Pest Control Strategies Against Trypophloeus binodulus. Insects, 15(12), 964. https://doi.org/10.3390/insects15120964











