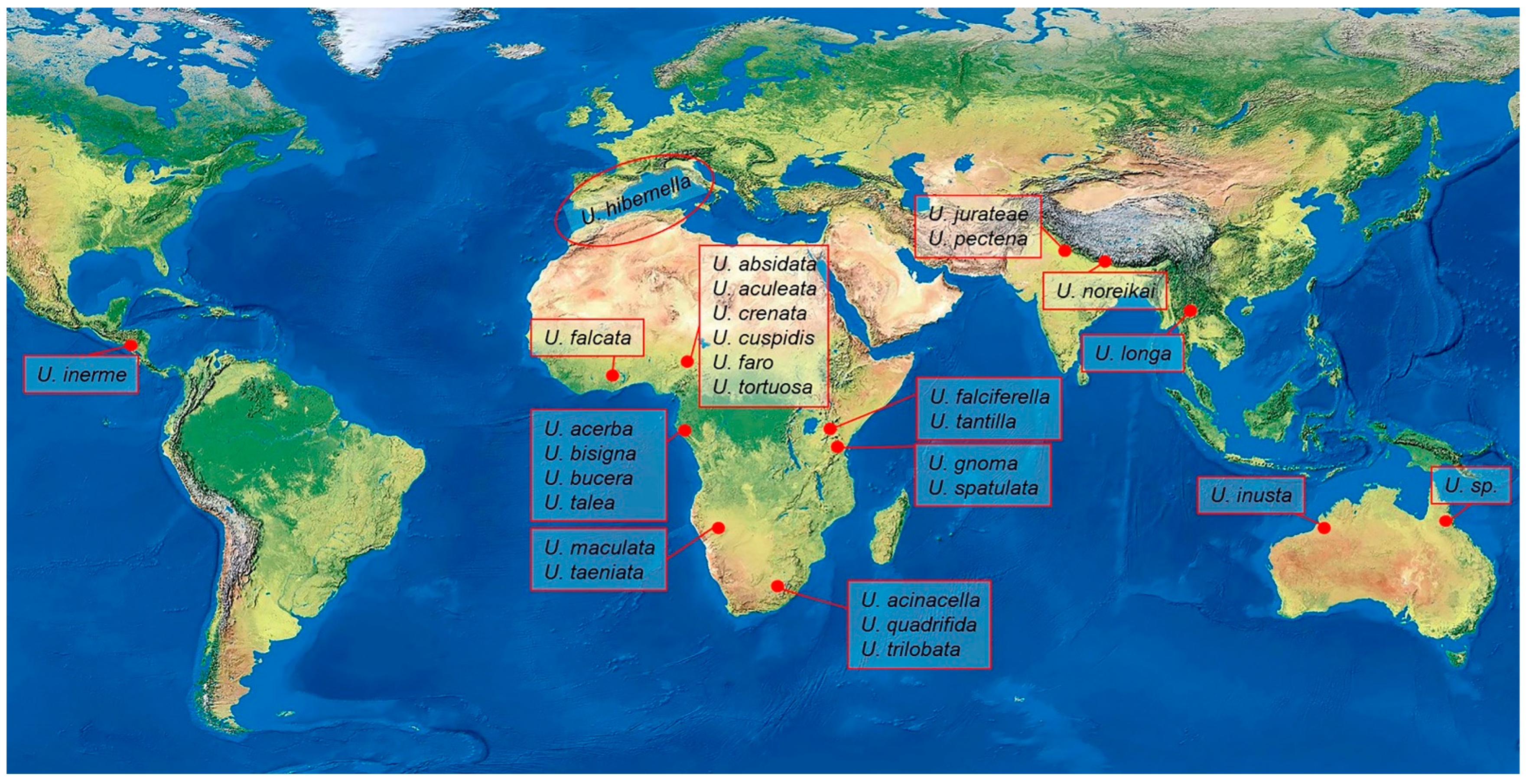
The First Neotropical Record of the Genus Urodeta (Lepidoptera: Elachistidae: Elachistinae) with Keys to the World Species and a Description of a New Species from Honduras
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
- Spinose knob of gnathos reduced ([18]: Figure 54) ....................................... U. hibernella
- –
- Spinose knob of gnathos well developed, armed with spines directed cephalically ................................................................................................................................................. 2
- 2.
- Sacculus entirely separated from remaining valva as an elongate lobe ...................... 3
- –
- Sacculus not separated from remaining valva ................................................................. 5
- 3.
- Valva divided into three distinct lobes (sacculus entirely separated, and termen of the remaining part of valva deeply emarginated, so appear divided into long and narrow lobes) ([1]: Figures 21–23) ................................................................................... U. trilobata
- –
- Valva divided into two separate lobes (sacculus and the remaining part of the valva) ................................................................................................................................................. 4
- 4.
- Uncus well developed, as long as the width of the spinose knob of the gnathos; spinose knob of the gnathos large; vesica with different cornuti: one elongate plate-like, one near square plate-like, and numerous small spine-like cornuti ([9]: Figures 25–28) .................................................................................................................................... U. acerba
- –
- Uncus not developed; spinose knob of the gnathos very small; vesica with several minute spines and two clusters of large cornuti (about 15–20 and 18–26), slightly different in size ([2]: Figures 5–11) .......................................................................... U. noreikai
- 5.
- Ventral margin of sacculus distinctly serrated ([9]: Figures 52–55) ................ U. crenata
- –
- Ventral margin of sacculus not serrated ........................................................................... 6
- 6.
- Spinose knob of the gnathos divided into two separate lobes ([9]: Figures 39–41) ................................................................................................................................... U. bucera
- –
- Spinose knob of gnathos not divided ............................................................................... 7
- 7.
- Inner processes of valvae fused apically and embedded with many small cusp-shaped spines ([9]: Figures 15–20) ...................................................................... U. absidata
- –
- Valva without inner process embedded with spines ...................................................... 8
- 8.
- Phallus with a strongly sclerotized narrow band along the ventral margin ............... 9
- –
- Phallus without a strongly sclerotized band along the ventral margin ...................... 12
- 9.
- Valvae are tightly fused together dorso–proximally; the spinose knob of the gnathos is rounded ([9]: Figures 74–76) ................................................................................. U. talea
- –
- Valvae not fused together dorso–proximally; the spinose knob of the gnathos is elongated .................................................................................................................................... 10
- 10.
- Indentation of the distal margin of the juxta is wider than the width of the juxta lobe ([9]: Figures 35 and 36) ......................................................................................... U. aculeata
- –
- Distal margin of the juxta is not indented ....................................................................... 11
- 11.
- Vesica with a cluster of minute internal spines and four large spine-shaped cornuti ([7]: Figures 33 and 34) ....................................................................................... U. maculata
- –
- Vesica with a cluster of minute internal spines and two large claw-shaped cornuti ([10]: Figures 7–14) .................................................................................................. U. falcata
- 12.
- Vesica without cornuti ...................................................................................................... 13
- –
- Vesica with cornuti ............................................................................................................ 14
- 13.
- Costa of the valva wrinkled, tapered to a sharp curved tip; cucullus short, about 1.5 times longer than wide ([6]: Figure 97) ....................... U. sp. reared from Terminalia sp.
- –
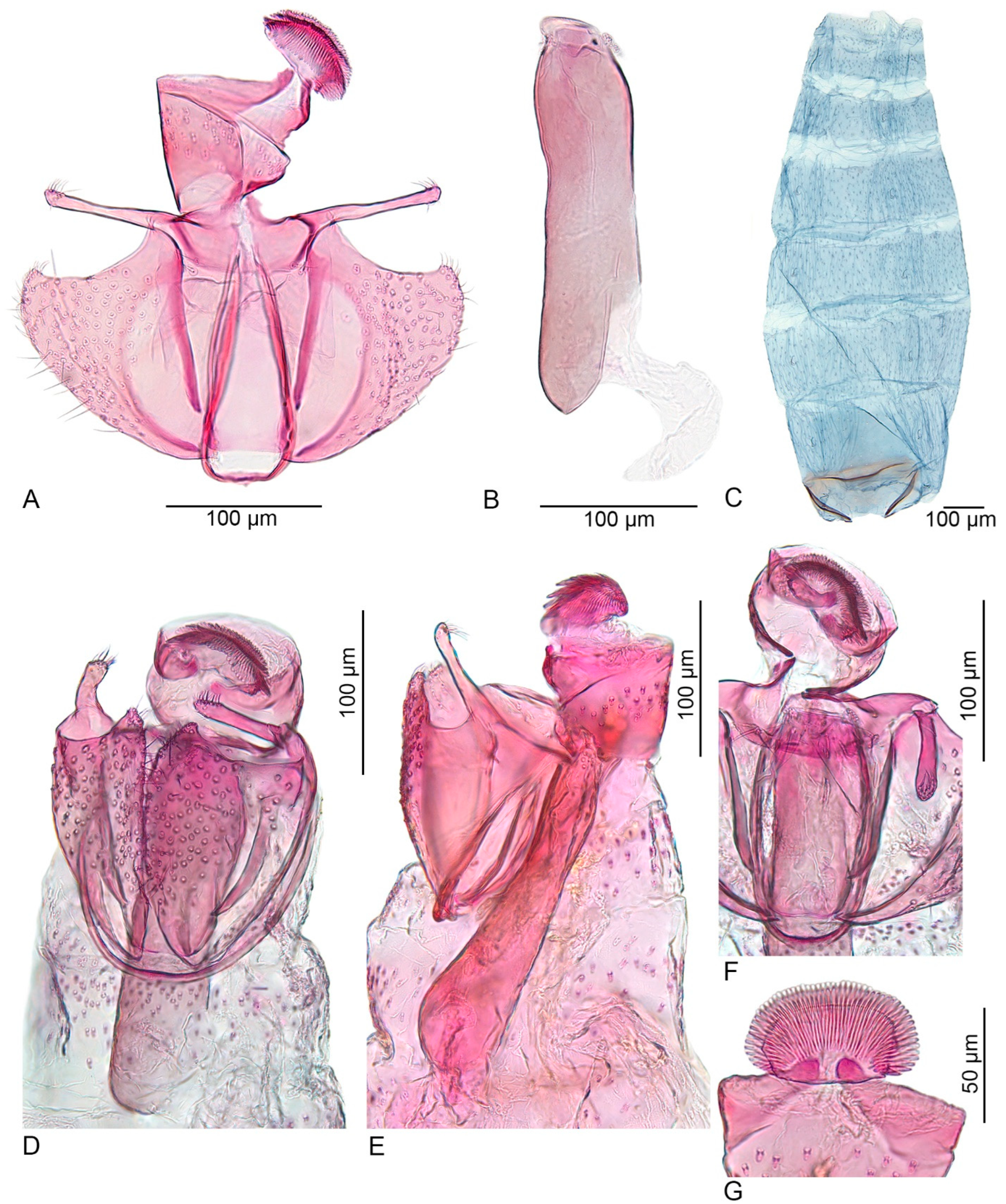
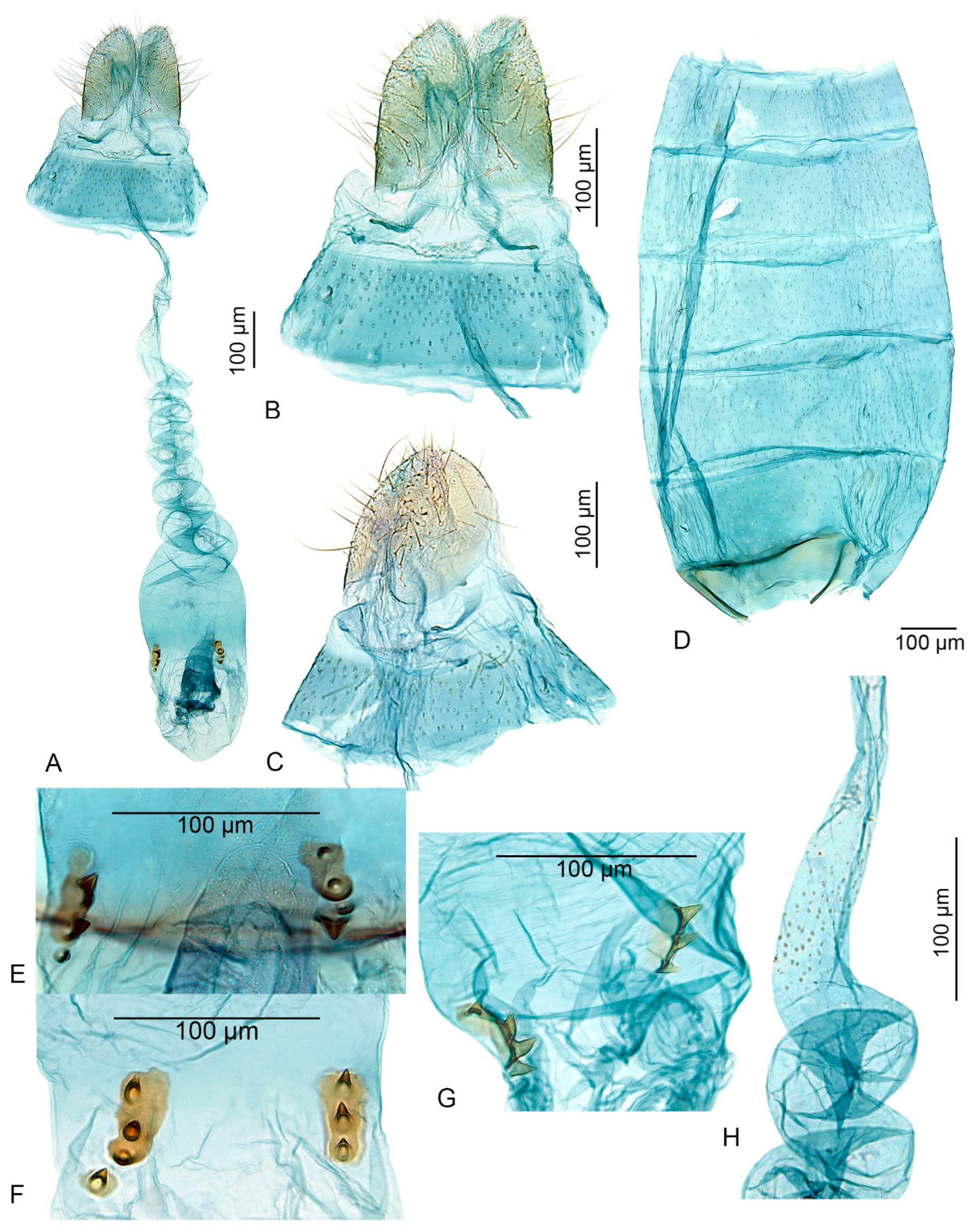
- Costa of the valva smooth, without a sharp curved tip; cucullus long, about 10 times longer than wide (this paper, Figure 3) ................................................. U. inerme sp. nov.
- 14.
- Vesica with one cornutus .................................................................................................. 15
- –
- Vesica with more than one cornutus ................................................................................ 16
- 15.
- Phallus about six–seven times longer than wide ([7]: Figures 35 and 36) ...... U. taeniata
- –
- Phallus about three–four times longer than wide ([9]: Figures 58–63) ........... U. cuspidis
- 16.
- Phallus gradually tapered towards pointed apex.......................................................... 17
- –
- Phallus apically not pointed ............................................................................................. 19
- 17.
- Cucullus narrow and long, about four times longer than broad; the spinose knob of the gnathos is oval ([8]: Figures 37–40) ................................................................. U. gnoma
- –
- Cucullus short, about two times longer than broad; the spinose knob of the gnathos is rounded ............................................................................................................................ 18
- 18.
- Ventral shield of the juxta with the caudal margin mesially incised ([6]: Figures 95 and 96) ...................................................................................................................... U. inusta
- –
- Ventral shield of the juxta with the caudal margin mesially not incised ([11]: Figures 9–13) ....................................................................................................................... U. jurateae
- 19.
- Ventral shield of the juxta with distinct apically rounded lobes; vesica with six large cornuti and numerous tiny spines ([8]: Figures 50 and 51) ............................... U. tantilla
- –
- Ventral shield of the juxta without distinct lobes; vesica with two rows of large cornuti (4–5 in each row) and numerous tiny spines ([8]: Figures 44–47) ......... U. spatulata
- Corpus bursae with a signum ............................................................................................ 2
- –
- Corpus bursae without a signum ..................................................................................... 14
- 2.
- Both pairs of apophyses (anteriores and posteriores) present ...................................... 3
- –
- Apophyses anteriores absent ............................................................................................ 11
- 3.
- Apophyses posteriores well developed, with length not less than 0.7 of the length of abdominal segment 7 ........................................................................................................... 4
- –
- Apophyses posteriores vestigial, not longer than 0.3 of length of abdominal segment 7 .............................................................................................................................................. 8
- 4.
- Ostium area covered by small spines ................................................................................ 5
- –
- Ostium area without spines ................................................................................................ 6
- 5.
- Ductus bursae with longitudinal folds; the signum is sickle-shaped ([1]: Figures 6–10) ........................................................................................................................ U. acinacella
- –
- Ductus bursae without longitudinal folds; the signum is formed by two weakly connected plates, each with a large spine and a few smaller ones ([1]: Figures 13–16) .............................................................................................................................. U. quadrifida
- 6.
- Ductus bursae coiled ........................................................................................................... 7
- –
- Ductus bursae not coiled ([18]: Figure 55) ...................................................... U. hibernella
- 7.
- Signum formed by a weakly sclerotized plate with four large teeth in a row; ostium without sclerotized plates; antrum without internal spines ([6]: Figure 98) .... U. inusta
- –
- Signum formed by one sickle-shaped spine and a weakly sclerotized transverse plate covered with tiny spines; ostium surrounded by longitudinal sclerotized plates; antrum with strong spines in the posterior part ([8]: Figures 41–43) .............. U. falciferella
- 8.
- Ductus bursae not coiled ([9]: Figures 42–49) ...................................................... U. bucera
- –
- Ductus bursae coiled ........................................................................................................... 9
- 9.
- Antrum with weakly sclerotized longitudinal folds; the signum is rounded, dentate, and surrounded by spines arranged radially ([2]: Figures 12–17) .................. U. noreikai
- –
- Antrum without sclerotized longitudinal folds; the signum is not rounded ............ 10
- 10.
- Signum formed from nine stout teeth, which slightly vary in size ([11]: Figures 18 and 19) ............................................................................................................................ U. pectena
- –
- Signa consisting of two irregularly oval sclerotized plates, each with two or three short, stout spines (this paper, Figure 4) ............................................. U. inerme sp. nov.
- 11.
- Corpus bursae with two signa ([10]: Figures 17 and 18) ................................... U. bisigna
- –
- Corpus bursae with one signum ...................................................................................... 12
- 12.
- Ductus bursae coiled ([9]: Figures 77–82) ............................................................... U. talea
- –
- Ductus bursae not coiled ................................................................................................... 13
- 13.
- Corpus bursae with internal spines arranged in rows of 3–8; signum formed by an oval sclerotized plate with one large and several small spines ([1]: Figures 24–28) ................................................................................................................................ U. trilobata
- –
- Corpus bursae without minute internal spines; signum formed from four stout teeth ([7]: Figures 30 and 31) ....................................................................................... U. maculata
- 14.
- Papillae anales with round, semitransparent spots where long setae arise; corpus bursae divided by a narrow prolonged constriction into two parts ([9]: Figures 29–32) .................................................................................................................................... U. acerba
- –
- Papillae anales without round, semitransparent spots where long setae arise; corpus bursae not divided into two parts .................................................................................... 15
- 15.
- Corpus bursae narrow and long, about four times longer than wide ([9]: Figures 21 and 22) .................................................................................................................... U. absidata
- –
- Corpus bursae rounded .................................................................................................... 16
- 16.
- Apophyses posteriores about 13 times longer than its width ([8]: Figures 48 and 49) ............................................................................................................................... U. spatulata
- –
- Apophyses posteriores absent or not longer than seven times its width ............................................................................................................................................... 17
- 17.
- Posterior margin of sternum 8 strongly sclerotized and folded, forming a wide pocket ([9]: Figures 85–88) .............................................................................................. U. tortuosa
- –
- Posterior margin of sternum 8 not folded ...................................................................... 18
- 18.
- Apophyses anteriores short, about 3–6 time longer than wide ................................... 19
- –
- 19.
- Antrum large, oval, and strongly sclerotized, with about 24 large and several small internal spines ([12]: Figures 3–6) ........................................................................... U. longa
- –
- Antrum without internal spines ....................................................................................... 20
- 20.
- Colliculum membranous, with dense internal spines; dorsal wall with a large, strongly sclerotized paired plate ([11]: Figures 14 and 15) .............................. U. jurateae
- –
- Colliculum strongly sclerotized, with minute internal spines; dorsal wall without a strongly sclerotized paired plate ([9]: Figures 66–71) ............................................. U. faro
4. Discussion
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Prins, J.; Sruoga, V. A review of the taxonomic history and biodiversity of the genus Urodeta (Lepidoptera: Elachistidae: Elachistinae), with description of new species. Zootaxa 2012, 3488, 41–62. [Google Scholar] [CrossRef]
- Sruoga, V.; De Prins, J. A new species of Urodeta (Lepidoptera: Elachistidae: Elachistinae) from Nepal, the first record of the genus from Asia, showing an ancient distribution pattern. Zootaxa 2013, 3599, 94–100. [Google Scholar] [CrossRef]
- Stainton, H.T. The Tineina of Southern Europe; John van Voorst: London, UK, 1869; pp. viii + 370. [Google Scholar] [CrossRef][Green Version]
- Zerkowitz, A. The Lepidoptera of Portugal. J. N. Y. Entomol. Soc. 1946, 54, 115–165. [Google Scholar]
- Lhomme, L. Catalogue des Lépidoptères de France et de Belgique. Tineina; par Douelle (Lot): Le Carriol, France, 1946–1963; Volume 2, pp. 489–1253. [Google Scholar]
- Kaila, L. Elachistine Moths of Australia (Lepidoptera: Gelechioidea: Elachistidae). Monographs on Australian Lepidoptera; CSIRO Publishing: Melbourne, Australia, 2011; Volume 11, 443p. [Google Scholar] [CrossRef]
- Mey, W. Microlepidoptera: Smaller families. In Esperiana Memoir: The Lepidoptera of the Brandberg Massif in Namibia; Part 2; Mey, W., Ed.; 2007; Volume 4, pp. 9–30. Available online: https://www.esperiana.info/ESPERIANA-Memoir/Esperiana-Memoir-4/ (accessed on 26 November 2024).
- Sruoga, V.; De Prins, J. The Elachistinae (Lepidoptera: Elachistidae) of Kenya with descriptions of eight new species. Zootaxa 2009, 2172, 1–31. [Google Scholar] [CrossRef]
- Sruoga, V.; De Prins, J. New species of Elachistinae (Lepidoptera: Elachistidae) from Cameroon and the Democratic Republic of the Congo. Zootaxa 2011, 3008, 1–32. [Google Scholar] [CrossRef]
- Sruoga, V.; De Prins, J. New species of Urodeta Stainton, 1869 (Lepidoptera, Elachistidae, Elachistinae) from Ghana and Democratic Republic of the Congo, with identification keys to the Afrotropical species of the genus. ZooKeys 2022, 1089, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Sruoga, V.; Rocienė, A. Three new species of Elachistidae (Lepidoptera: Gelechioidea) from India. Zootaxa 2018, 4394, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Sruoga, V.; Kaila, L.; Rocienė, A. The Elachistinae (Lepidoptera: Gelechioidea, Elachistidae) of Thailand, with description of eight new species. Eur. J. Taxon. 2019, 574, 1–34. [Google Scholar] [CrossRef]
- Stonis, J.R.; Remeikis, A.; Diškus, A.; Dobrynina, V.; Orlovskytė, S. A Phenomenon: What Are the Minuscule Grey Moths Abundant in the Dry Season in the Tropical Dry Forests of the Pacific Coast of Honduras? Insects 2024, 15, 641. [Google Scholar] [CrossRef] [PubMed]
- Brehm, G. A new LED lamp for the collection of nocturnal Lepidoptera and a spectral comparison of light-trapping lamps. Nota Lepidopterol. 2017, 40, 87–108. [Google Scholar] [CrossRef]
- Robinson, G.S. The preparation of slides of Lepidoptera genitalia with special reference to the Microlepidoptera. Entomol. Gaz. 1976, 27, 127–132. [Google Scholar]
- Traugott-Olsen, E.; Nielsen, E.S. The Elachistidae (Lepidoptera) of Fennoscandia and Denmark. Fauna Entomol. Scand. 1977, 6, 1–299. [Google Scholar]
- Kaila, L. Phylogeny and classification of the Elachistidae s.s. (Lepidoptera: Gelechioidea). Syst. Entomol. 1999, 24, 139–169. [Google Scholar] [CrossRef]
- Koster, S.; Sinev, S. Momphidae, Batrachedridae, Stathmopodidae, Agonoxenidae, Cosmopterigidae, Chrysopeleiidae. In Microlepidoptera of Europe; Huemer, P., Karsholt, O., Lyneborg, L., Eds.; Apollo Books: Stenstrup, Denmark, 2003; Volume 5, pp. 1–387. [Google Scholar] [CrossRef]
- Sruoga, V.; Puplesis, R. A remarkable new species of Stephensia Stainton (Insecta: Lepidoptera: Elachistidae) from Belize rainforest (Central America). Zool. Sci. 2003, 20, 1273–1277. [Google Scholar] [CrossRef] [PubMed][Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sruoga, V. The First Neotropical Record of the Genus Urodeta (Lepidoptera: Elachistidae: Elachistinae) with Keys to the World Species and a Description of a New Species from Honduras. Insects 2024, 15, 941. https://doi.org/10.3390/insects15120941
Sruoga V. The First Neotropical Record of the Genus Urodeta (Lepidoptera: Elachistidae: Elachistinae) with Keys to the World Species and a Description of a New Species from Honduras. Insects. 2024; 15(12):941. https://doi.org/10.3390/insects15120941
Chicago/Turabian StyleSruoga, Virginijus. 2024. "The First Neotropical Record of the Genus Urodeta (Lepidoptera: Elachistidae: Elachistinae) with Keys to the World Species and a Description of a New Species from Honduras" Insects 15, no. 12: 941. https://doi.org/10.3390/insects15120941
APA StyleSruoga, V. (2024). The First Neotropical Record of the Genus Urodeta (Lepidoptera: Elachistidae: Elachistinae) with Keys to the World Species and a Description of a New Species from Honduras. Insects, 15(12), 941. https://doi.org/10.3390/insects15120941





