Spätzle Regulates Developmental and Immune Trade-Offs Induced by Bacillus thuringiensis Priming in Rhynchophorus ferrugineus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Entomopathogen
2.2. Preparation of Bt Priming Suspension
2.3. Effect of Bt Priming on Biological Parameters
2.4. Hormone Content Measurement
2.5. RT-qPCR Analysis
2.6. RNA Interference
2.7. Detection of Bt Priming Effects After SPZ Interference
2.8. Statistical Analysis
3. Results
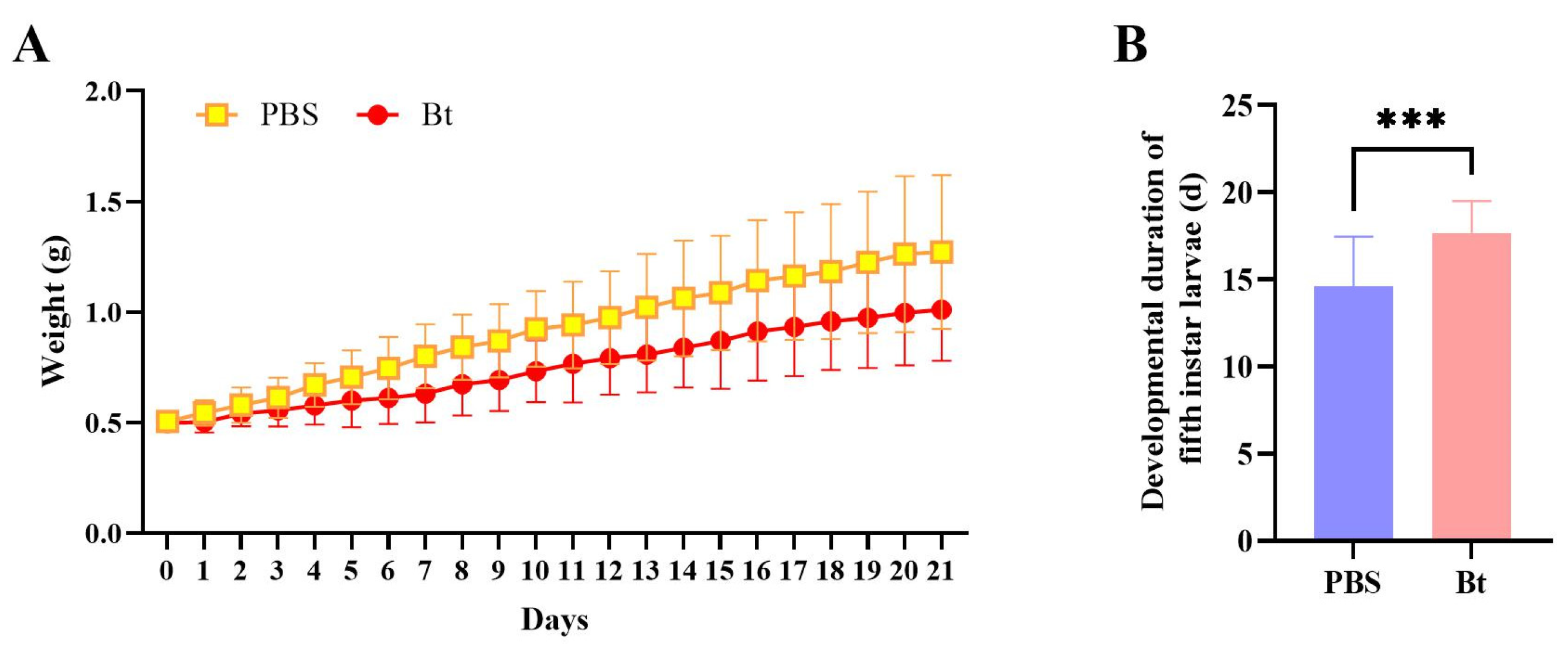
3.1. Effect of Bt Priming on RPW Development
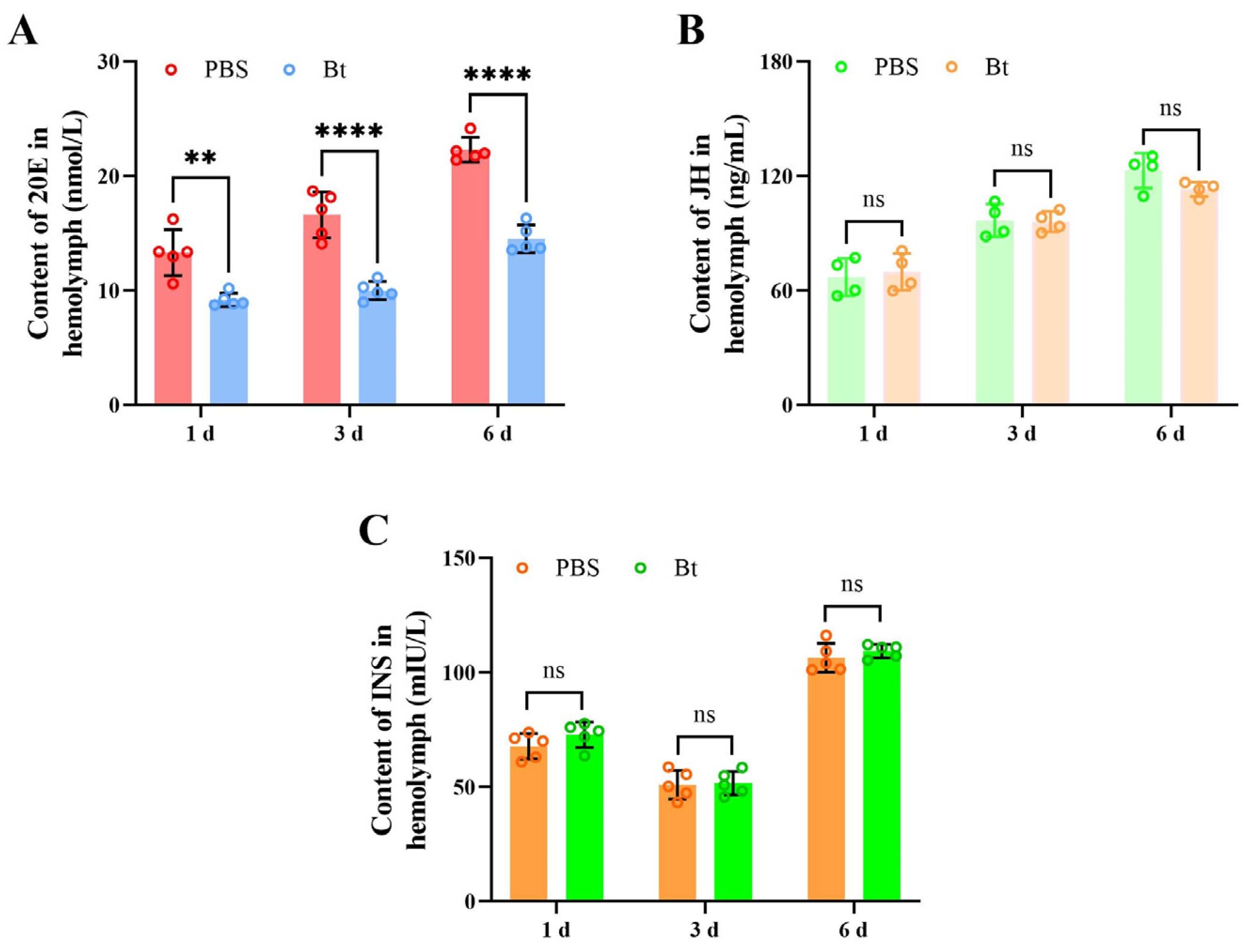
3.2. Effects of Bt Priming on Hormone Contents
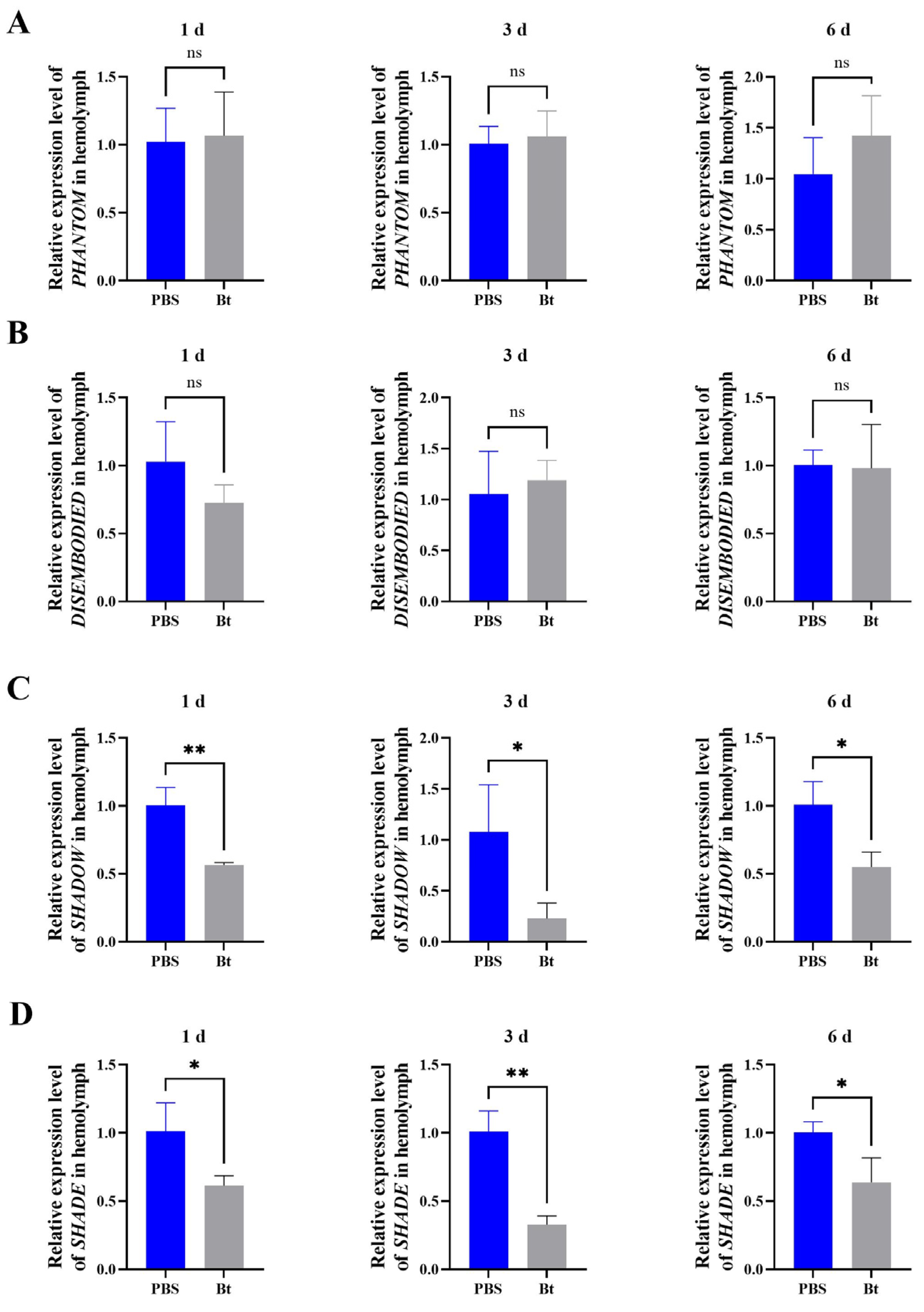
3.3. Effects of Bt Priming on Transcription of 20E Synthesis Pathway Genes
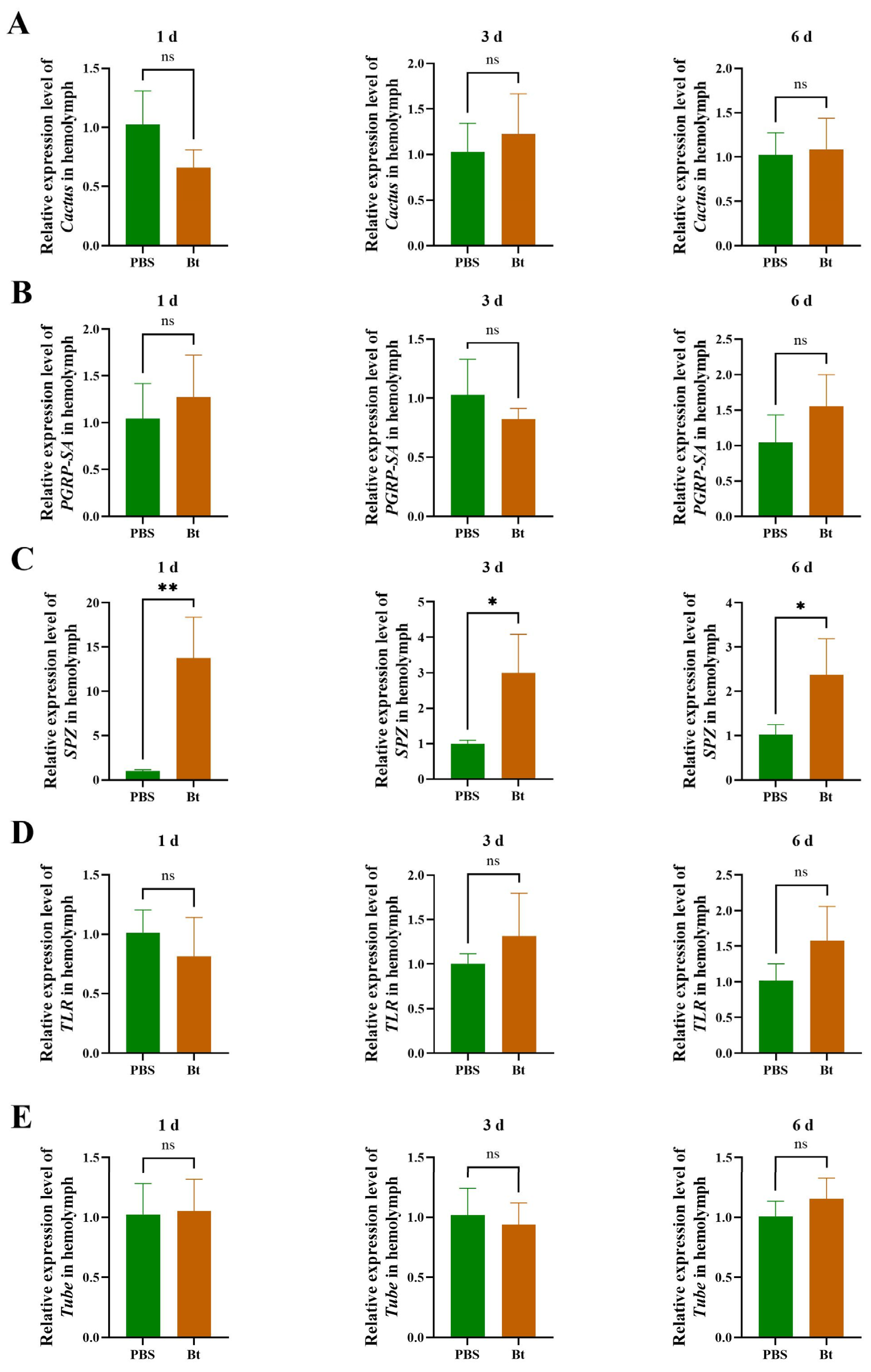
3.4. Effects of Bt Priming on Transcription of Toll Pathway Genes
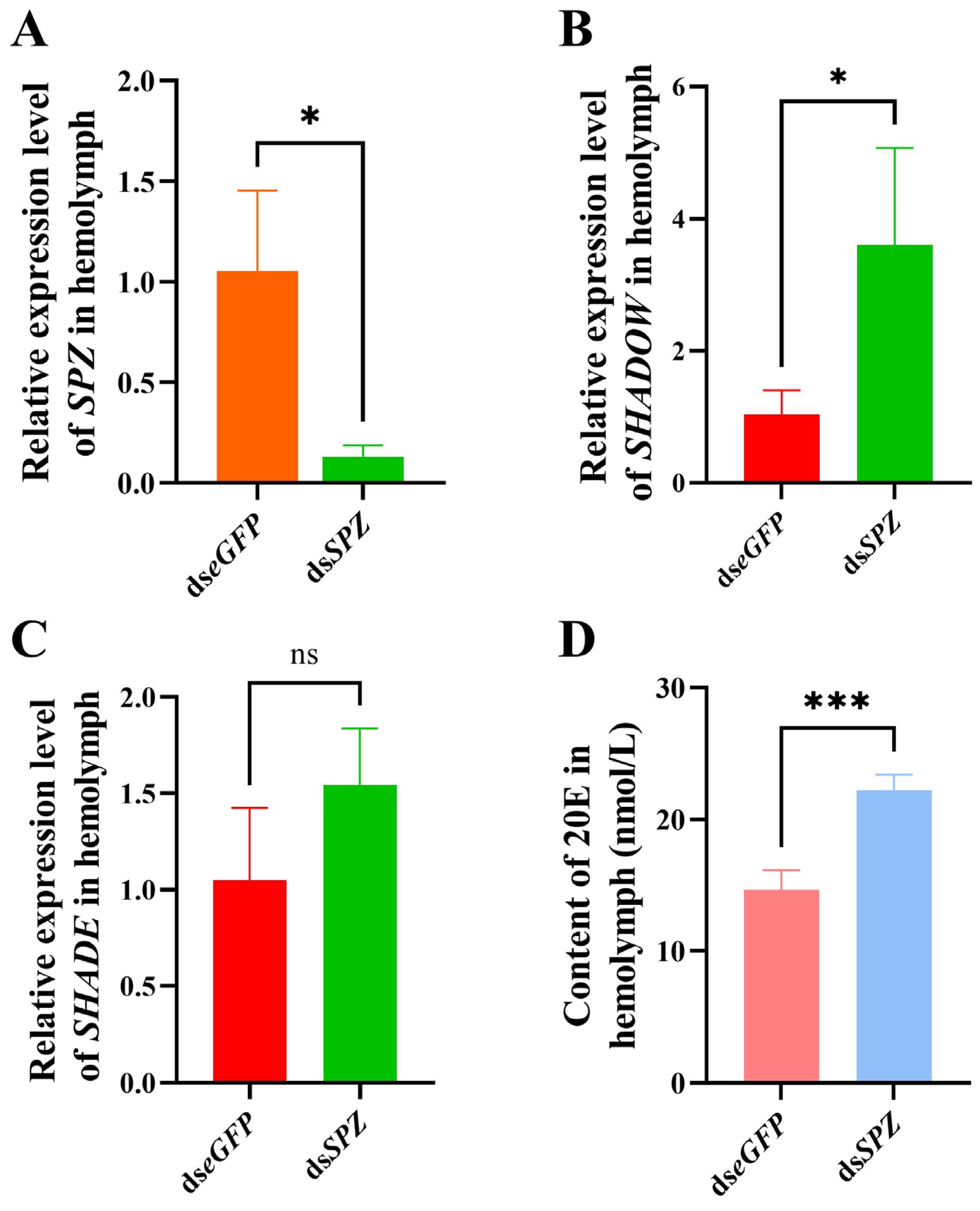
3.5. Effect of SPZ Silencing on SHADOW Expression and 20E Synthesis
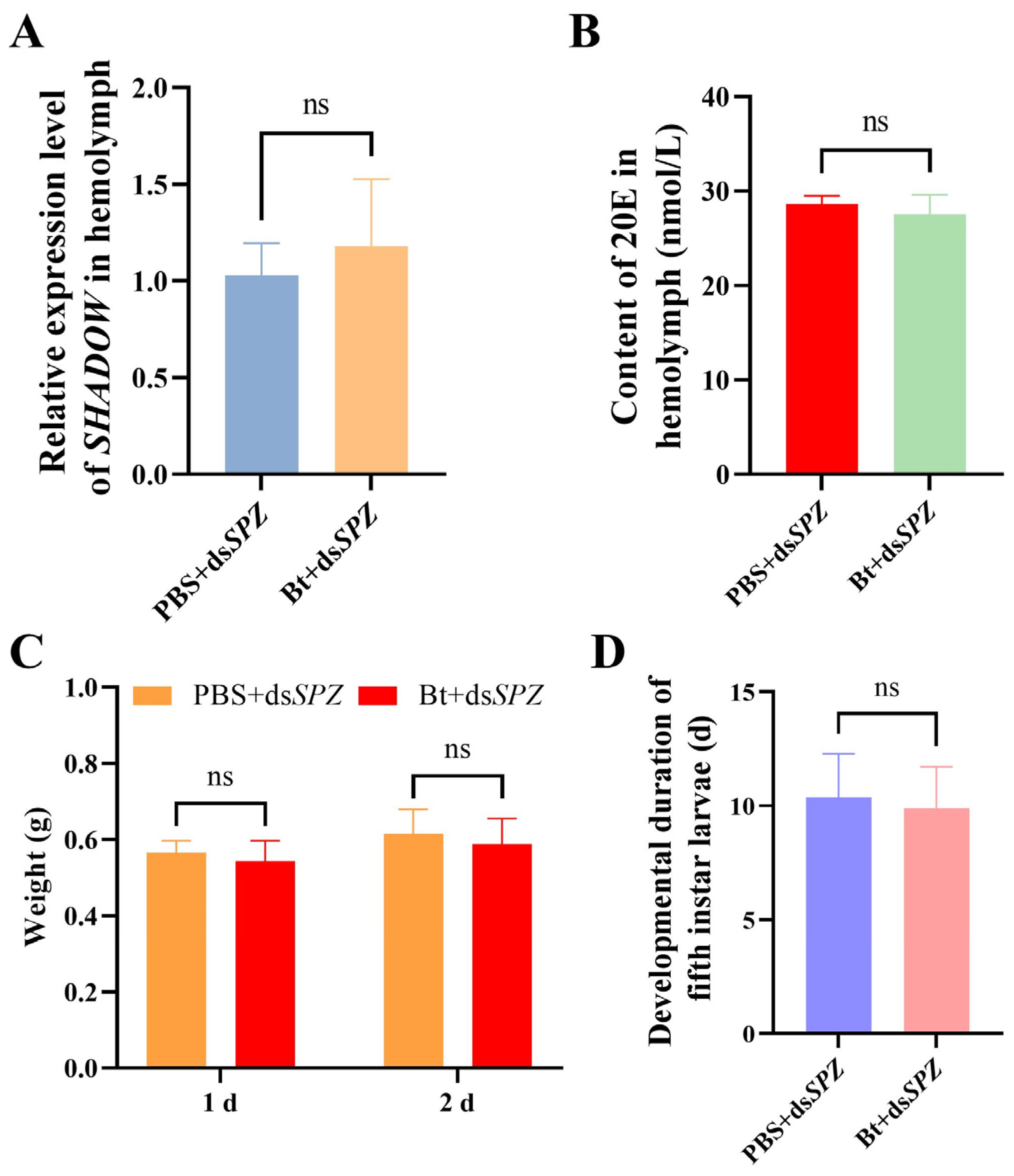
3.6. SPZ Interference Led to Clearance of Bt Priming on Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vilmos, P.; Kurucz, E. Insect immunity: Evolutionary roots of the mammalian innate immune system. Immunol. Lett. 1998, 62, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Silverman, N.; Cherry, S. Immunity in Drosophila melanogaster—From microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 2014, 14, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, G.; Farrell, G.; Kavanagh, K. Immune priming: The secret weapon of the insect world. Virulence 2020, 11, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.N.; Meng, J.Y.; Wang, Z.; Lin, S.Y.; Shen, J.; Yan, S. Research progress of aphid immunity system: Potential effective target for green pest management. Insect Sci. 2024; Advance online publication. [Google Scholar] [CrossRef]
- Cooper, D.; Eleftherianos, I. Memory and specificity in the insect immune system: Current perspectives and future challenges. Front. Immunol. 2017, 8, 539. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, A.R.; Li, P.J.; Lu, S.P.; Jia, Q.C.; Huang, S.N.; Bai, J.; Hou, Y.M. A specific primed immune response in red palm weevil, Rhynchophorus ferrugineus, is mediated by hemocyte differentiation and phagocytosis. Dev. Comp. Immunol. 2022, 131, 104380. [Google Scholar] [CrossRef]
- Jang, S.; Ishigami, K.; Mergaert, P.; Kikuchi, Y. Ingested soil bacteria breach gut epithelia and prime systemic immunity in an insect. Proc. Natl. Acad. Sci. USA 2024, 121, e2315540121. [Google Scholar] [CrossRef]
- Pham, L.N.; Dionne, M.S.; Shirasu-Hiza, M.; Schneider, D.S. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 2007, 3, e26. [Google Scholar] [CrossRef]
- Miyashita, A.; Kizaki, H.; Kawasaki, K.; Sekimizu, K.; Kaito, C. Primed immune responses to gram-negative peptidoglycans confer infection resistance in silkworms. J. Biol. Chem. 2014, 289, 14412–14421. [Google Scholar] [CrossRef]
- Milutinović, B.; Peuß, R.; Ferro, K.; Kurtz, J. Immune priming in arthropods: An update focusing on the red flour beetle. Zoology 2016, 119, 254–261. [Google Scholar] [CrossRef]
- Shi, Z.H.; Lin, Y.T.; Hou, Y.M. Mother-derived trans-generational immune priming in the red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera, Dryophthoridae). Bull. Entomol. Res. 2014, 104, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Dhinaut, J.; Chogne, M.; Moret, Y. Immune priming specificity within and across generations reveals the range of pathogens affecting evolution of immunity in an insect. J. Anim. Ecol. 2018, 87, 448–463. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.B.; van Lieshout, E.; Simmons, L.W. The effect of maternal and paternal immune challenge on offspring immunity and reproduction in a cricket. J. Evol. Biol. 2014, 27, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Mikonranta, L.; Mappes, J.; Kaukoniitty, M.; Freitak, D. Insect immunity: Oral exposure to a bacterial pathogen elicits free radical response and protects from a recurring infection. Front. Zool. 2014, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Ardia, D.R.; Gantz, J.E.; Schneider, B.C.; Schneider, S.; Strebel, S. Costs of immunity in insects: An induced immune response increases metabolic rate and decreases antimicrobial activity. Funct. Ecol. 2012, 26, 732–739. [Google Scholar] [CrossRef]
- Stearns, S.C. Life history evolution: Successes, limitations, and prospects. Naturwissenschaften 2000, 87, 476–486. [Google Scholar] [CrossRef]
- Rolff, J. Bateman’s principle and immunity. Proc. Biol. Sci. 2002, 269, 867–872. [Google Scholar] [CrossRef]
- Little, T.J.; Kraaijeveld, A.R. Ecological and evolutionary implications of immunological priming in invertebrates. Trends Ecol. Evol. 2004, 19, 58–60. [Google Scholar] [CrossRef]
- Flatt, T.; Kawecki, T.J. Juvenile hormone as a regulator of the trade-off between reproduction and life span in Drosophila melanogaster. Evolution 2007, 61, 1980–1991. [Google Scholar] [CrossRef]
- Khan, I.; Prakash, A.; Agashe, D. Experimental evolution of insect immune memory versus pathogen resistance. Proc. Biol. Sci. 2017, 284, 20171583. [Google Scholar] [CrossRef]
- Gourbal, B.; Pinaud, S.; Beckers, G.J.M.; van Der Meer, J.W.M.; Conrath, U.; Netea, M.G. Innate immune memory: An evolutionary perspective. Immunol. Rev. 2018, 283, 21–40. [Google Scholar] [CrossRef] [PubMed]
- van Boven, M.; Weissing, F.J. The evolutionary economics of immunity. Am. Nat. 2004, 163, 277–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kangassalo, K.; Valtonen, T.M.; Roff, D.; Pölkki, M.; Dubovskiy, I.M.; Sorvari, J.; Rantala, M.J. Intra-and trans-generational effects of larval diet on susceptibility to an entomopathogenic fungus, Beauveria bassiana, in the greater wax moth, Galleria mellonella. J. Evol. Biol. 2015, 28, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Kangassalo, K.; Valtonen, T.M.; Sorvari, J.; Kecko, S.; Pölkki, M.; Krams, I.; Krama, T.; Rantala, M.J. Independent and interactive effects of immune activation and larval diet on adult immune function, growth and development in the greater wax moth (Galleria mellonella). J. Evol. Biol. 2018, 31, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, C.; Troussard, J.P.; Martinaud, G.; Moreau, J.; Moret, Y. Differential expression and costs between maternally and paternally derived immune priming for offspring in an insect. J. Anim. Ecol. 2011, 80, 1174–1183. [Google Scholar] [CrossRef]
- Roth, O.; Kurtz, J. The stimulation of immune defence accelerates development in the red flour beetle (Tribolium castaneum). J. Evol. Biol. 2008, 21, 1703–1710. [Google Scholar] [CrossRef]
- Flatt, T.; Tu, M.P.; Tatar, M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. BioEssays 2005, 27, 999–1010. [Google Scholar] [CrossRef]
- Schwenke, R.A.; Lazzaro, B.P.; Wolfner, M.F. Reproduction-Immunity trade-offs in insects. Annu. Rev. Entomol. 2016, 61, 239–256. [Google Scholar] [CrossRef]
- Iga, M.; Smagghe, G. Identification and expression profile of Halloween genes involved in ecdysteroid biosynthesis in Spodoptera littoralis. Peptides 2010, 31, 456–467. [Google Scholar] [CrossRef]
- Yamanaka, N.; Rewitz, K.F.; O’Connor, M.B. Ecdysone control of developmental transitions: Lessons from Drosophila research. Annu. Rev. Entomol. 2013, 58, 497–516. [Google Scholar] [CrossRef]
- Flatt, T.; Heyland, A.; Rus, F.; Porpiglia, E.; Sherlock, C.; Yamamoto, R.; Garbuzov, A.; Palli, S.R.; Tatar, M.; Silverman, N. Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. J. Exp. Biol. 2008, 211, 2712–2724. [Google Scholar] [CrossRef] [PubMed]
- Harshman, L.G.; Zera, A.J. The cost of reproduction: The devil in the details. Trends Ecol. Evol. 2007, 22, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Palli, S.R.; Ladd, T.R.; Tomkins, W.L.; Shu, S.; Ramaswamy, S.B.; Tanaka, Y.; Arif, B.; Retnakaran, A. Choristoneura fumiferana entomopoxvirus prevents metamorphosis and modulates juvenile hormone and ecdysteroid titers. Insect Biochem. Mol. Biol. 2000, 30, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Kinjo, H.; Takatsuka, J.; Shiotsuki, T.; Kamita, S.G.; Kunimi, Y. Entomopoxvirus infection induces changes in both juvenile hormone and ecdysteroid levels in larval Mythimna separata. J. Gen. Virol. 2016, 97, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Dembilio, O.; Quesada-Moraga, E.; Santiago-Alvarez, C.; Jacas, J.A. Potential of an indigenous strain of the entomopathogenic fungus Beauveria bassiana as a biological control agent against the red palm weevil, Rhynchophorus ferrugineus. J. Invertebr. Pathol. 2010, 104, 214–221. [Google Scholar] [CrossRef]
- Pu, Y.C.; Hou, Y.M. Isolation and identification of bacterial strains with insecticidal activities from Rhynchophorus ferrugineus Oliver (Coleoptera: Curculionidae). J. Appl. Entomol. 2016, 140, 617–626. [Google Scholar] [CrossRef]
- Pu, Y.C.; Ma, T.L.; Hou, Y.M.; Sun, M. An entomopathogenic bacterium strain, Bacillus thuringiensis, as a biological control agent against the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Pest Manag. Sci. 2017, 73, 1494–1502. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhao, S.; Wu, X. BmNPV-induced hormone metabolic disorder in silkworm leads to enhanced locomotory behavior. Dev. Comp. Immunol. 2021, 121, 104036. [Google Scholar] [CrossRef]
- Li, J.; Mao, T.; Wang, H.; Lu, Z.; Qu, J.; Fang, Y.; Chen, J.; Li, M.; Cheng, X.; Hu, J.; et al. The CncC/keap1 pathway is activated in high temperature-induced metamorphosis and mediates the expression of Cyp450 genes in silkworm, Bombyx mori. Biochem. Biophys. Res. Commun. 2019, 514, 1045–1050. [Google Scholar] [CrossRef]
- Xie, J.; Chen, H.; Zheng, W.; Cai, Z.; Li, X.; Zhang, H. miR-275/305 cluster is essential for maintaining energy metabolic homeostasis by the insulin signaling pathway in Bactrocera dorsalis. PLoS Genet. 2022, 18, e1010418. [Google Scholar] [CrossRef]
- Huang, X.; Warren, J.T.; Gilbert, L.I. New players in the regulation of ecdysone biosynthesis. J. Genet. Genom. 2008, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, S.A.; Wasserman, S.A. Conventional and non-conventional Drosophila Toll signaling. Dev. Comp. Immunol. 2014, 42, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yu, N.; Smagghe, G. FoxO mediates the timing of pupation through regulating ecdysteroid biosynthesis in the red flour beetle, Tribolium castaneum. Gen. Comp. Endocrinol. 2018, 258, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Cime-Castillo, J.; Vargas, V.; Hernández-Tablas, J.M.; Quezada-Ruiz, E.; Díaz, G.; Lanz-Mendoza, H. The costs of transgenerational immune priming for homologous and heterologous infections with different serotypes of dengue virus in Aedes aegypti mosquitoes. Front. Immunol. 2023, 14, 1286831. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Brayner, F.A.; Alves, L.C.; Dixit, R.; Barillas-Mury, C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science 2010, 329, 1353–1355. [Google Scholar] [CrossRef]
- Wu, G.; Liu, J.; Li, M.; Xiao, Y.; Yi, Y. Prior infection of Galleria mellonella with sublethal dose of Bt elicits immune priming responses but incurs metabolic changes. J. Insect Physiol. 2022, 139, 104401. [Google Scholar] [CrossRef]
- Kurtz, J.; Franz, K. Innate defence: Evidence for memory in invertebrate immunity. Nature 2003, 425, 37–38. [Google Scholar] [CrossRef]
- Beckage, N.E.; Gelman, D.B. Wasp parasitoid disruption of host development: Implications for new biologically based strategies for insect control. Annu. Rev. Entomol. 2004, 49, 299–330. [Google Scholar] [CrossRef]
- Karlhofer, F.M.; Schafellner, M.; Hoch, G. Reduced activity of juvenile hormone esterase in microsporidia-infected Lymantria dispar larvae. J. Invertebr. Pathol. 2012, 110, 126–128. [Google Scholar] [CrossRef]
- Down, R.E.; Bell, H.A.; Bryning, G.; Kirkbride-Smith, A.E.; Edwards, J.P.; Weaver, R.J. Infection by the microsporidium Vairimorpha necatrix (Microspora: Microsporidia) elevates juvenile hormone titres in larvae of the tomato moth, Lacanobia oleracea (Lepidoptera: Noctuidae). J. Invertebr. Pathol. 2008, 97, 223–229. [Google Scholar] [CrossRef]
- Petryk, A.; Warren, J.T.; Marqués, G.; Jarcho, M.P.; Gilbert, L.I.; Kahler, J. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc. Natl. Acad. Sci. USA 2003, 100, 13773–13778. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, L.; Zou, M.M.; Vasseur, L.; Chu, L.N.; Qin, Y.D.; Zhai, Y.L.; You, M.S. Identification of Halloween genes and RNA interference-mediated functional characterization of a Halloween gene shadow in Plutella xylostella. Front. Physiol. 2019, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Wan, P.J.; Zhou, L.T.; Mu, L.L.; Li, G.Q. Knockdown of a putative Halloween gene Shade reveals its role in ecdysteroidogenesis in the small brown planthopper Laodelphax striatellus. Gene 2013, 531, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Tzou, P.; De Gregorio, E.; Lemaitre, B. How Drosophila combats microbial infection: A model to study innate immunity and host-pathogen interactions. Curr. Opin. Microbiol. 2002, 5, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D. Drosophila immunity: Paths and patterns. Curr. Opin. Immunol. 2003, 15, 12–19. [Google Scholar] [CrossRef]
- Brennan, C.A.; Anderson, K.V. Drosophila: The genetics of innate immune recognition and response. Annu. Rev. Immunol. 2004, 22, 457–483. [Google Scholar] [CrossRef]
- Lemaitre, B.; Reichhart, J.M.; Hoffmann, J.A. Drosophila host defense: Differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl. Acad. Sci. USA 1997, 94, 14614–14619. [Google Scholar] [CrossRef]
- Leulier, F.; Parquet, C.; Pili-Floury, S.; Ryu, J.H.; Caroff, M.; Lee, W.J.; Mengin-Lecreulx, D.; Lemaitre, B. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat. Immunol. 2003, 4, 478–484. [Google Scholar] [CrossRef]
- Valanne, S.; Wang, J.H.; Rämet, M. The Drosophila Toll signaling pathway. J. Immunol. 2011, 186, 649–656. [Google Scholar] [CrossRef]
- Muhammad, A.; Habineza, P.; Wang, X.; Xiao, R.; Ji, T.; Hou, Y.; Shi, Z. Spätzle Homolog-mediated Toll-like pathway regulates innate immune responses to maintain the homeostasis of gut microbiota in the red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae). Front. Microbiol. 2020, 11, 846. [Google Scholar] [CrossRef]
- Hamilton, W.D.; Zuk, M. Heritable true fitness and bright birds: A role for parasites? Science 1982, 218, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, B.C.; Verhulst, S. Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996, 11, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Nava-Sánchez, S.; González-Tokman, D.; Munguía-Steyer, R.; Córdoba-Aguilar, A. Does mating activity impair phagocytosis-mediated priming immune response? A test using the house cricket, Acheta domesticus. Acta Ethol. 2015, 18, 295–299. [Google Scholar] [CrossRef]
- Shikano, I.; Hua, K.N.; Cory, J.S. Baculovirus-challenge and poor nutrition inflict within-generation fitness costs without triggering transgenerational immune priming. J. Invertebr. Pathol. 2016, 136, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Prigot-Maurice, C.; Depeux, C.; Paulhac, H.; Braquart-Varnier, C.; Beltran-Bech, S. Immune priming in Armadillidium vulgare against Salmonellaenterica: Direct or indirect costs on life history traits? ZooKeys 2022, 1101, 131–158. [Google Scholar] [CrossRef]
- Rolff, J.; Siva-Jothy, M.T. Invertebrate ecological immunology. Science 2003, 301, 472–475. [Google Scholar] [CrossRef]
- Schmid-Hempel, P. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 2005, 50, 529–551. [Google Scholar] [CrossRef]
- Luu, H.; Tate, A.T. Recovery and immune priming modulate the evolutionary trajectory of infection-induced reproductive strategies. J. Evol. Biol. 2017, 30, 1748–1762. [Google Scholar] [CrossRef]
- Contreras-Garduño, J.; Rodríguez, M.C.; Rodríguez, M.H.; Alvarado-Delgado, A.; Lanz-Mendoza, H. Cost of immune priming within generations: Trade-off between infection and reproduction. Microbes Infect. 2014, 16, 261–267. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhang, H.; Tan, A.; Hu, Z.; Peng, L.; Hou, Y. Spätzle Regulates Developmental and Immune Trade-Offs Induced by Bacillus thuringiensis Priming in Rhynchophorus ferrugineus. Insects 2024, 15, 925. https://doi.org/10.3390/insects15120925
Li P, Zhang H, Tan A, Hu Z, Peng L, Hou Y. Spätzle Regulates Developmental and Immune Trade-Offs Induced by Bacillus thuringiensis Priming in Rhynchophorus ferrugineus. Insects. 2024; 15(12):925. https://doi.org/10.3390/insects15120925
Chicago/Turabian StyleLi, Pengju, He Zhang, Anran Tan, Zhuolin Hu, Lu Peng, and Youming Hou. 2024. "Spätzle Regulates Developmental and Immune Trade-Offs Induced by Bacillus thuringiensis Priming in Rhynchophorus ferrugineus" Insects 15, no. 12: 925. https://doi.org/10.3390/insects15120925
APA StyleLi, P., Zhang, H., Tan, A., Hu, Z., Peng, L., & Hou, Y. (2024). Spätzle Regulates Developmental and Immune Trade-Offs Induced by Bacillus thuringiensis Priming in Rhynchophorus ferrugineus. Insects, 15(12), 925. https://doi.org/10.3390/insects15120925




