Parasitoids of Insect Pests Feeding on Scaevola taccada (Goodeniaceae) from Yongxing Island in South China Sea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection and Rearing
2.2. Species Identification
2.3. Imaging
2.4. Data Analysis
3. Results
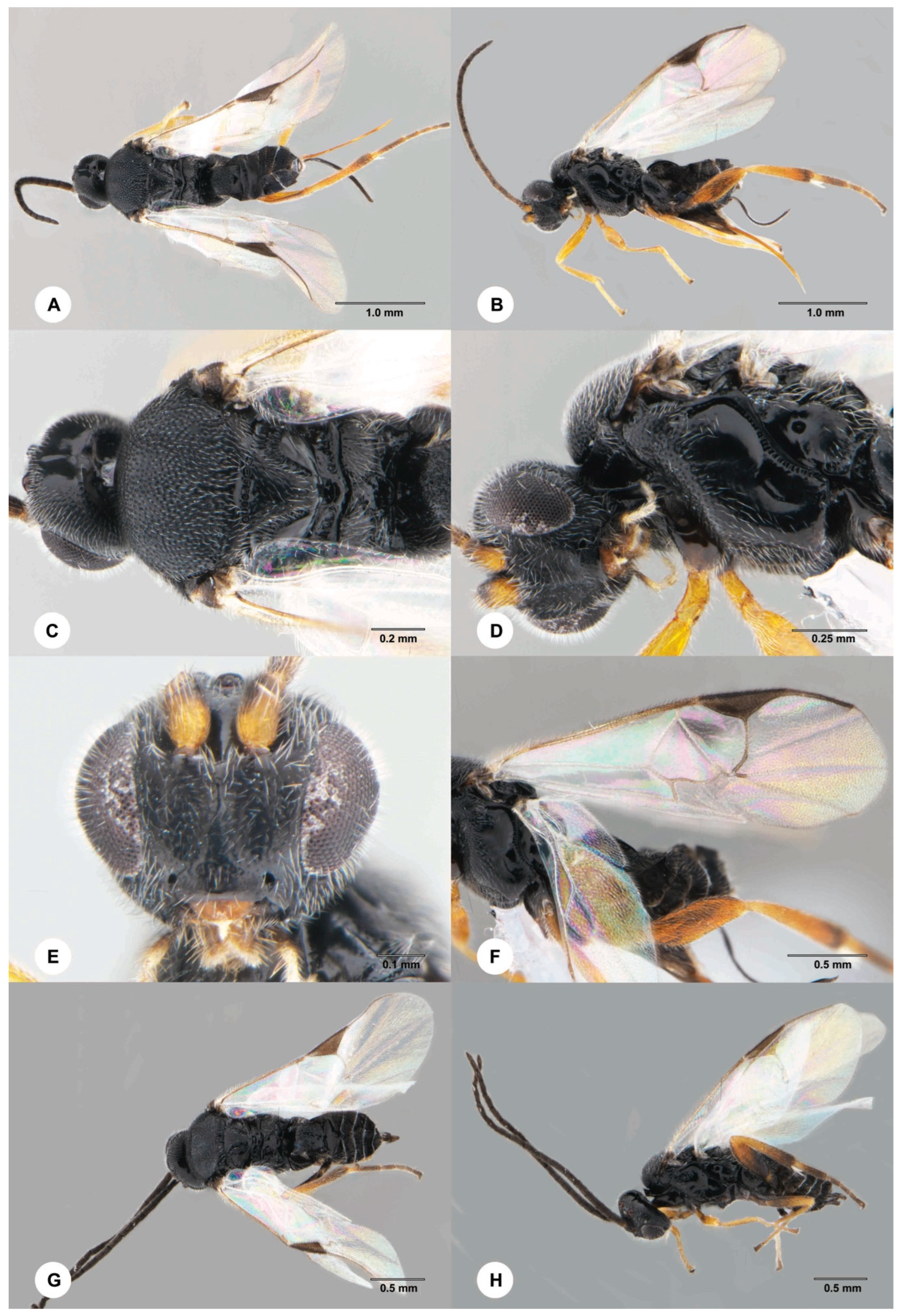
- Dolichogenidea stantoni (Ashmead, 1904) (Braconidae)
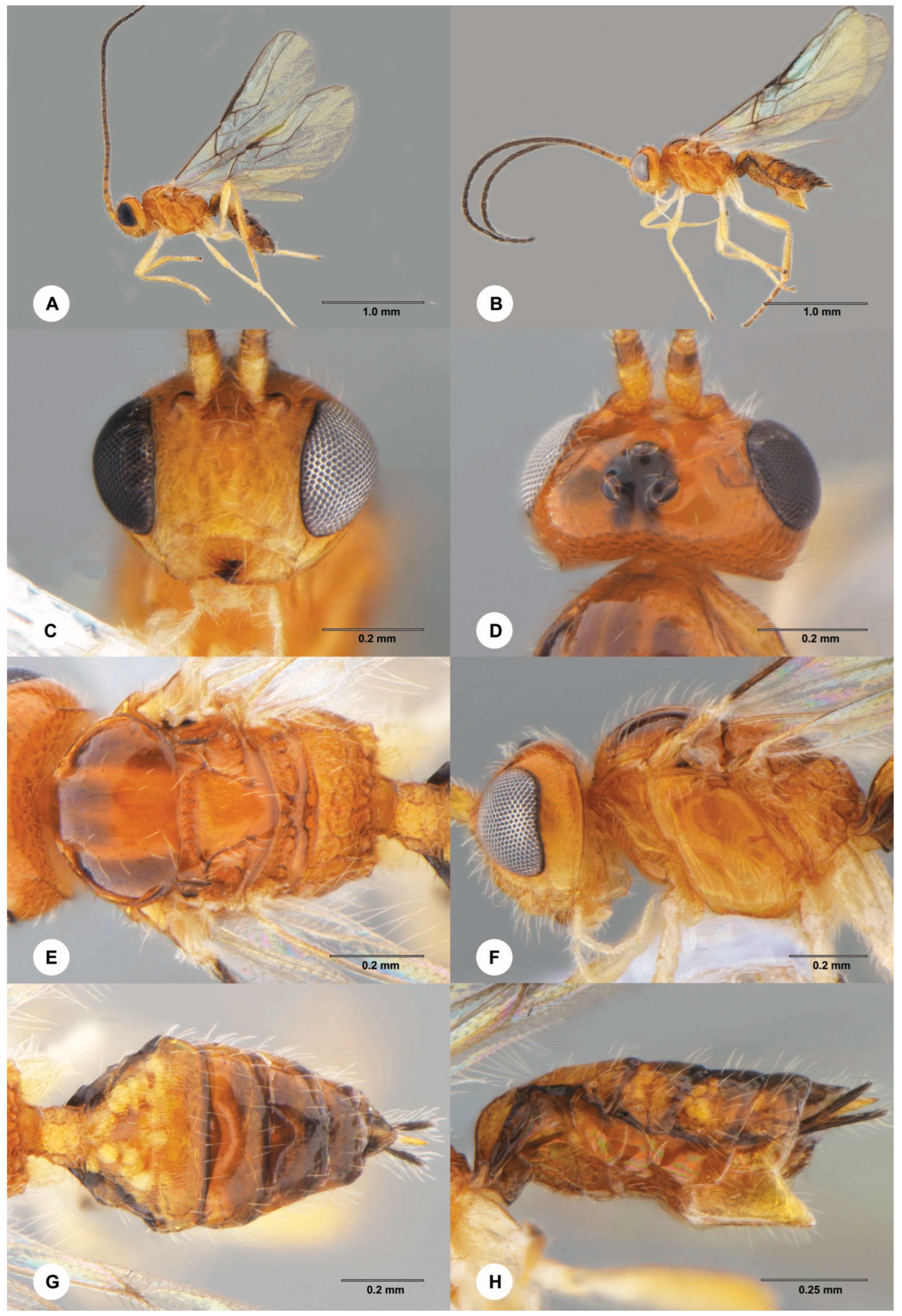
- Opius biroi Fischer, 1960 (Braconidae)
- Euderus albitarsis (Zetterstedt, 1838) (Eulophidae)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howarth, D.G.; Gustafsson, M.H.G.; Baum, D.A.; Motley, T.J. Phylogenetics of the Genus Scaevola (Goodeniaceae): Implication for Dispersal Patterns across the Pacific Basin and Colonization of the Hawaiian Islands. Am. J. Bot. 2003, 90, 915–923. [Google Scholar] [CrossRef]
- Howarth, D.G.; Baum, D.A. Genealogical Evidence of Homoploid Hybrid Speciation in an Adaptive Radiation of Scaevola (Goodeniaceae) in the Hawaiian Islands. Evolution 2005, 59, 948–961. [Google Scholar]
- Castillo-Campos, G.; García-Franco, J.; Martínez, L. First Record of Naturalization of Scaevola taccada (Gaertn.) Roxb. (Goodeniaceae) in Southeastern Mexico. BioInvasions Rec. 2021, 10, 425–435. [Google Scholar] [CrossRef]
- Li, S.; Mao, X.; He, Z.; Xu, S.; Guo, Z.; Shi, S. Chromosomal-Scale Genome Assemblies of Two Coastal Plant Species, Scaevola taccada and S. hainanensis—Insight into Adaptation Outside of the Common Range. Int. J. Mol. Sci. 2023, 24, 7355. [Google Scholar] [CrossRef]
- Shiao, S.F.; Wu, W.J. Four new agromyzid species from Taiwan (Diptera: Agromyzidae). Trans. Am. Entomol. Soc. 1996, 122, 213–226. [Google Scholar]
- Wijesekara, A. Synopsis of the Agromyzidae (Diptera) of Sri Lanka. Cey. J. Sci. (Bio. Sci.) 2002, 29, 41–62. [Google Scholar]
- Chen, Q.; Liang, X.; Wu, C.L.; Chen, Q. Pest survey and safety assessment on five Islands of Yongle Archipelago. Chin. J. Trop. Crops 2020, 41, 148–156. (In Chinese) [Google Scholar]
- Hill, M.G. Susceptibility of Scaevola taccada (Gaertn.) Roxb. Bushes to attack by the coccid Icerya seychellarum Westwood: The effects of leaf loss. Ecol. Entomol. 1980, 5, 345–352. [Google Scholar] [CrossRef]
- Masumoto, M.; Okajima, S. Three new species of the genus Thrips (Thysanoptera, Thripidae) in Japan. Zootaxa 2019, 4614, 575–584. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Huang, G. Notes on three Herpetogramma species feeding on Alternanthera philoxeroides (Mart.) Griseb. Chin. Agric. Sci. Bull. 2010, 26, 302–304. (In Chinese) [Google Scholar]
- Nixon, G.E.J. The Indo-Australian species of the ultor-group of Apanteles Förster (Hymenoptera: Braconidae). Bull. Br. Mus. (Nat. Hist.) Entomol. Ser. 1967, 21, 1–34. [Google Scholar]
- Fischer, M. Hymenoptera, Braconidae (Opiinae I). Das Tierreich 1972, 91, 1–620. [Google Scholar]
- Askew, R.R. Handbooks for the identification of British Insects, Hymenoptera Chalcidoidea Section (b). R. Entomol. Soc. Lond. 1968, 8, 1–39. [Google Scholar]
- Taekul, C.; Valerio, A.A.; Austin, A.D.; Klompen, H.; Johnson, N.F. Molecular phylogeny of telenomine egg parasitoids (Hymenoptera: Platygastridae s.l.: Telenominae): Evolution of host shifts and implications for classification. Syst. Entomol. 2014, 39, 24–35. [Google Scholar] [CrossRef]
- Yan, C.J.; Talamas, E.; Lahey, Z.; Chen, H.Y. Protelenomus Kieffer is a derived lineage of Trissolcus Ashmead (Hymenoptera, Scelionidae), with comments on the evolution of phoresy in Scelionidae. J. Hymenopt. Res. 2022, 94, 121–137. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoch, W.; Lutz, R.; Vrijenoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Ashmead, W.H. A list of Hymenoptera of the Philippine Islands with descriptions of new species. J. N. Y. Entomol. Soc. 1904, 12, 1–22. [Google Scholar]
- Wilkinson, D.S. A revision of the Indo-Australian species of the genus Apanteles (Hym. Bracon.). Part I. Bull. Entomol. Res. 1928, 19, 79–105, 109–146. [Google Scholar] [CrossRef]
- Yu, D.S.; van Achterberg, C.; Horstmann, K. Taxapad 2016: World Ichneumonoidea 2015; Taxapad Database; Taxapad: Ottawa, ON, Canada, 2016. [Google Scholar]
- Ku, D.S.; Belokobylskij, S.A.; Cha, J.Y. Economic Insects of Korea 16: Braconidae (Hymenoptera); Insecta Koreana; National Institute of Agricultural Science and Technology: Suwon, Republic of Korea, 2001; 283p. [Google Scholar]
- Wen, J.Z.; Lei, Z.R.; Wang, Y. Opiinae parasitoids of the leafminer Liriomyza spp. in China. Entomol. Knowl. 2002, 39, 14–16. (In Chinese) [Google Scholar]
- Fischer, M. Die europäischen Arten der Gattung Opius Wesm. Teil 1Va. Ann. Zool. 1960, 19, 33–112. [Google Scholar]
- Papp, J. A checklist of the Braconidae of Hungary (Hymenoptera). Folia Entomol. Hung. 2005, 66, 137–194. [Google Scholar]
- Ghahari, H.; Fischer, M.; Sakenin, H.; Imani, S. A contribution to the Agathidinae, Alysinae, Aphidiinae, Braconinae, Microgastrinae and Opiinae (Hymenoptera: Braconidae) from cotton fields and surrounding grasslands of Iran. Linz. Biol. Beitraege 2011, 43, 1269–1276. [Google Scholar]
- Fischer, M. Die Opiinae des Museo Civico di Storia Naturale in Genau. Ann. Mus. Civ. Stor. Nat. Genova 1962, 73, 71–97. [Google Scholar]
- Báez, M.; Koponen, M.; Gracia, A.; Martin, E. Lista de Especies Silvestres de Canarias (Hongos, Plantas y Animales Terrestres); Hymenoptera; Gobierno de Canarias: Canarias, Spain, 2001; pp. 267–279. [Google Scholar]
- Jiménez Peydró, R. Opiinae de la provincia de Valencia (Hymenoptera, Braconidae). Bol. Asoc. Española Entomol. 1982, 6, 277–283. [Google Scholar]
- Avinent, L.; Jiménez, R. Opiinae from the collection of the Zoology Department in the University of Valencia: I. Madrid, Palencia and Segovia (Spain). Bol. Asoc. Española Entomol. 1987, 11, 121–134. [Google Scholar]
- Fischer, M.; Beyarslan, A. A survey of Opiinae (Hymenoptera: Braconidae) of Turkey. Fragm. Faun. 2005, 48, 27–62. [Google Scholar] [CrossRef]
- Bouček, Z.; Askew, R.R. Hym. Chalcidoidea. Palearctic Eulophidae (excl. Tetrastichinae). In Index of Entomophagous Insects; Delucchi, V., Remaudière, G., Eds.; Le François: Paris, France, 1968; Volume 3, 260p. [Google Scholar]
- Gibson, G.A.P.; Gates, M.W.; Buntin, G.D. Parasitoids (Hymenoptera: Chalcidoidea) of the cabbage seedpod weevil (Coleoptera: Curculionidae) in Georgia, USA. J. Hymenopt. Res. 2006, 15, 187–207. [Google Scholar]
- Deng, S.; Li, C. Description of A New Species and A New Country Record Species of the Genus Euderus (Hymenoptera: Eulophidae) from China. J. Northeast For. Univ. 2022, 50, 121–124. [Google Scholar]
- Yefremova, Z.A. An annotated checklist of the Eulophidae (excl. Tetrastichinae) (Hymenoptera: Chalcidoidea) of Israel. Zootaxa 2015, 3957, 001–036. [Google Scholar] [CrossRef]
- Ren, H.; Jian, S.G.; Zhang, Q.M.; Wang, F.G.; Shen, T.; Wang, J. Plants and vegetation on South China Sea Islands. Ecol. Environ. Sci. 2017, 26, 1639–1648. (In Chinese) [Google Scholar]
- Santos, A.M.C. Ecology and biogeography of island parasitoid faunas. Front. Biogeogr. 2012, 4, 19–24. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Sanchez Garcia, F.J.; Santos, A.M.C.; Canal, N.A.; Furlong, M.J.; Melo, M.C.; GC, D.Y.; Pozsgai, G. Island and Mountain Ecosystems as Testbeds for Biological Control in the Anthropocene. Front. Ecol. Evol. 2022, 10, 912628. [Google Scholar] [CrossRef]
- Baeckens, S.; Van Damme, R. The island syndrome. Curr. Biol. 2020, 30, 338–339. [Google Scholar] [CrossRef] [PubMed]
- Soumya, K.; Visalakshy, P.N.G.; Krishnamoorthy, A.; Pillai, K.G. Dolichogenidea stantoni (Hymenoptera: Braconidae) a potential biocontrol agent for melon borer, Diaphania indica. Entomon 2017, 42, 1–6. [Google Scholar]





| Host Species | Parasitoid Species | % Parasitism | % Female Parasitoids |
|---|---|---|---|
| Herpetogramma submarginale | Dolichogenidea stantoni | 48.90 ± 8.40 | 56.67 ± 2.08 |
| Ophiomyia scaevolana | Opius biroi | 5.80 ± 1.15 | 67.23 ± 7.51 |
| Euderus albitarsis | 64.40 ± 1.61 | 55.57 ± 6.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; van Achterberg, C.; Li, Y.; Liu, Z.; Wang, J.; Luo, S. Parasitoids of Insect Pests Feeding on Scaevola taccada (Goodeniaceae) from Yongxing Island in South China Sea. Insects 2024, 15, 926. https://doi.org/10.3390/insects15120926
Chen H, van Achterberg C, Li Y, Liu Z, Wang J, Luo S. Parasitoids of Insect Pests Feeding on Scaevola taccada (Goodeniaceae) from Yongxing Island in South China Sea. Insects. 2024; 15(12):926. https://doi.org/10.3390/insects15120926
Chicago/Turabian StyleChen, Huayan, Cornelis van Achterberg, Yang Li, Zhen Liu, Jun Wang, and Shixiao Luo. 2024. "Parasitoids of Insect Pests Feeding on Scaevola taccada (Goodeniaceae) from Yongxing Island in South China Sea" Insects 15, no. 12: 926. https://doi.org/10.3390/insects15120926
APA StyleChen, H., van Achterberg, C., Li, Y., Liu, Z., Wang, J., & Luo, S. (2024). Parasitoids of Insect Pests Feeding on Scaevola taccada (Goodeniaceae) from Yongxing Island in South China Sea. Insects, 15(12), 926. https://doi.org/10.3390/insects15120926






