Comparative Transcriptome Analysis of the Pest Galeruca daurica (Coleoptera: Chrysomelidae) Larvae in Response to Six Main Metabolites from Allium mongolicum (Liliaceae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Sample Processing
2.3. cDNA Library Construction and Transcriptome Sequencing
2.4. Analysis and Annotation of Differentially Expressed Genes
2.5. Verification of Differentially Expressed Genes by qRT-PCR
3. Results
3.1. The Basic Characteristics of Transcriptome Sequencing Analysis
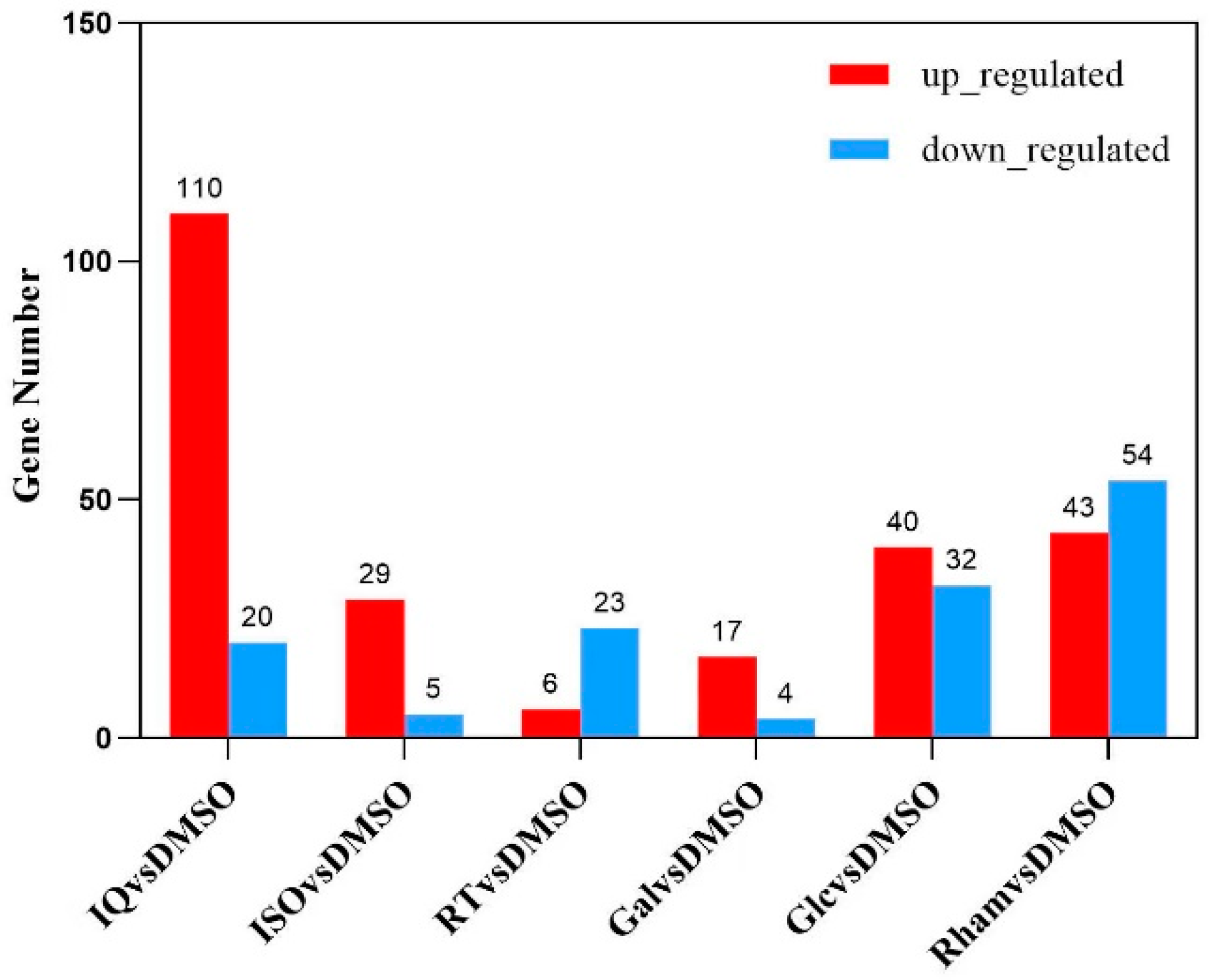
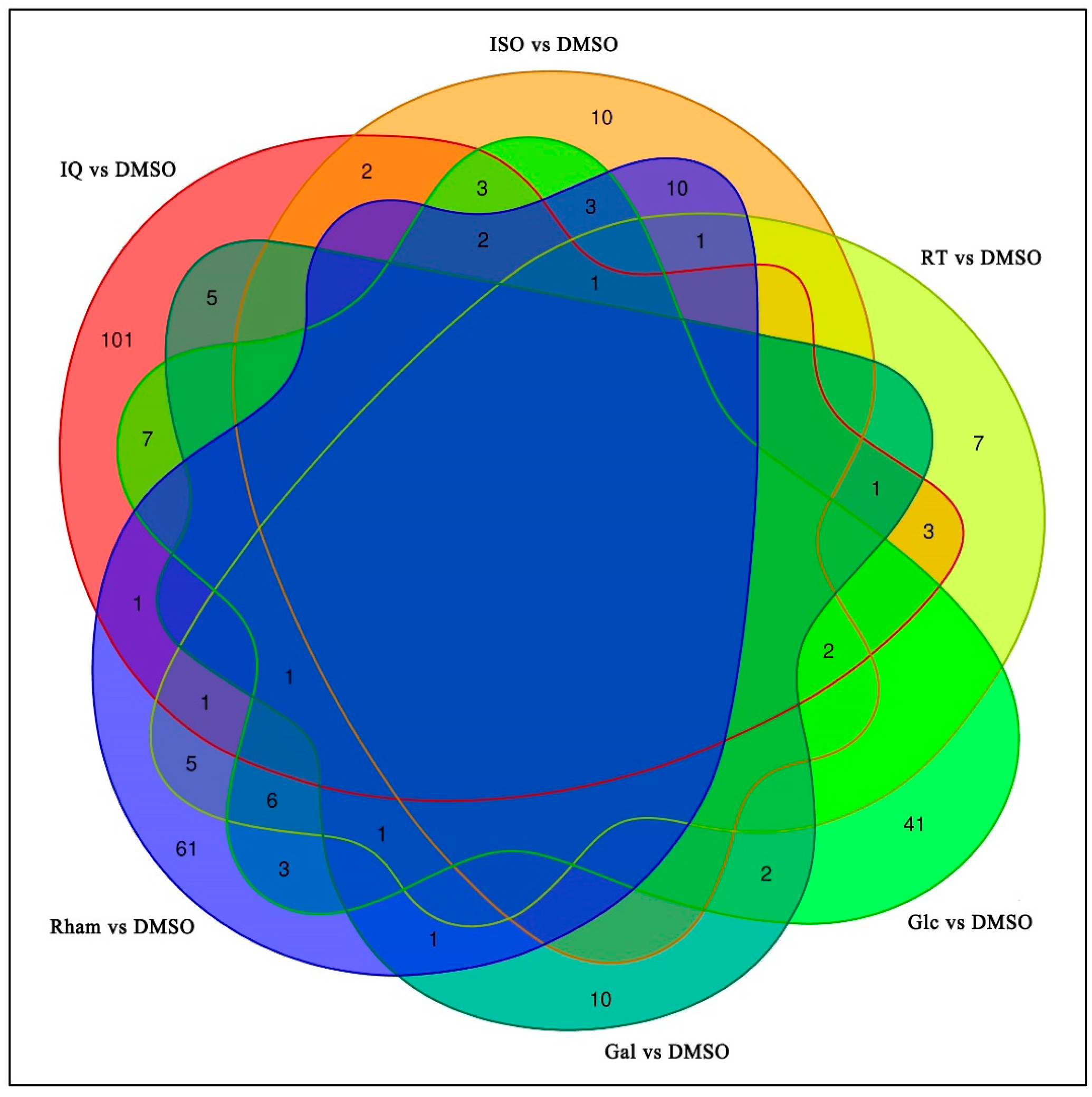
3.2. Analysis and Annotation of Differentially Expressed Genes (DEGs)
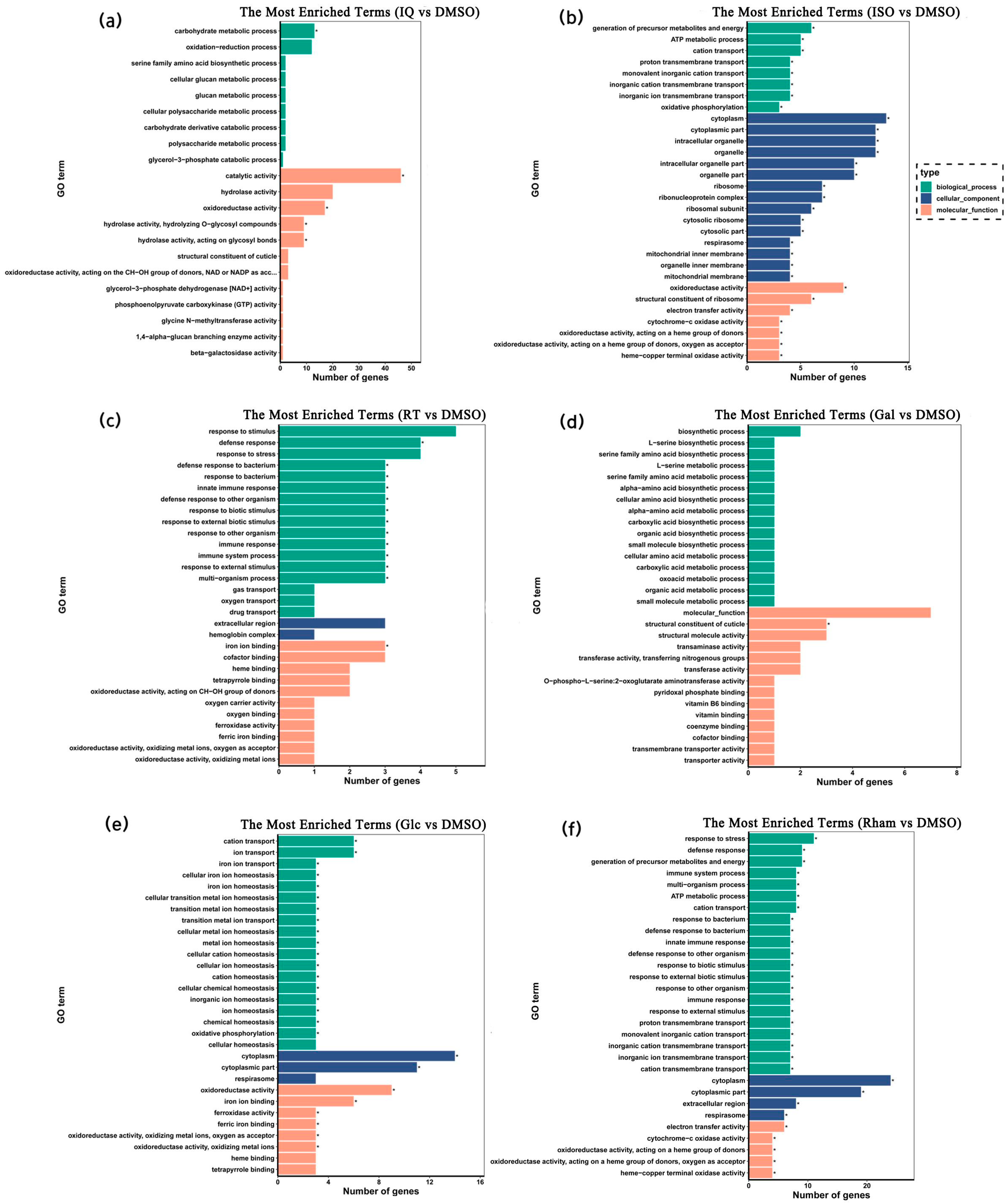
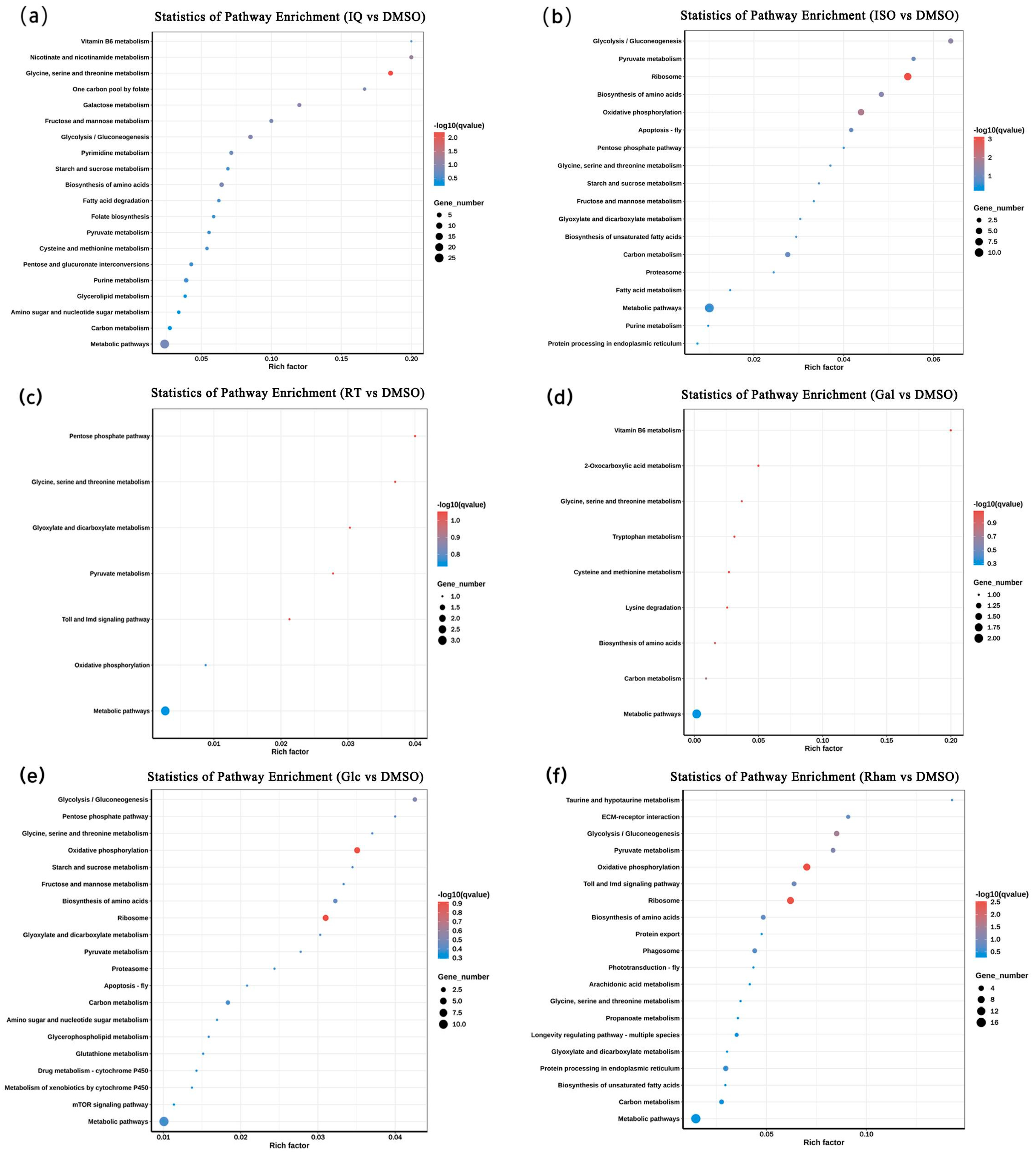
3.3. GO and KEGG Enrichment Analysis of Differentially Expressed Genes (DEGs)
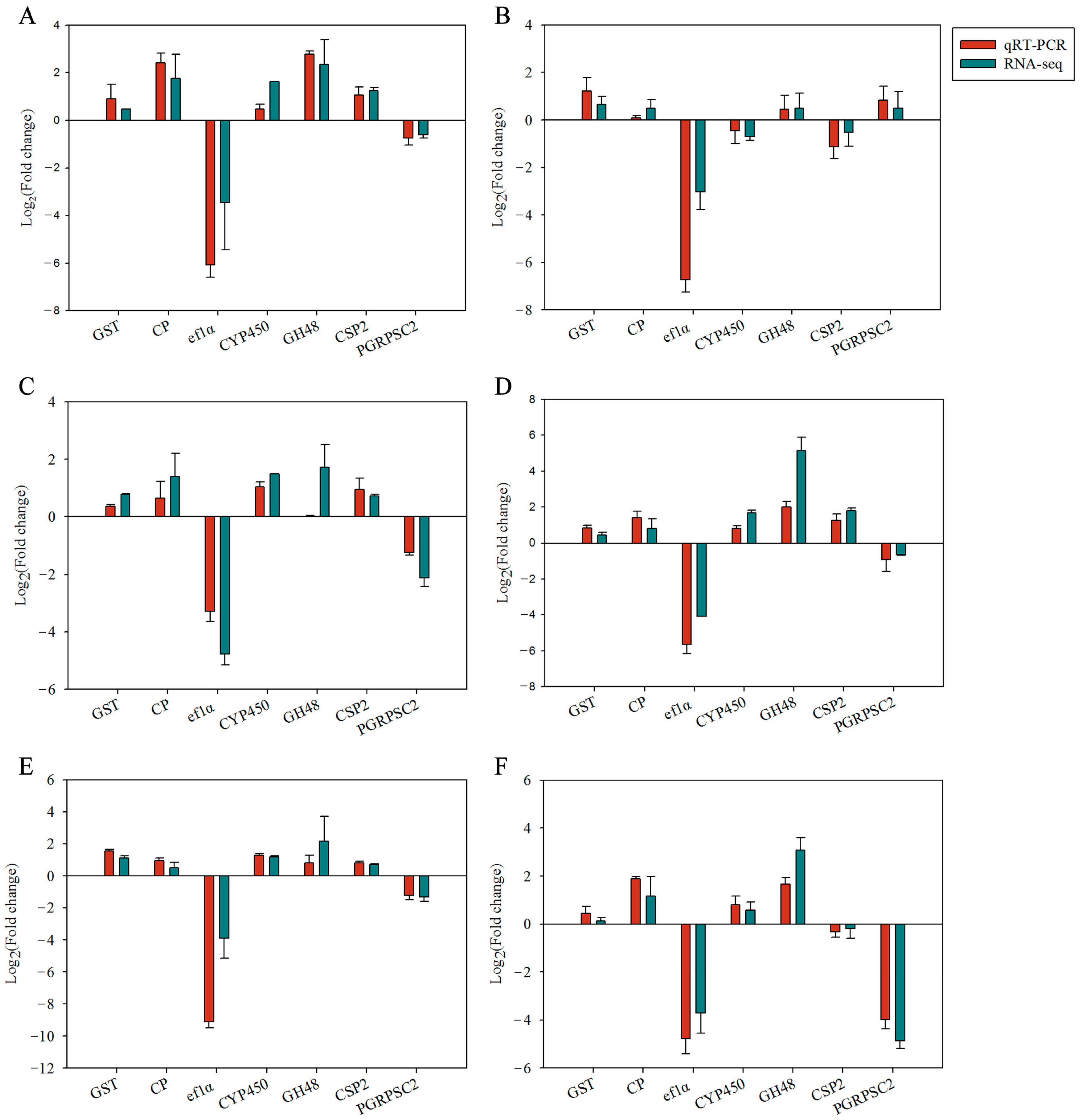
3.4. Validation of Differentially Expressed Genes (DEGs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hwang, S.Y.; Liu, C.H.; Shen, T.C. Effects of plant nutrient availability and host plant species on the performance of two Pieris butterflies (Lepidoptera: Pieridae). Biochem. Syst. Ecol. 2008, 36, 505–513. [Google Scholar] [CrossRef]
- Shahbaz, M.; Nouri-Ganbalani, G.; Naseri, B. Comparative damage and digestive enzyme activity of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on 12 tomato cultivars. Entomol. Res. 2019, 49, 401–408. [Google Scholar] [CrossRef]
- Després, L.; David, J.-P.; Gallet, C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Pauchet, Y.; Wilkinson, P.; Vogel, H.; Nelson, D.R.; Reynolds, S.E.; Heckel, D.G.; French-Constant, R.H. Pyrosequencing the Manduca sexta larval midgut transcriptome: Messages for digestion, detoxification and defense. Insect Mol. Biol. 2010, 19, 61–75. [Google Scholar] [CrossRef]
- Wäckers, F.L. Gustatory response by the Hymenopteran parasitoid Cotesia glomerata to a range of nectar and honeydew sugars. J. Chem. Ecol. 1999, 25, 2863–2877. [Google Scholar] [CrossRef]
- Naziri, A.M.; Kassim, N.F.A.; Jong, Z.W.; Webb, C.E. Effects of sugar concentration on fecundity, biting behavior and survivability of female Aedes (stegomyia) albopictus (Skuse). Southeast Asian J. Trop. Med. Public Health 2016, 47, 1160–1166. [Google Scholar]
- Singh, S.; Kaur, I.; Kariyat, R. The multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Wach, A.; Pyrzyńska, K.; Biesaga, M. Quercetin content in some food and herbal samples. Food Chem. 2005, 100, 699–704. [Google Scholar] [CrossRef]
- Simmonds, M.S. Flavonoid-insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Simmonds, M.S.; Stevenson, P.C. Effects of isoflavonoids from Cicer on larvae of Heliocoverpa armigera. J. Chem. Ecol. 2001, 27, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Bentivenha, J.P.F.; Canassa, V.F.; Baldin, E.L.L.; Borguini, M.G.; Lima, G.P.P.; Lourenção, A.L. Role of the rutin and genistein flavonoids in soybean resistance to Piezodorus guildinii (Hemiptera: Pentatomidae). Arthropod-Plant Interact. 2018, 12, 311–320. [Google Scholar] [CrossRef]
- Dixit, G.; Praveen, A.; Tripathi, T.; Yadav, V.K.; Verma, P.C. Herbivore-responsive cotton phenolics and their impact on insect performance and biochemistry. J. Asia-Pacific Entonnol. 2017, 20, 341–351. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Nenaah, G.E. Toxic and antifeedant activities of prenylated flavonoids isolated from Tephrosia apollinea L. against three major coleopteran pests of stored grains with reference to their structure–activity relationship. Nat. Prod. Res. 2014, 28, 2245–2252. [Google Scholar] [CrossRef]
- Yan, J.-K.; Zhu, J.; Liu, Y.-J.; Chen, X.; Wang, W.-H.; Zhang, H.-N.; Li, L. Recent advances in research on Allium plants: Functional ingredients, physiological activities, and applications in agricultural and food sciences. Crit. Rev. Food Sci. Nutr. 2022, 63, 21–29. [Google Scholar] [CrossRef]
- Zhao, C.-Y.; Ao, C.-J.; Zhang, X.-F. Isolation and purification, structure identification and effect of flavonoids in shallot on immune antioxidant function in mice. Feed Industry. 2010, 31, 11–14. [Google Scholar]
- Dong, Y.-Z.; Qu, L.; Li, X.-X.; Han, L.-F.; Wang, T.; Zhang, Y. Isolation and identification of constituents from Allium mongolicum Regel I. Chin. J. Med. Chem. 2015, 4, 298–302. [Google Scholar]
- Dong, Y.-Z.; Shi, W.-Z.; Yang, S.-C.; Li, X.-X.; Zhang, Y.; Wang, T. Isolation and identification of constituents from Allium mongolicum Regel II. J. Tianjin Univ. Tradit. Chin. Med. 2016, 35, 404–408. [Google Scholar]
- Sobolewska, D.; Michalska, K.; Podolak, I.; Grabowska, K. Steroidal saponins from the genus Allium. Phytochem. Rev. 2016, 15, 1–35. [Google Scholar] [CrossRef]
- Turunen, S. Efficient use of dietary galactose in Pieris brassicae. J. Insect Physiol. 1992, 38, 503–509. [Google Scholar] [CrossRef]
- Hossein, H.; Marjan, N. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J. Endocrinol. Investig. 2014, 37, 783–788. [Google Scholar]
- Debarati, C.; Kavitha, T. Longevity-promoting efficacies of rutin in high fat diet fed Drosophila melanogaster. Biogerontology 2020, 21, 653–668. [Google Scholar]
- Silva, T.R.F.B.; Almeida, A.C.S.; Moura, T.L.; Silva, A.R.; Freitas, S.S.; Jesus, F.G. Effect of the flavonoid rutin on the biology of Spodoptera frugiperda (Lepidoptera: Noctuidae). Acta Sci. Agron. 2016, 38, 165–170. [Google Scholar] [CrossRef]
- Jadhav, D.R.; Mallikarjuna, N.; Rathore, A.; Pokle, D. Effect of some flavonoids on survival and development of Helicoverpa armigera (Hübner) and Spodoptera litura (Fab) (Lepidoptera: Noctuidae). Asian J. Agric. Sci. 2012, 4, 298–307. [Google Scholar]
- Golawska, S.; Sprawka, I.; Lukasik, I.; Golawski, A. Are naringenin and quercetin useful chemicals in pest-management strategies? J. Pest Sci. 2014, 87, 173–180. [Google Scholar] [CrossRef]
- Selin-Rani, S.; Senthil-Nathan, S.; Thanigaivel, A.; Vasantha-Srinivasan, P.; Edwin, E.-S.; Ponsankar, A.; Lija-Escaline, J.; Kalaivani, K.; Abdel-Megeed, A.; Hunter, W.B.; et al. Toxicity and physiological effect of quercetin on generalist herbivore, Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Chemosphere 2016, 165, 257–267. [Google Scholar] [CrossRef]
- Diaz Napal, G.N.; Defagó, M.T.; Valladares, G.R.; Palacios, S.M. Response of Epilachna paenulata to two flavonoids, pinocembrin and quercetin, in a comparative study. J. Chem. Ecol. 2010, 36, 898–904. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect P450 enzymes. Annu. Rev. Entomol. 1999, 44, 507–533. [Google Scholar] [CrossRef]
- Giraudo, M.; Hilliou, F.; Fricaux, T.; Audant, P.; Feyereisen, R.; Le, G.G. Cytochrome P450s from the fall armyworm (Spodoptera frugiperda): Responses to plant allelochemicals and pesticides. Insect Mol. Biol. 2015, 24, 115–128. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect P450 inhibitors and insecticides: Challenges and opportunities. Pest Manage. Sci. 2015, 71, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Cianfrogna, J.A.; Zangerl, A.R.; Berenbaum, M.R. Effects of furanocoumarins on feeding behavior of parsnip webworms Depressaria pastinacella. J. Chem. Ecol. 2002, 28, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-M.; Zhang, Y.-J.; Cui, G.-Y.; Ji, R.; Zhang, Y.-J.; He, L. Effects of tea saponin on the detoxification and protective enzyme activity of Locusta migratoria. Chin. J. Appl. Entomol. 2023, 60, 1412–1422. [Google Scholar]
- Yang, X.K.; Huang, D.C.; Ge, S.Q.; Bai, M.; Zhang, R.Z. One million mu of meadow in Inner Mongolia suffer from the harm of breaking out of Galeruca daurica (Joannis). Chin. Bull. Entomol. 2010, 47, 812. [Google Scholar]
- Hao, X.; Zhou, X.-R.; Pang, B.-P.; Zhang, Z.-R.; Ma, C.-Y. Effects of host plants on the growth, development and feeding of firefly larvae of Galeruca daurica. Acta Agrestia Sin. 2014, 4, 854–858. [Google Scholar]
- Li, J.-W.; Li, L.; Li, N.; Pang, B.-P. Effect of host plant metabolites on feeding by Galeruca daurica. J. North. Agric. 2024, in press.
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, X.-R.; Pang, B.-P. Reference gene selection and evaluation for expression analysis using qRT-PCR in Galeruca daurica (Joannis). Bull. Entomol. Res. 2016, 107, 359–368. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-R.; Li, W.-Z.; Dong, J.-F.; Ding, S.-B.; Zhou, Z.; Song, N.; Ma, J.-S. An introduction to phytophagous insect host-plant selection hypotheses. Chin. J. Appl. Entomol. 2021, 58, 1245–1256. [Google Scholar]
- Qi, Q.; Sun, L.-L.; Xu, L.-S.; Cao, C.-W. Response of glutathione S-transferase (GST) genes in Lymantria dispar to flavone and quercetin stresses based on RNAi analysis. J. Environ. Entomol. 2021, 43, 1359–1367. [Google Scholar]
- Chen, R.; Gao, H.; Zhang, G.-J.; Zhu, K.-Y.; Cheng, W.-N. Effects of secondary metabolites in wheat kernels on activities of three detoxifying enzymes and related gene expression in Sitodiplosis moselena. Sci. Agric. Sin. 2020, 53, 4204–4214. [Google Scholar]
- Howell, N. Evolutionary conservation of protein regions in the protonmotive cytochrome b and their possible roles in redox catalysis. J. Mol. Evol. 1989, 29, 157–169. [Google Scholar] [CrossRef]
- Dennerlein, S.; Rehling, P.; Richter-Dennerlein, R. Cytochrome c oxidase biogenesis—From translation to early assembly of the core subunit COX1. FEBS Letters. 2023, 597, 1569–1578. [Google Scholar] [CrossRef]
- Yu, M.; Chen, Y.-X.; Liu, Y.-J.; Yu, M.; Xu, Y.; Wang, B. Efficient polysaccharides from Crinum asiaticum L.’ structural characterization and anti-tumor effect. Saudi J. Biol. Sci. 2019, 26, 2085–2090. [Google Scholar] [CrossRef]
- Gao, P.-Y.; Bian, J.; Xu, S.-S.; Liu, C.-F.; Sun, Y.-Q.; Zhang, G.-L.; Li, D.-Q.; Liu, X.-G. Structural features, selenization modification, antioxidant and anti-tumor effects of polysaccharides from alfalfa roots. Int. J. Biol. Macromol. 2020, 149, 207–214. [Google Scholar] [CrossRef]
- Giraud, M.F.; Naismith, J.H. The rhamnose pathway. Curr. Opin. Struct. Biol. 2000, 10, 687–696. [Google Scholar] [CrossRef]
- Pan, Z.-Q.; Yao, Q.; Tang, B.; Zheng, X.-X.; Zhang, W.-Q. Cloning and sequence analysis of three 60S ribosomal protein genes from Spodoptera exigua. J. Environ. Entomol. 2008, 30, 310–317. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, J.; Wang, H.; Li, Y.; Dong, R.; Pang, B. Comparative Transcriptome Analysis of the Pest Galeruca daurica (Coleoptera: Chrysomelidae) Larvae in Response to Six Main Metabolites from Allium mongolicum (Liliaceae). Insects 2024, 15, 847. https://doi.org/10.3390/insects15110847
Li L, Li J, Wang H, Li Y, Dong R, Pang B. Comparative Transcriptome Analysis of the Pest Galeruca daurica (Coleoptera: Chrysomelidae) Larvae in Response to Six Main Metabolites from Allium mongolicum (Liliaceae). Insects. 2024; 15(11):847. https://doi.org/10.3390/insects15110847
Chicago/Turabian StyleLi, Ling, Jinwei Li, Haichao Wang, Yanyan Li, Ruiwen Dong, and Baoping Pang. 2024. "Comparative Transcriptome Analysis of the Pest Galeruca daurica (Coleoptera: Chrysomelidae) Larvae in Response to Six Main Metabolites from Allium mongolicum (Liliaceae)" Insects 15, no. 11: 847. https://doi.org/10.3390/insects15110847
APA StyleLi, L., Li, J., Wang, H., Li, Y., Dong, R., & Pang, B. (2024). Comparative Transcriptome Analysis of the Pest Galeruca daurica (Coleoptera: Chrysomelidae) Larvae in Response to Six Main Metabolites from Allium mongolicum (Liliaceae). Insects, 15(11), 847. https://doi.org/10.3390/insects15110847




