Revealing the Immune Response of Sitona callosus Gyllenhal to Entomopathogenic Fungi Beauveria bassiana Infection Through Integrative Analyses of Transcriptomics and Metabolomics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Insects
2.2. Spore Suspension and Preparation of S. callosus Samples
2.3. Experimental Method and Analysis Process of RNA Sequencing from S. callosus
2.4. Widely Untargeted Metabolomics Analysis of S. callosus
2.5. Real-Time Fluorescence Quantitative PCR Detection
2.6. Statistical Analysis
3. Results and Analysis
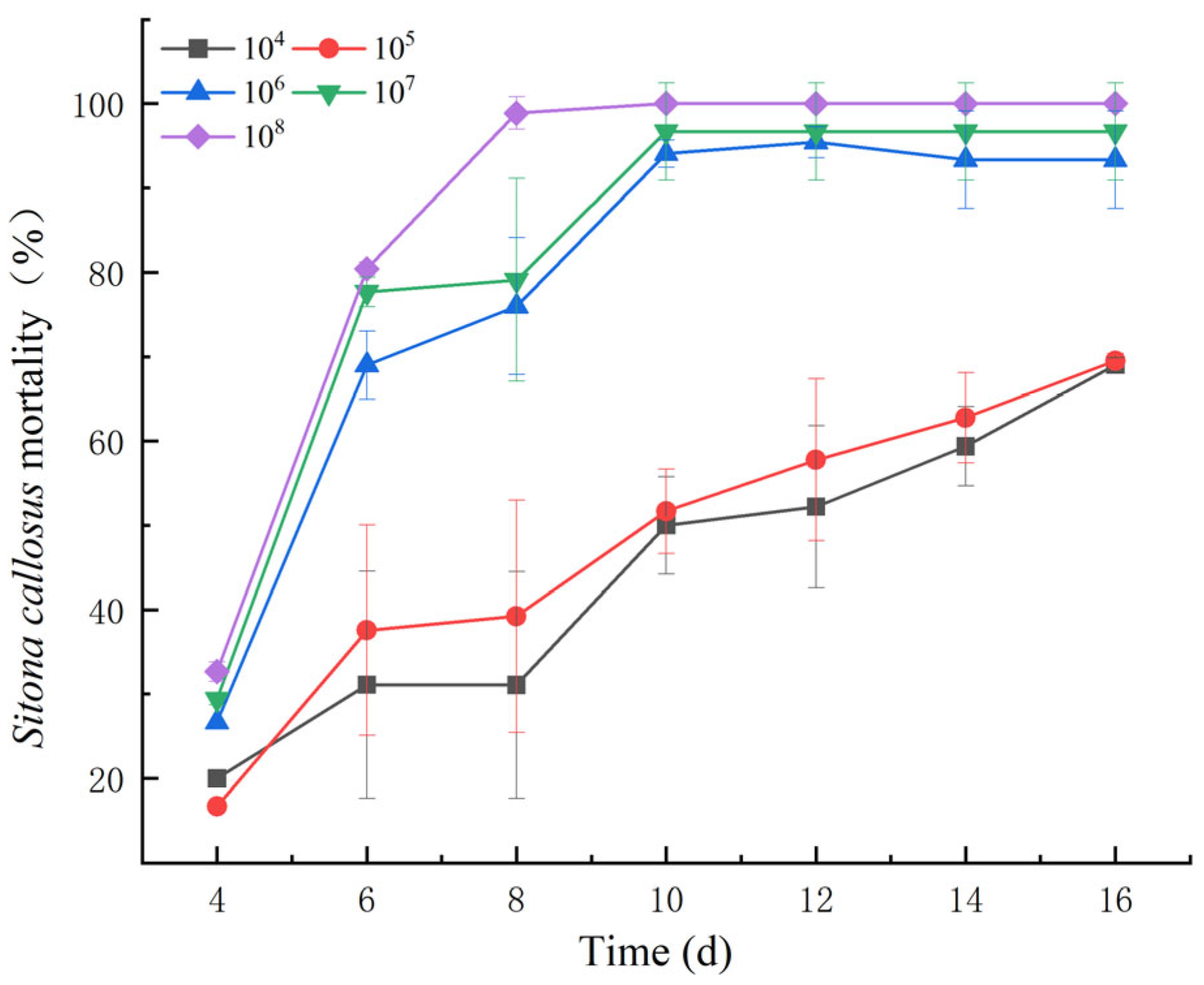
3.1. Changes in S. callosus Infected with B. bassiana
3.2. Transcriptome Data Quality Control and Analysis
3.3. Gene Function
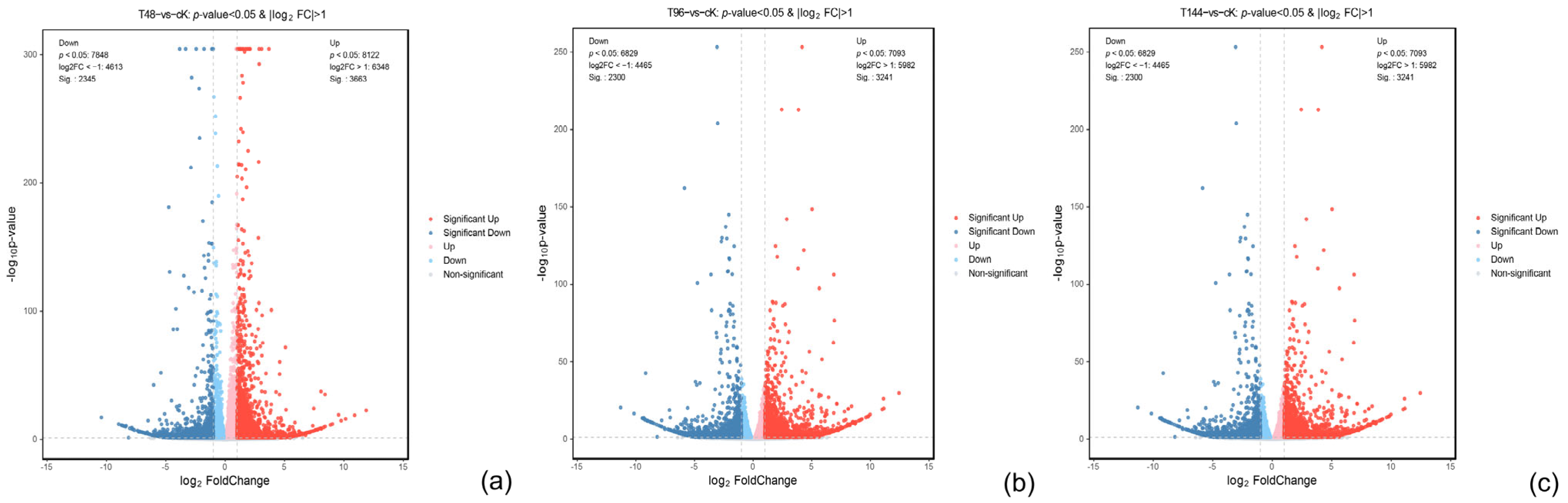
3.4. Differential Gene Expression Analysis
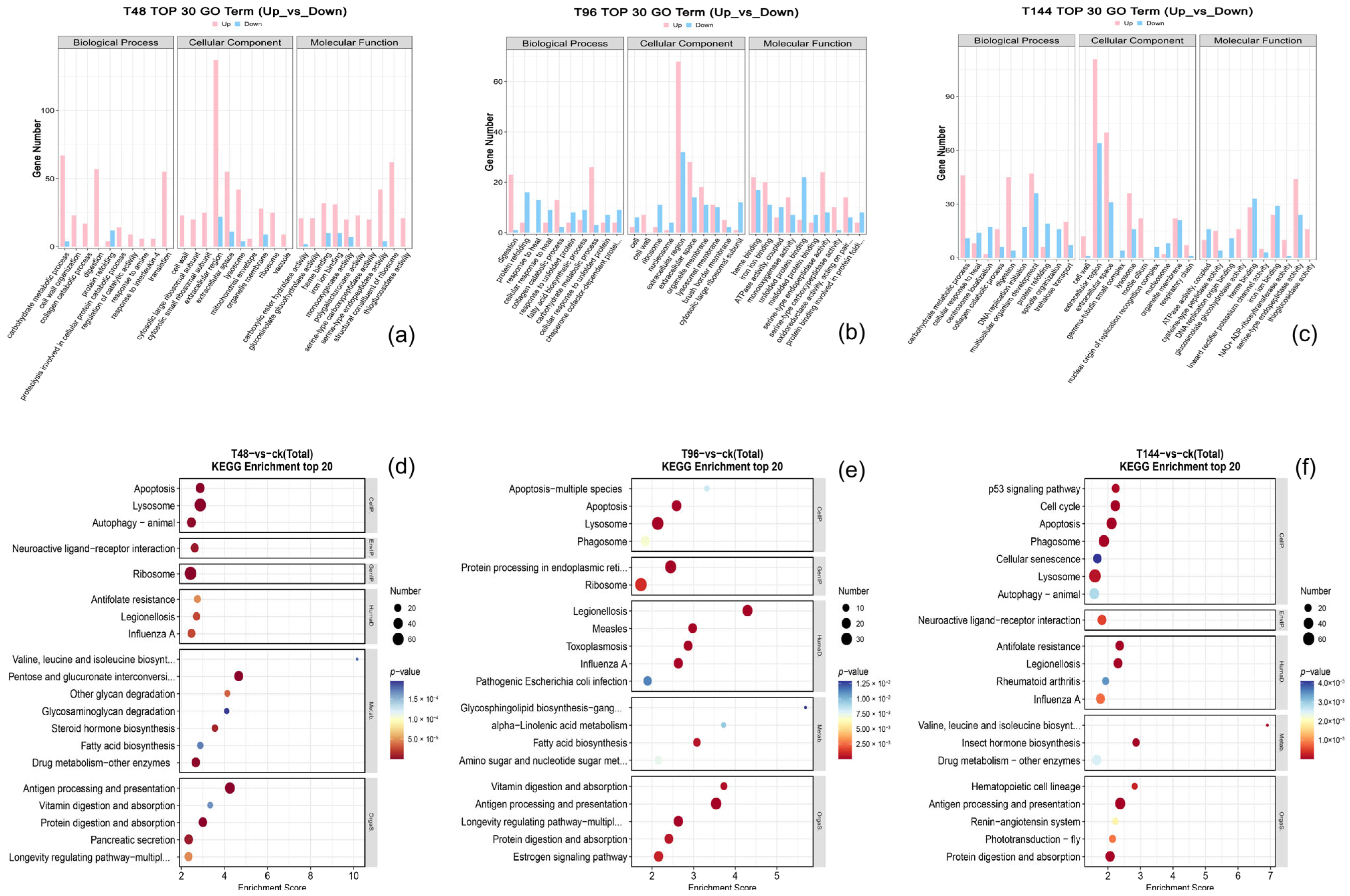
3.5. Enrichment Analysis of Differentially Expressed Genes: GO and KEGG
3.6. Metabolomic Analysis of the Response of S. callosus After Infection with B. bassiana
3.7. Immune Response of S. callosus After Infection
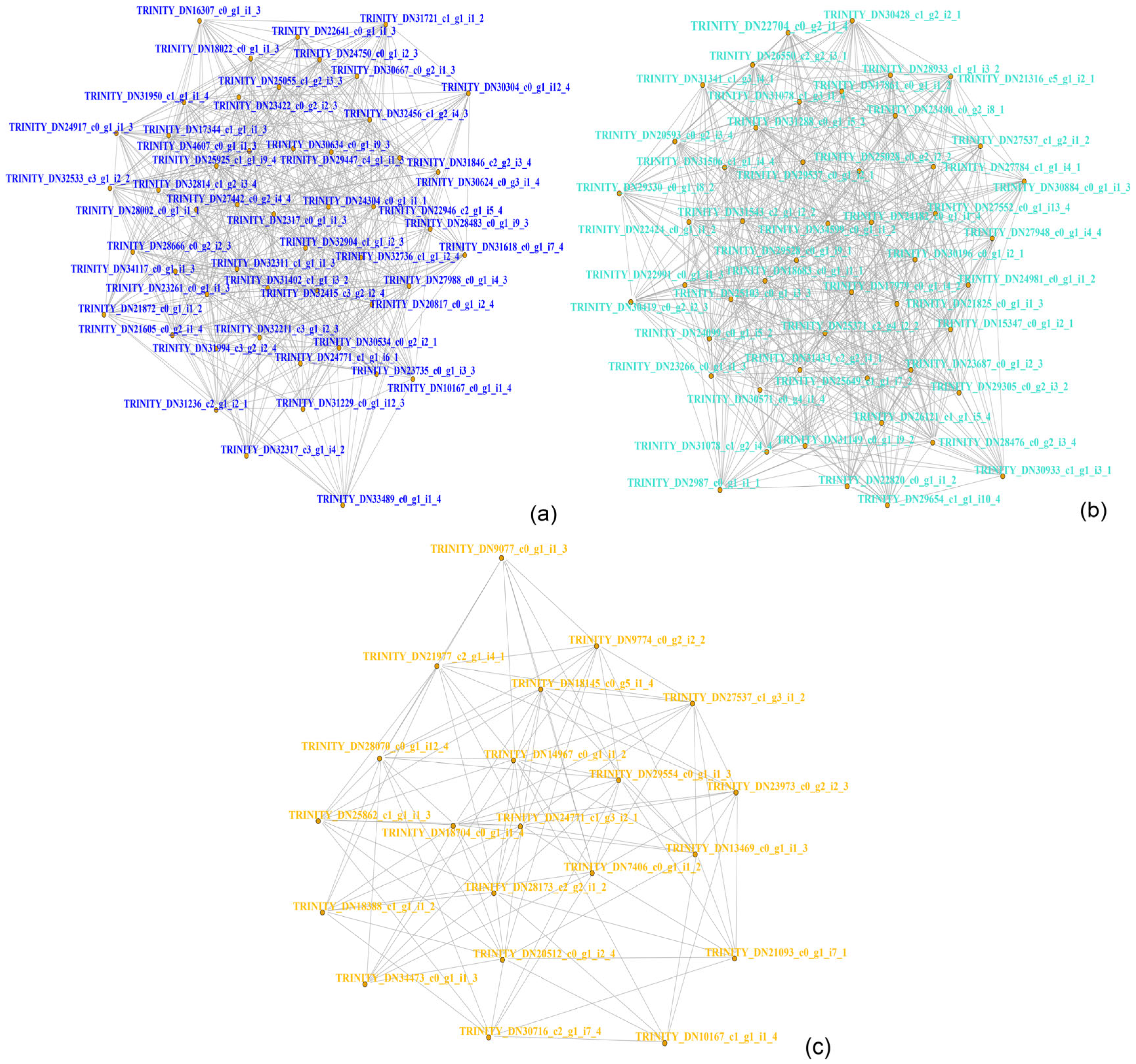
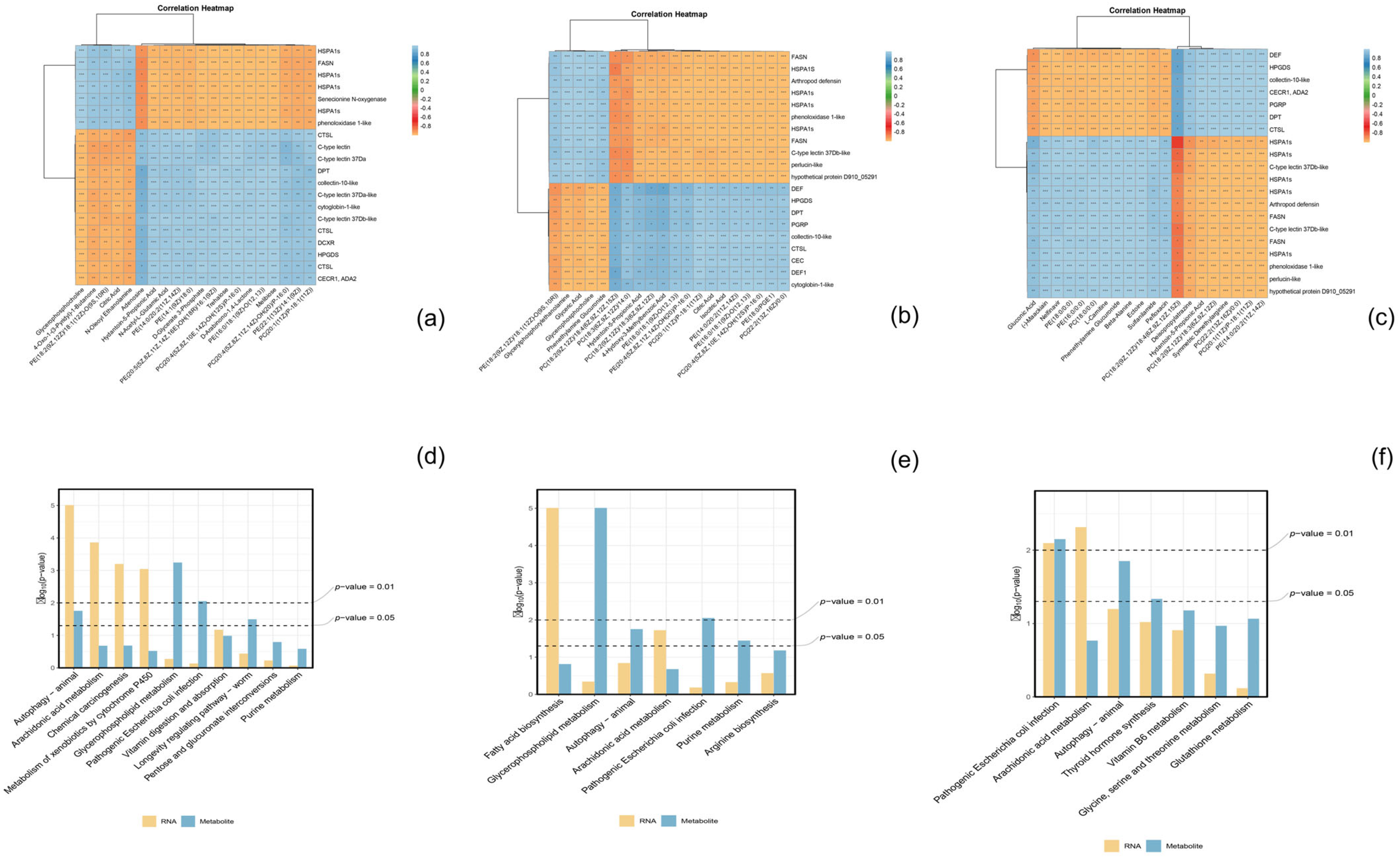
3.8. Integrative Analysis of Immune-Related Genes and Metabolites
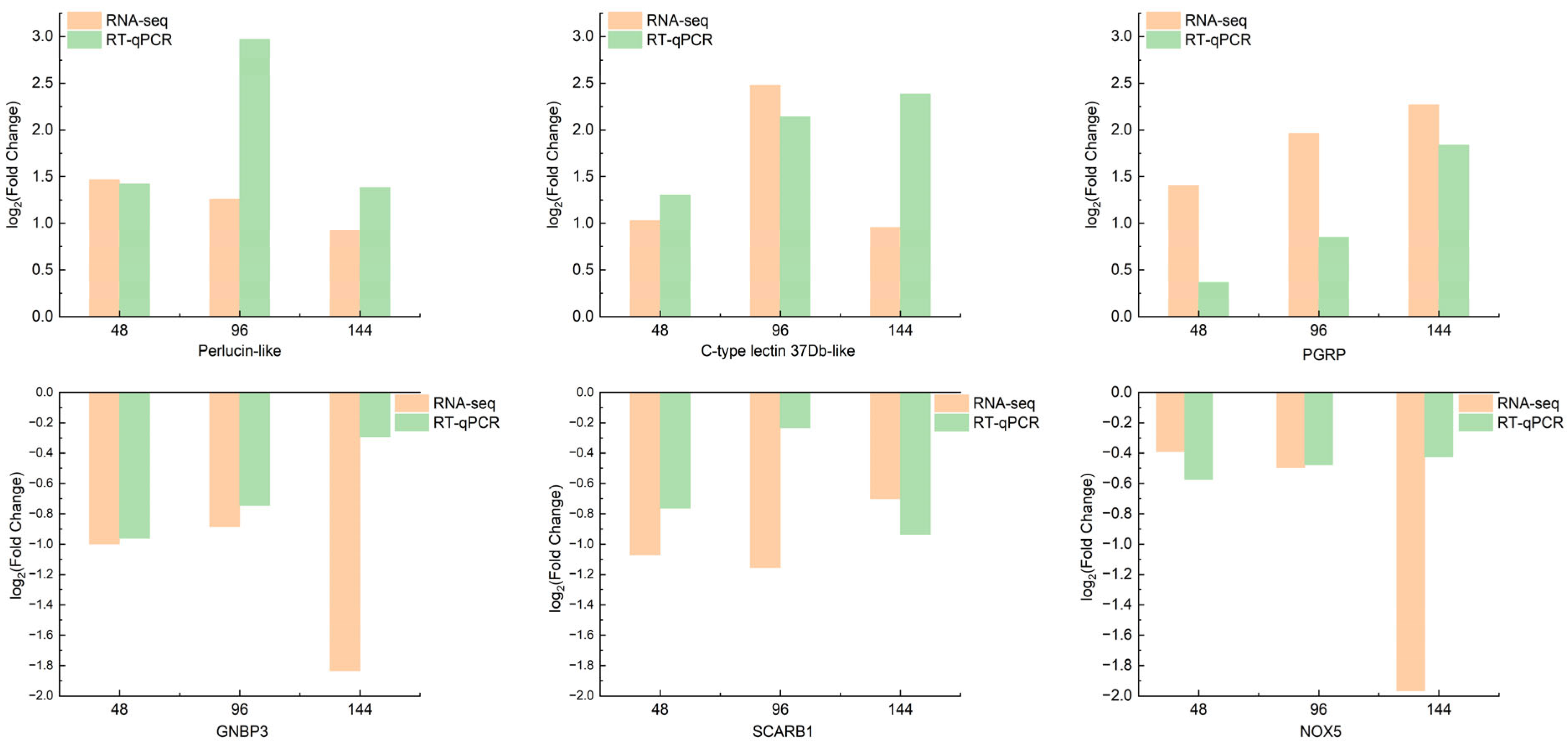
3.9. RT-qPCR Validation of Transcriptome Sequencing Data
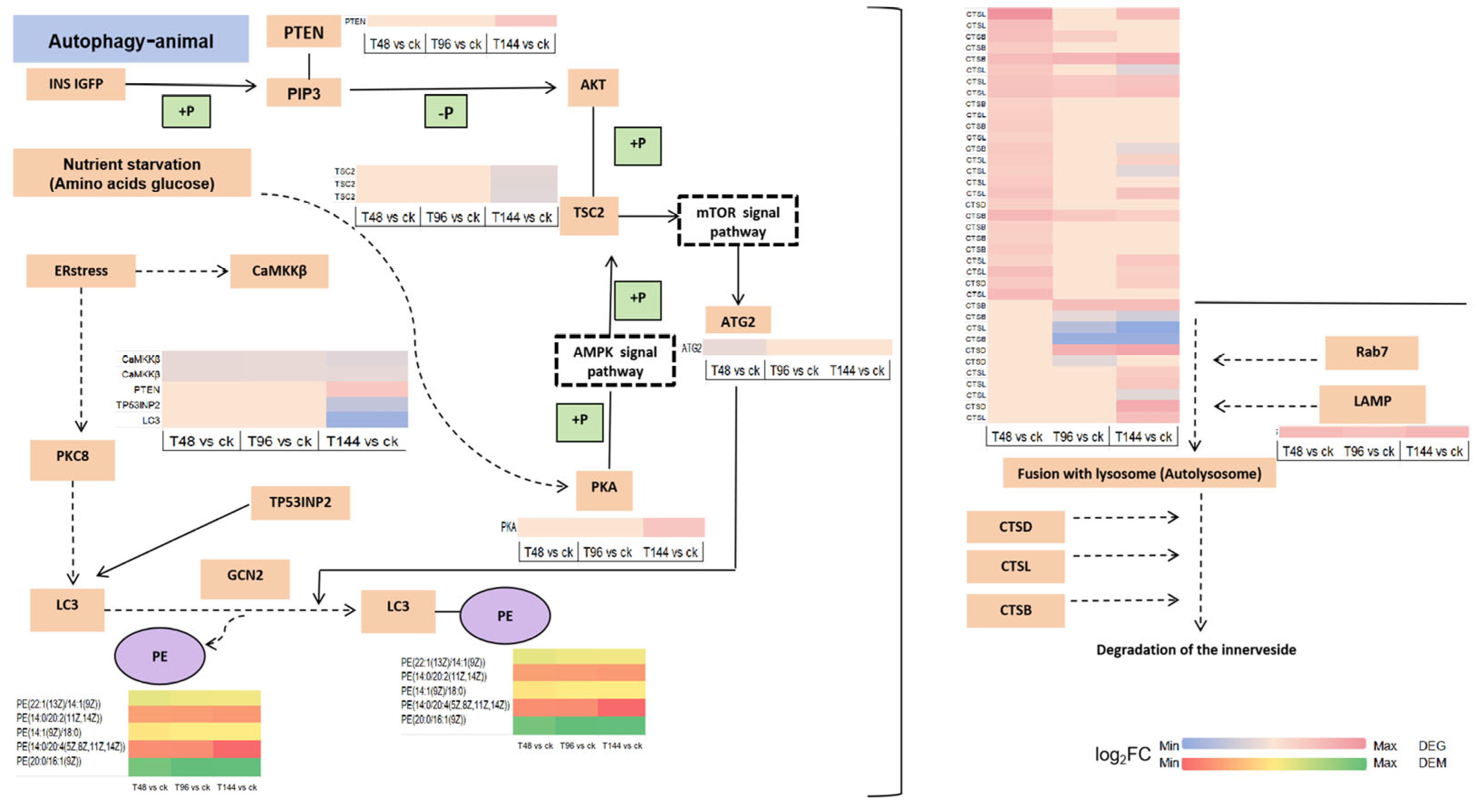
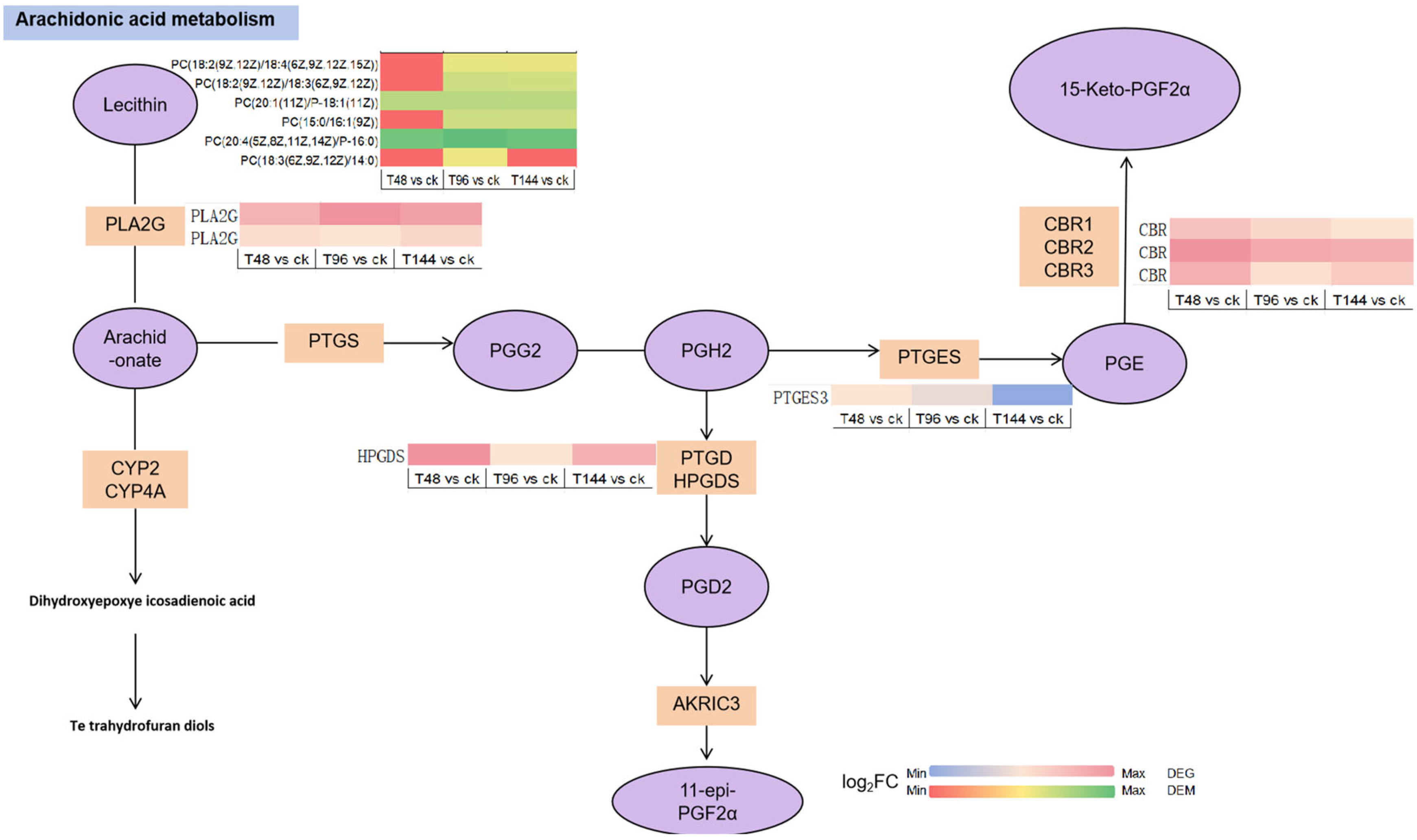
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Müjgan, K. Tekirdağ ilinde baklagil yem bitkilerinde bulunan Sitona Gm. (Coleoptera, Curculionidae) türleri, konukçuları ve yayılışları üzerine ön araştırmalar. Türkiye Entomoloji Derg. 1995, 19, 299–304. [Google Scholar]
- Sanaei, E.; Seiedy, M.; de Castro, A.J.V. Distribution of weevils (Coleoptera: Curculionidae) in alfalfa fields of Iran’s northern provinces with a new record for the country. Zool. Ecol. 2015, 25, 129–135. [Google Scholar] [CrossRef]
- Toshova, T.B.; Subchev, M.A.; Atanasova, D.I.; de Castro, A.J.V.; Smart, L. Sitona weevils (Coleoptera: Curculionidae) caught by traps in alfalfa fields in Bulgaria. Biotechnol. Biotechnol. Equip. 2009, 23, 132–135. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Wang, J.; Yang, X.-Z.; Li, X.-P.; Feng, R.-Q.; Yuan, M.-L. Mitochondrial genome of Sitona callosus (Coleoptera: Curculionidae) and phylogenetic analysis within Entiminae. Mitochondrial DNA Part B 2017, 2, 538–539. [Google Scholar] [CrossRef] [PubMed]
- Wiech, K.; Clements, R.O. Studies on the Sitona spp. and Apion spp. weevils feeding on white clover foliage at a site in S.E. England. J. Appl. Entomol. 1992, 113, 437–440. [Google Scholar] [CrossRef]
- Mowat, D.J.; Shakeel, M.A. The effect of some invertebrate species on persistence of white clover in ryegrass swards. Grass Forage Sci. 1989, 44, 117–124. [Google Scholar] [CrossRef]
- Dintenfass, L.P.; Brown, G.C. Feeding rate of larval clover root curculio, Sitona hispidulus (Coleoptera: Curculionidae), on alfalfa taproots. J. Econ. Entomol. 1986, 79, 506–510. [Google Scholar] [CrossRef]
- Manglitz, G.R.; Anderson, D.M.; Gorz, H.J. Observations on the larval feeding habits of two species of Sitona (Coleoptera: Curculionidae) in sweetclover fields. Entomol. Soc. Am. 1963, 56, 831–885. [Google Scholar] [CrossRef]
- Pesho, G.R. Clover root curculio: Estimates of larval injury to alfalfa tap roots. J. Econ. Entomol. 1975, 68, 61–65. [Google Scholar] [CrossRef]
- Cárcamo, H.A.; Herle, C.E.; Lupwayi, N.Z. Sitona lineatus (Coleoptera: Curculionidae) larval feeding on Pisum sativum L. affects soil and plant nitrogen. J. Insect Sci. 2015, 15, 74. [Google Scholar] [CrossRef]
- Gözüaçik, C. Diversity of Sitona species (Coleoptera, Curculionidae) in cultivated plants belonging to the Fabaceae family. Appl. Ecol. Environ. Res. 2023, 21, 3275–3284. [Google Scholar] [CrossRef]
- Islam, M.T.; Omar, D.B. Combined effect of Beauveria bassiana with neem on virulence of insect in case of two application approaches. J. Anim. Plant Sci. 2012, 22, 77–82. [Google Scholar]
- Wraight, S.P.; Lopes, R.B.; Faria, M. Microbial control of insect and mite pests: Chapter 16-Microbial control of mite and insect pests of greenhouse crops. In Microbial Control of Insect and Mite Pests; Academic Press: New York, NY, USA, 2017; pp. 237–252. [Google Scholar] [CrossRef]
- Arthurs, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, S.; Yadav, P.K. Entomopathogenic fungi and their relevance in sustainable agriculture: A review. Cogent Food Agric. 2023, 9, 2180857. [Google Scholar] [CrossRef]
- Kaushal, K.S.; Ajoy, K.C.; Priyanka, K. Chapter 15-Entomopathogenic fungi. In Ecofriendly Pest Management for Food Security; Academic Press: New York, NY, USA, 2016; pp. 475–505. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Zhong, S.; Fei, Z.; Gao, S.; Zhang, S.; Li, Z.; Wang, P.; Blissard, G.W. Transcriptome responses of the host Trichoplusia ni to infection iy the baculovirus Autographa californica multiple nucleopolyhedrovirus. J. Virol. 2014, 88, 13781–13797. [Google Scholar] [CrossRef]
- Sagri, E.; Reczko, M.; Tsoumani, K.T.; Gregoriou, M.-E.; Harokopos, V.; Mavridou, A.-M.; Tastsoglou, S.; Athanasiadis, K.; Ragoussis, J.; Mathiopoulos, K.D. The molecular biology of the olive fly comes of age. BMC Genet. 2014, 15, S8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Khalil, S.M.; Mitchell, R.D.; Bissinger, B.W.; Egekwu, N.; Sonenshine, D.E.; Roe, R.M. Mevalonate-farnesal biosynthesis in ticks: Comparative synganglion transcriptomics and a new perspective. PLoS ONE 2016, 11, e0141084. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Su, L.; Yang, C.; Meng, J.; Zhou, L.; Zhang, C. Comparative transcriptome and metabolome analysis of Ostrinia furnacalis female adults under UV-A exposure. Sci. Rep. 2021, 11, 6797. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, H.A.; Knee, J.M.; Dennis, A.B.; Udaka, H.; Marshall, K.E.; Merritt, T.J.S.; Sinclair, B.J. Sinclair Cold acclimation wholly reorganizes the Drosophila melanogaster transcriptome and metabolome. Sci. Rep. 2016, 6, 28999. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Qin, Q.; Zhang, H.; Li, X.; Wang, H.; Meng, Q. Transcriptome and metabolome analyses of Thitarodes xiaojinensis in response to Ophiocordyceps sinensis infection. Microorganisms 2023, 11, 2361. [Google Scholar] [CrossRef]
- Hou, Z. Study on Occurrence and Control of Two Main Alfalfa Weevils in Ningxia. Master’s Thesis, Ningxia University, Yinchuan, China, 2022. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Regev, A. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Pachter, L. Streaming fragment assignment for real-time analysis of sequencing experiments. Nat. Methods 2013, 10, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Xuan, H.; Huang, Y.; Zhou, L.; Deng, S.; Wang, C.; Xu, J.; Wang, H.; Zhao, J.; Guo, N.; Xing, H. Key soybean seedlings drought-responsive genes and pathways revealed by comparative transcriptome analyses of two cultivars. Int. J. Mol. Sci. 2022, 23, 2893. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Liu, X.M.; Yuan, M.L. Progress in innate immunity-related genes in insects. Hereditas 2018, 40, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, Z.; Liu, L. Research progress in the immunity of insects and the immune mechanisms of five important invasive insects. J. Plant Prot. 2019, 46, 6–16. [Google Scholar]
- Bao, S.C.; Liu, X.; Hou, X.H. Research progress on innate immune regulatory mechanisms in insects. Acta Parasitol. Med. Entomol. Sin. 2024, 31, 115–122. [Google Scholar]
- Lecaille, F.; Kaleta, J.; Brömme, D. Human and parasitic papain-like cysteine proteases: Their role in physiology and pathology and recent developments in inhibitor design. Chem. Rev. 2002, 102, 4459–4488. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-D.; Cai, Q.-F.; Yan, L.-J.; Du, C.-H.; Liu, G.-M.; Su, W.-J.; Ke, C.; Cao, M.-J. Cathepsin L is an immune-related protein in pacific abalone (Haliotis discus hannai)—Purification and characterization. Fish Shellfish. Immunol. 2015, 47, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Vandenbulcke, F.; Bocquet, B.; Tasiemski, A.; Desmons, A.; Verstraete, M.; Salzet, M.; Cocquerelle, C. Cathepsin L and cystatin B gene expression discriminates immune cœlomic cells in the leech Theromyzon tessulatum. Dev. Comp. Immunol. 2008, 32, 795–807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santana, B.G.; Dalton, J.P.; Camargo, F.V.; Parkinson, M.; Ndao, M. The diagnosis of human fascioliasis by enzyme-linked immunosorbent assay (ELISA) using recombinant cathepsin L protease. PLoS Negl. Trop. Dis. 2013, 7, e2414. [Google Scholar] [CrossRef]
- Saikhedkar, N.; Summanwar, A.; Joshi, R.; Giri, A. Cathepsins of lepidopteran insects: Aspects and prospects. Insect Biochem. Mol. Biol. 2015, 64, 51–59. [Google Scholar] [CrossRef]
- Shu, B.S.; Zou, Y.; Zhang, W.Y.; Feng, J.; Wu, Z.Z.; Lin, J.T. Effects of sublethal doses of imidacloprid and chlorpyrifos on the expression profiles of cathepsins in Diaphorina citri Kuwayama. J. Environ. Entomol. 2022, 44, 1027–1036. [Google Scholar] [CrossRef]
- Shu, B.; Wu, Y.; Qu, M.; Pu, X.; Wu, Z.; Lin, J. Comparative transcriptomic analyses revealed genes and pathways responsive to heat stress in Diaphorina citri. Gene 2020, 727, 144246. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Cai, Z.; Wu, H.; Luo, L. Changes in midgut gene expression following Bacillus thuringiensis (Bacillales: Bacillaceae) infection in Monochamus alternatus (Coleoptera: Cerambycidae). Fla. Entomol. 2016, 99, 60–66. [Google Scholar] [CrossRef]
- Iwanaga, S.; Lee, B.-L. Recent advances in the innate immunity of invertebrate animals. BMB Rep. 2005, 38, 128–150. [Google Scholar] [CrossRef] [PubMed]
- Drickamer, K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature 1992, 360, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Dambuza, I.M.; Brown, G.D. C-type lectins in immunity: Recent developments. Curr. Opin. Immunol. 2015, 32, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Pees, B.; Yang, W.; Zárate-Potes, A.; Schulenburg, H.; Dierking, K. High innate immune specificity through diversified C-type lectin-like domain proteins in invertebrates. J. Innate Immun. 2016, 8, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Chen, M.; Liang, Z.; Yue, X.; Ge, H.; Zhang, W. Functional analysis of CYP6ER1, a P450 gene associated with imidacloprid resistance in Nilaparvata lugens. Sci. Rep. 2016, 6, 34992. [Google Scholar] [CrossRef]
- Liang, Z.; Pang, R.; Dong, Y.; Sun, Z.; Ling, Y.; Zhang, W. Identification of SNPs involved in regulating a novel alternative transcript of P450 CYP6ER1 in the brown planthopper. Insect Sci. 2018, 25, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Matova, N.; Anderson, K.V. Rel/NF-κB double mutants reveal that cellular immunity is central to Drosophila host defense. Proc. Natl. Acad. Sci. USA 2006, 103, 16424–16429. [Google Scholar] [CrossRef]
- Hanson, M.A.; Cohen, L.B.; Marra, A.; Iatsenko, I.; Wasserman, S.A.; Lemaitre, B. The Drosophila Baramicin polypeptide gene protects against fungal infection. PLoS Pathog. 2021, 17, e1009846. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.C.; Kim, Y. Toll signal pathway activating eicosanoid biosynthesis shares its conserved upstream recognition components in a lepidopteran Spodoptera exigua upon infection by Metarhizium rileyi, an entomopathogenic fungus. J. Invertebr. Pathol. 2022, 188, 107707. [Google Scholar] [CrossRef] [PubMed]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef]
- Codogno, P.; Mehrpour, M.; Proikas-Cezanne, T. Canonical and non-canonical autophagy: Variations on a common theme of self-eating? Nat. Rev. Mol. Cell Biol. 2011, 13, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Papinski, D.; Kraft, C. Atg1 kinase organizes autophagosome formation by phosphorylating Atg9. Autophagy 2014, 10, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 2008, 8, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Silverman, N.; Cherry, S. Immunity in Drosophila melanogaster—From microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 2014, 14, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.A.; Ahmed, S.; Kim, Y. Biosynthetic pathway of arachidonic acid in Spodoptera exigua in response to bacterial challenge. Insect Biochem. Mol. Biol. 2019, 111, 103179. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Nguyen, T.; Stanley-Samuelson, D.W. Eicosanoids mediate insect nodulation responses to bacterial infections. Proc. Natl. Acad. Sci. USA 1994, 91, 12418–12422. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, K.; Park, J.; Stanley, D.W.; Kim, Y. Plasmatocyte-spreading peptide influences hemocyte behavior via eicosanoids. Arch. Insect Biochem. Physiol. 2011, 78, 145–160. [Google Scholar] [CrossRef]
- Yajima, M.; Takada, M.; Takahashi, N.; Kikuchi, H.; Natori, S.; Oshima, Y.; Kurata, S. A newly established in vitro culture using transgenic Drosophila reveals functional coupling between the phospholipase A2-generated fatty acid cascade and lipopolysaccharide-dependent activation of the immune deficiency (imd) pathway in insect immunity. Biochem. J. 2003, 371, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Kim, Y. Eicosanoids mediate prophenoloxidase release from oenocytoids in the beet armyworm Spodoptera exigua. Insect Biochem. Mol. Biol. 2008, 38, 99–112. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Gu, X.; Xin, M.; Wang, X. Revealing the Immune Response of Sitona callosus Gyllenhal to Entomopathogenic Fungi Beauveria bassiana Infection Through Integrative Analyses of Transcriptomics and Metabolomics. Insects 2024, 15, 940. https://doi.org/10.3390/insects15120940
Li N, Gu X, Xin M, Wang X. Revealing the Immune Response of Sitona callosus Gyllenhal to Entomopathogenic Fungi Beauveria bassiana Infection Through Integrative Analyses of Transcriptomics and Metabolomics. Insects. 2024; 15(12):940. https://doi.org/10.3390/insects15120940
Chicago/Turabian StyleLi, Nan, Xin Gu, Ming Xin, and Xinpu Wang. 2024. "Revealing the Immune Response of Sitona callosus Gyllenhal to Entomopathogenic Fungi Beauveria bassiana Infection Through Integrative Analyses of Transcriptomics and Metabolomics" Insects 15, no. 12: 940. https://doi.org/10.3390/insects15120940
APA StyleLi, N., Gu, X., Xin, M., & Wang, X. (2024). Revealing the Immune Response of Sitona callosus Gyllenhal to Entomopathogenic Fungi Beauveria bassiana Infection Through Integrative Analyses of Transcriptomics and Metabolomics. Insects, 15(12), 940. https://doi.org/10.3390/insects15120940




