Utilizing Insects as Bioindicators: An Approximation for Conservation in Urban Lentic Ecosystems in Central Chile
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
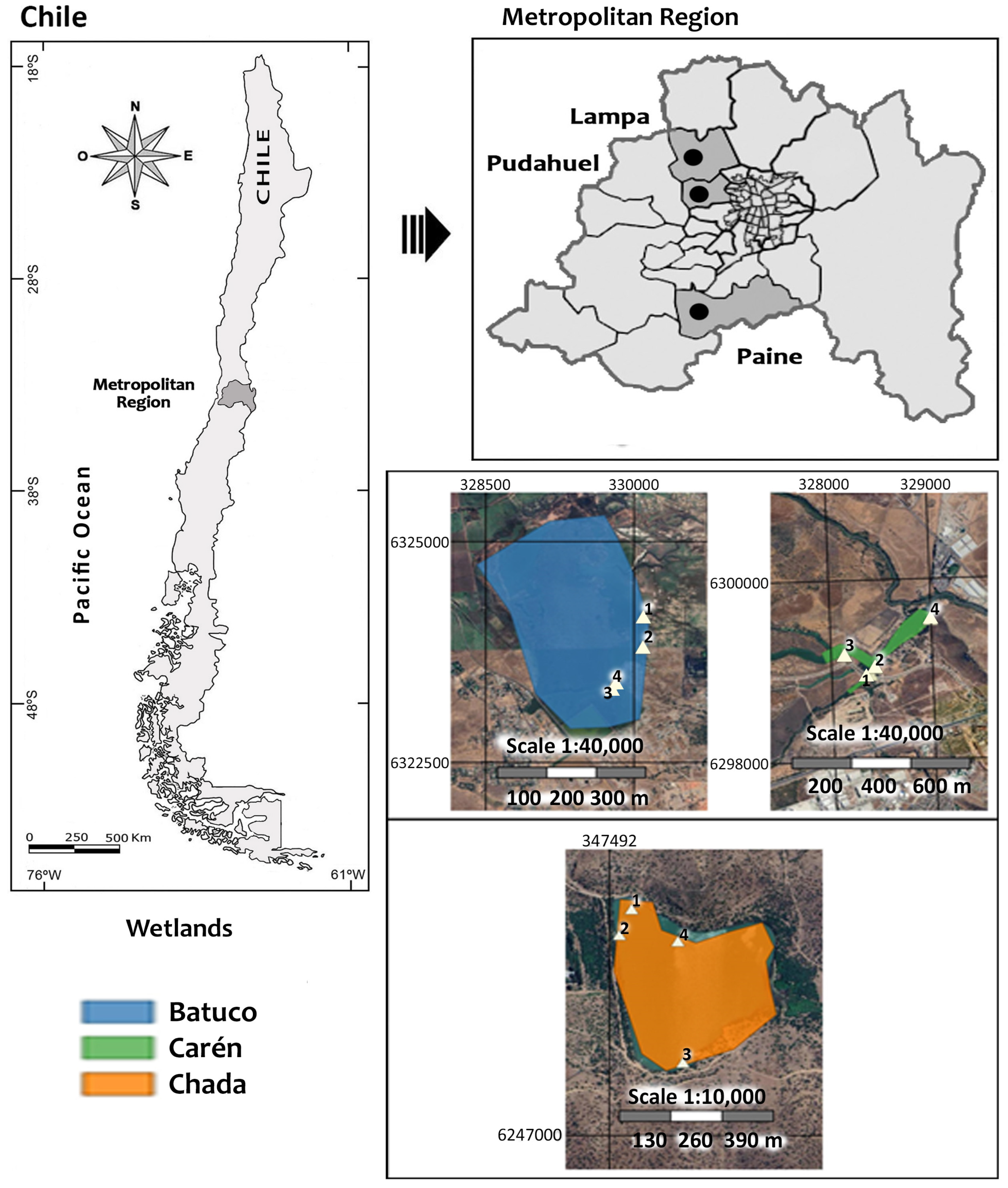
2.1. Study Areas
2.2. Sampling and Identification of Aquatic Insects
2.3. Determination of Water Quality Classes by Biotic Indices
2.4. Measurement and Determination of Water Quality Classes Using Physicochemical Variables
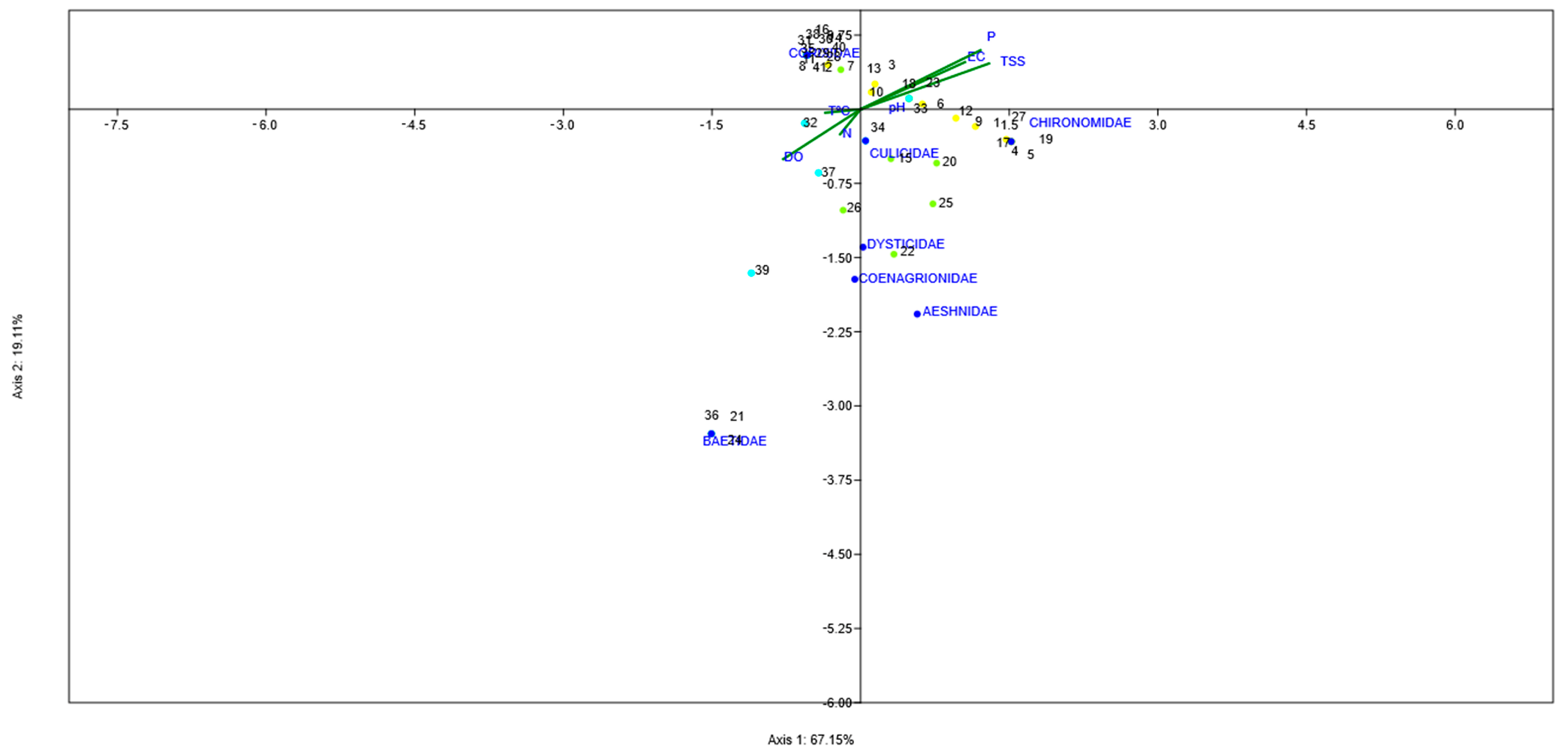
2.5. Global Analysis of Physicochemical and Biological Variables
3. Results
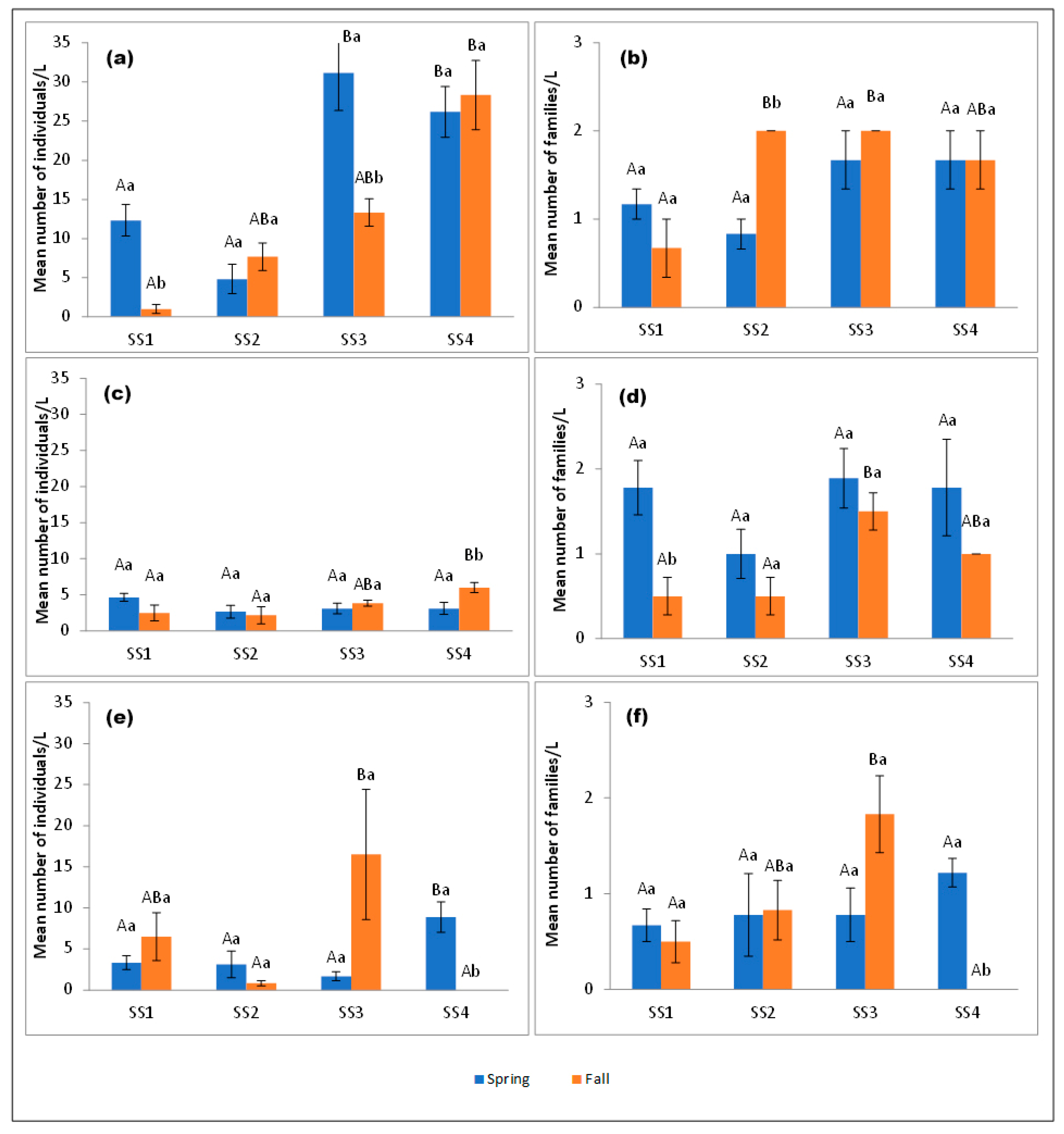
3.1. Abundance and Richness of Aquatic Insect Family Assemblages
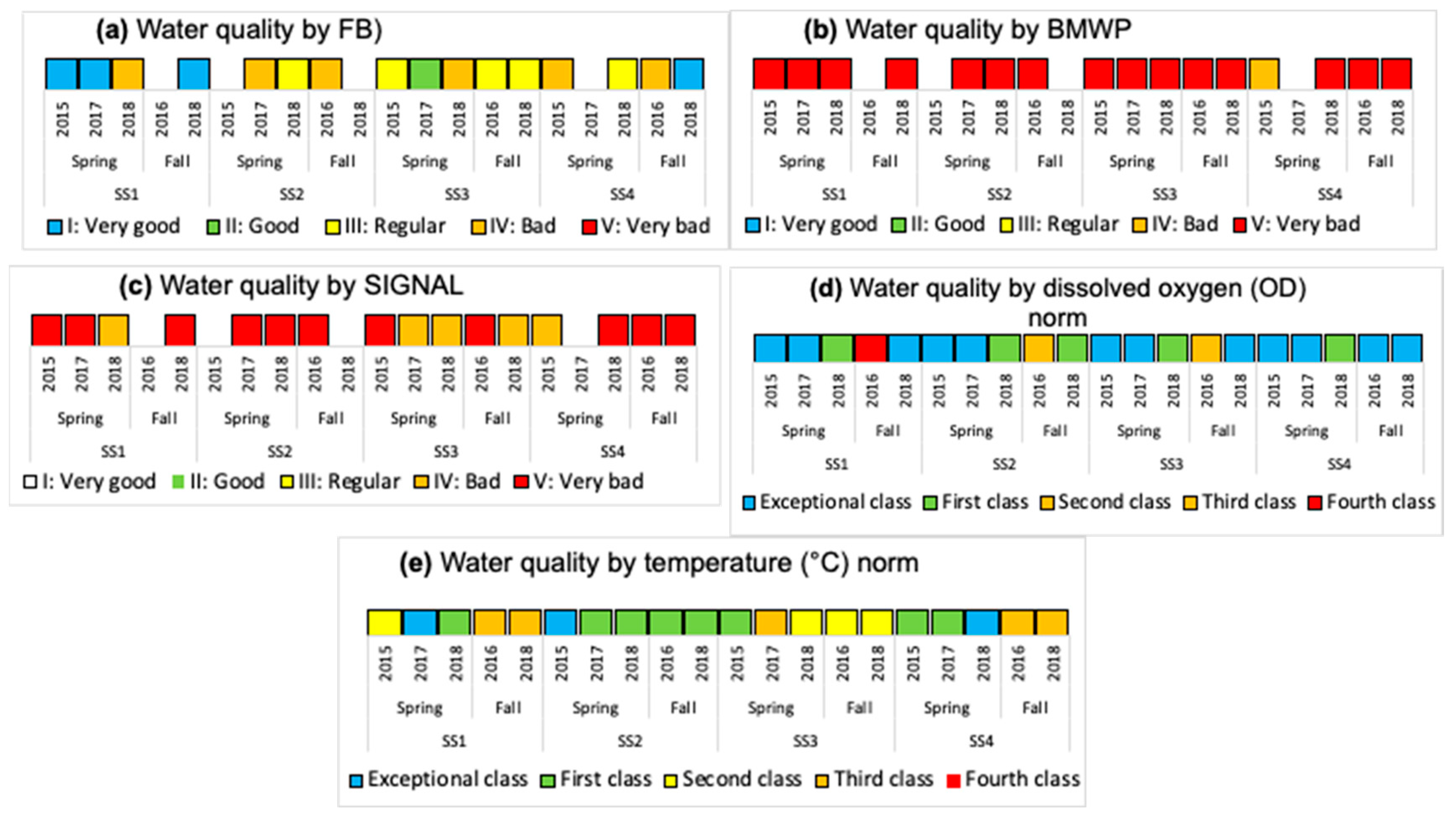
3.2. Water Quality from Physicochemical and Biological Variables
3.3. Global Results of the Three Water Bodies
4. Discussion
4.1. Analysis by Water Body
4.1.1. Batuco
4.1.2. Carén
4.1.3. Chada
4.2. A Global Analysis of the Three Water Bodies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Formetta, G.; Marra, F.; Dallan, E.; Zaramella, M.; Borga, M. Differential orographic impact on sub-hourly, hourly, and daily extreme precipitation. Adv. Water Resour. 2022, 159, 104084. [Google Scholar] [CrossRef]
- Tesser-Obregón, C. El agua y los territorios hídricos en la Región Metropolitana de Santiago de Chile. Casos de estudio: Tiltil, Valle de Mallarauco y San Pedro de Melipilla. Estud. Geográficos 2013, 74, 255–285. [Google Scholar] [CrossRef]
- Barría, P.; Chadwick, C.; Ocampo-Melgar, A.; Galleguillos, M.; Garreaud, R.; Díaz-Vasconcellos, R.; Poblete, D.; Rubio-Álvarez, E.; Poblete-Caballero, D. Water management or megadrought: What caused the Chilean Aculeo Lake drying? Reg. Environ. Chang. 2021, 21, 19. [Google Scholar] [CrossRef]
- Rey, O.; Eizaguirre, C.; Angers, B.; Baltazar-Soares, M.; Sagonas, K.; Prunier, J.G.; Blanchet, S. Linking epigenetics and biological conservation: Towards a conservation epigenetics perspective. Funct. Ecol. 2020, 34, 414–427. [Google Scholar] [CrossRef]
- INN Chile (Instituto Nacional de Normalización Chile). Norma Chilena Oficial NCh 1333. of. 78 Modificada en 1987 “Requisitos de Calidad de Agua para Diferentes Usos”; INN Chile: Santiago, Chile, 1987. [Google Scholar]
- CONAMA (Comisión Nacional del Medio Ambiente, Chile). Guía Para el Establecimiento de las Normas Secundarias de Calidad Ambiental Para Aguas Continentales Superficiales y Marinas; CONAMA: Santiago, Chile, 2004. [Google Scholar]
- Figueroa, R.; Soria, M.; Beltrán, M.; Correa-Araneda, J. Estudio de comunidades biológicas como bioindicadores de calidad de agua. In Estudio de Comunidades Biológicas como Bioindicadores de Calidad de Agua; Chatata, B., Talavera, C., Villasante, F., Eds.; Universidad Nacional de San Agustín-CONCYTEC: Arequipa, Peru, 2016; pp. 23–34. [Google Scholar]
- Alvarado-Orellana, A.; Huerta-Fuentes, A.; Palma-Muñoz, A.; Rodríguez-Tobar, S. Variación estacional de la diversidad de coleópteros epigeos en la Laguna Carén (Santiago, Chile). Rev. Colomb. Entomol. 2018, 44, 266–272. [Google Scholar] [CrossRef]
- Chowdhury, S.; Dubey, V.K.; Choudhury, S.; Das, A.; Jeengar, D.; Sujatha, B.; Kumar, A.; Kumar, N.; Semwal, A.; Kumar, V. Insects as bioindicator: A hidden gem for environmental monitoring. Front. Environ. Sci. 2023, 11, 1146052. [Google Scholar] [CrossRef]
- Figueroa, R.; Palma, A.; Ruiz, V.; Niell, X. Análisis comparativo de índices bióticos utilizados en la evaluación de la calidad de las aguas en un río mediterráneo de Chile: Río Chillán, VIII Región. Rev. Chil. Hist. Nat. 2007, 80, 225–242. [Google Scholar] [CrossRef]
- Gobierno de Chile. Definición Contenida, Guía para la Conservación y Seguimiento Ambiental de Humedales Andinos. 2011. Available online: https://bibliotecadigital.ciren.cl/server/api/core/bitstreams/45a24e5b-1b80-476d-9660-a01d14e971c7/content (accessed on 8 August 2024).
- Laino-Guanes, R.M.; Bello-Mendoza, R.; González-Espinosa, M.; Ramírez-Marcial, N.; Jiménez-Otárola, F.; Musálem-Castillejos, K. Concentración de metales en agua y sedimentos de la cuenca alta del río Grijalva, frontera México-Guatemala. Tecnol. Cienc. Agua 2015, 6, 61–74. [Google Scholar]
- Segnini, S.; Correa, I.; Chacón, M. Evaluación de la calidad del agua de ríos en los Andes venezolanos usando el índice biótico BMWP. In Enfoques y Temáticas en Entomología; Arrivillaga, J.C., El Souki, M., Herrera, B., Eds.; Sociedad Venezolana de Entomología: Caracas, Venezuela, 2009; pp. 217–254. [Google Scholar]
- Hinselhoff, W.L. Rapid field assessment of organic pollution with a family-level biotic index. J. N. Am. Benthol. Soc. 1988, 7, 65–68. [Google Scholar]
- Armitage, P.D.; Moss, D.; Wright, J.F.; Furse, M.T. The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running-water sites. Water Res. 1983, 17, 333–347. [Google Scholar] [CrossRef]
- Chessman, B.C. New sensitivity grades for Australian river macroinvertebrates. Mar. Freshw. Res. 2003, 54, 95–103. [Google Scholar] [CrossRef]
- Ndatimana, G.; Nantege, D.; Arimoro, F.O. A review of the application of the macroinvertebrate-based multimetric indices (MMIs) for water quality monitoring in lakes. Environ. Sci. Pollut. Res. 2023, 30, 73098–73115. [Google Scholar] [CrossRef]
- Obolewski, K.; Glińska-Lewczuk, K.; Srzelczak, A. The use of benthic macroinvertebrate metrics in the assessment of ecological status of floodplain lakes. J. Freshw. Ecol. 2014, 29, 225–242. [Google Scholar] [CrossRef]
- Abhijna, U.G.; Ratheesh, R.; Kumar, B. Distribution and diversity of aquatic insects of Vellayani lake in Kerala. J. Environ. Biol. 2013, 34, 605–611. [Google Scholar]
- Dalal, A.; Gupta, S. A comparative study of the aquatic insect diversity of two ponds located in Cachar District, Assam, India. Turk. J. Zool. 2016, 40, 392–401. [Google Scholar] [CrossRef]
- Choudhury, D.; Gupta, S. Impact of waste dump on surface water quality and aquatic insect diversity of Deepor Beel (Ramsar site), Assam, North-east India. Environ. Monit. Assess. 2017, 189, 540. [Google Scholar] [CrossRef]
- Dalal, A.; Gupta, S. Aquatic Insects as Pollution Indicator—A Study in Cachar, Assam, Northeast India. In Environmental Pollution: Select Proceedings of ICWEES-2016; Springer: Singapore, 2018; pp. 103–124. [Google Scholar]
- Hershey, A.E.; Lamberti, G.A.; Chaloner, D.T.; Northington, R.M. Aquatic Insect Ecology, Chapter 17. In Ecology and Classification of North American Freshwater Invertebrates, 3rd ed.; Thorp, J.H., Covich, A.P., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 659–694. [Google Scholar]
- Pintar, M.; Resetarits, W.J. Aquatic beetles colonization of disparate taxa in small lentic systems. Ecol. Evol. 2020, 10, 12170–12182. [Google Scholar] [CrossRef] [PubMed]
- Hayasshi, M. Insect diversity of lentic water surface in Japan. Jpn. J. Syst. Entomol. 2023, 29, 199–210. [Google Scholar]
- Reyes-Morales, F. Macroinvertebrados acuáticos de los cuerpos lénticos de la Región Maya. Guatemala. Rev. Científica 2013, 23, 7–16. [Google Scholar] [CrossRef]
- Badaracco, C.; Huerta, A.; Palma, A. Assemblage of riparian epigeal coleopteran of the Batuco wetland (Metropolitan Region, Chile). Gayana 2021, 85, 46–54. [Google Scholar]
- Ministerio del Medio Ambiente (MMA). Declara Santuario de la Naturaleza Laguna de Batuco. Diario Oficial de la República de Chile Nº43.105. 2021. Available online: https://www.monumentos.gob.cl/sites/default/files/decretos/sn_01770_2021_d20_do.pdf (accessed on 15 August 2024).
- Gobernación Provincial de Maipo. Tranque de Chada, Riego y Vida para la Zona. 2015. Available online: www.gobernacionmaipo.gov.cl/noticias/tranque-de-chada-riego-y-vida-para-la-zona/ (accessed on 8 August 2024).
- Universidad de Chile. Parque Carén: Pionera planta Piloto de Alimentos Saludables Inició su Construcción. 2017. Available online: https://www.uchile.cl/noticias/137460/pionera-planta-piloto-de-alimentos-saludables-inicia-su-construccion (accessed on 8 August 2024).
- Universidad de Chile. Parque Carén-Universidad de Chile. 2024. Available online: https://caren.uchile.cl/presentacion/#parqueConclusions (accessed on 8 August 2024).
- INN Chile (Instituto Nacional de Normalización Chile). Norma Chilena Oficial NCh-ISO 5667/4 “Calidad del agua—Muestreo—Parte 4: Guía para la toma de Muestras de lagos Naturales y Artificiales”; INN Chile: Santiago, Chile, 2016. [Google Scholar]
- Camousseight, A. Estado de conocimiento de los Ephemeroptera de Chile. Gayana 2006, 70, 50–56. [Google Scholar] [CrossRef]
- Thyssen, P.J. Keys for Identification of Immature Insects. In Current Concepts in Forensic Entomology; Amendt, J., Goff, M., Campobasso, C., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 25–42. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020; Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina. Available online: http://www.infostat.com.ar (accessed on 8 August 2024).
- Engelmann, H.D. Zur Dominanzklassifizierung von Bodenarthropoden. Pedobiologia 1978, 18, 378–380. [Google Scholar] [CrossRef]
- MOP-DGA (Ministerio de Obras Públicas-Dirección General de Aguas). Valores de los Puntajes de FBI Modificados para Chile; MOP-DGA: Santiago, Chile, 2010. [Google Scholar]
- INN Chile. Norma Chilena Oficial de Calidad de Agua NCh 411/1-4; INN Chile: Santiago, Chile, 1996. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Anton-Pardo, M.; Ortega, J.C.; Melo, A.S.; Bini, L.M. Global meta-analysis reveals that invertebrate diversity is higher in permanent than in temporary lentic water bodies. Freshw. Biol. 2019, 64, 2234–2246. [Google Scholar] [CrossRef]
- Ye, Z.; Damgaard, J.; Hädicke, C.W.; Zhu, X.; Mazzucconi, S.A.; Hebsgaard, M.B.; Xie, T.; Yang, H.; Bu, W. Phylogeny and historical biogeography of the water boatmen (Insecta: Hemiptera: Heteroptera: Nepomorpha: Corixoidea). Mol. Phylogenetics Evol. 2023, 180, 107698. [Google Scholar] [CrossRef]
- Savage, A.A. Use of water boatmen (Corixidae) in the classification of lakes. Biol. Conserv. 1982, 23, 55–70. [Google Scholar] [CrossRef]
- Newall, P.; Tiller, D. Derivation of nutrient guidelines for streams in Victoria, Australia. Environ. Monit. Assess. 2002, 74, 85–103. [Google Scholar] [CrossRef]
- Martel-Cea, A.; Astorga, G.A.; Hernández, M.; Caputo, C.; Abarzúa, A.M. Modern chironomids (Diptera: Chironomidae) and the environmental variables that influence their distribution in the Araucanian lakes, south-central Chile. Hydrobiologia 2021, 848, 2551–2568. [Google Scholar] [CrossRef]
- Castellanos, K.; Pizarro, J.; Cuentas, K.; Costa, J.C.; Pino, Z.; Gutierrez, L.C.; Luiz, O.; Arboleda, J.W. Lentic water quality characterization using macroinvertebrates as bioindicators: An adapted BMWP index. Ecol. Indic. 2017, 7, 53–66. [Google Scholar] [CrossRef]
- Buffagni, A. The lentic and lotic characteristics of habitats determine the distribution of benthic macroinvertebrates in Mediterranean rivers. Freshw. Biol. 2020, 66, 13–34. [Google Scholar] [CrossRef]
- Gandouin, E.; Maasri, A.; Van Vliet-Lanoe, B.; Franquet, E. Chironomid (Insecta: Diptera) assemblages from a gradient of lotic and lentic waterbodies in River Floodplains of France: A Methodological Tool for Paleoecological Applications. J. Paleolimnol. 2006, 35, 149–166. [Google Scholar] [CrossRef]
- Mellado, C. Caracterización Hídrica y Gestión Ambiental del Humedal Batuco. Master’s Thesis, Universidad de Chile, Santiago, Chile, 2008. [Google Scholar]
- Mera, R.; Siccha-Ramirez, R.; Ramirez, J.L.; Núñez-Rodríguez, D.; Britzke, R.; Velásquez-Rodríguez, K.; Ramírez, R.; Huamantinco, A.A. Who is Andesiops peruvianus (Ulmer, 1920) (Ephemeroptera: Baetidae)? New insight from the type basin using morphological and molecular analyses. Zootaxa 2023, 5256, 371–382. [Google Scholar] [CrossRef]
- MOP-DGA. Decreto 53; MOP-DGA: Santiago, Chile, 2020. [Google Scholar]
- SMA (Superintendencia del Medio Ambiente—Gobierno de Chile). Normas Secundarias de Calidad Ambiental para la Protección de las Aguas de la Cuenca del Río Maipo. Informe Técnico de Cumplimiento de Normas de Calidad del Agua; SMA: Santiago, Chile, 2020. [Google Scholar]
- Instituto Nacional de Investigación de Recursos Naturales (Chile). Suelos. Descripciones. Proyecto Aerofotogramétrico Chile/OEA/BID; IREN-CORFO: Santiago, Chile, 1964. [Google Scholar]
- Sandoval, J. El Riego en Chile; Dirección de Obras Hidráulicas (DOH Chile)—Ministerio de Obras Públicas (MOP): Santiago, Chile, 2003. [Google Scholar]
- Posada, E.; Mojica, D.; Pino, N.; Bustamante, C.; Monzón, A. Establecimiento de índices de calidad ambiental de ríos con bases en el comportamiento del oxígeno disuelto y de la temperatura. Aplicación al caso del río Medellín, en el Valle de Aburrá en Colombia. Dyna 2013, 80, 192–200. [Google Scholar]
- Blocksom, K.A.; Kurtenbach, J.P.; Klemm, D.J.; Fulk, F.A.; Comier, S.M. Development and Evaluation of the Lake Macroinvertebrate Integrity Index (LMII) for New Jersey Lakes and Reservoirs. Environ. Monit. Assess. 2002, 77, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Michael-Kordatou, I.; Michael, C.; Duan, X.; He, X.; Dionysiou, D.D.; Mills, M.A.; Fatta-Kassinos, D. Dissolved effluent organic matter: Characteristics and potential implications in wastewater treatment and reuse applications. Water Res. 2015, 77, 213–248. [Google Scholar] [CrossRef]
- Boyd, C.E. Eutrophication. In Water Quality; Boyd, C.E., Ed.; Springer: Cham, Switzerland, 2020; pp. 311–322. [Google Scholar]
- Carrera, D.; Crisanto, T.; Maya, M. Relación entre la composición química inorgánica del agua, la precipitación y la evaporación. Enfoque UTE 2015, 6, 25–34. [Google Scholar] [CrossRef]
- Carrera-Villacrés, D.; Guerrón-Varela, E.; Cajas-Morales, L.; González-Farinango, T.; Guamán-Pineda, E.; Velarde-Salazar, P. Relación de temperatura, pH y CE en la variación de concentración de fosfatos en el Río Grande, Cantón Chone. Congr. De Cienc. Y Tecnol. ESPE 2018, 13, 37–40. [Google Scholar] [CrossRef]
- González, P.; Stehr, A.; Barra, R. Assessment of water quality trends through the application of an aggregated water quality index with historical monitored data in a Mediterranean Andean basin. Ecol. Indic. 2024, 166, 112373. [Google Scholar] [CrossRef]







| Taxon | Water Bodies | |||||
|---|---|---|---|---|---|---|
| Batuco | Carén | Chada | ||||
| No. of Specimens * | Abundance Rank | No. of Specimens | Abundance Rank | No. of Specimens | Abundance Rank | |
| COLEOPTERA Dytiscidae | 4 | Recedent | - | - | 4 | Recedent |
| Hydrophilidae | - | - | - | - | 2 | Subrecedent |
| DIPTERA | ||||||
| Chironomidae | 211 | Eudominant | 88 | Eudominant | 18 | Subdominant |
| Culicidae EPHEMEROPTERA | 1 | Sporadic | - | - | 3 | Recedent |
| Baetidae | - | - | 26 | Dominant | 43 | Dominant |
| HEMIPTERA | ||||||
| Corixidae | 382 | Eudominant | 82 | Eudominant | 226 | Eudominant |
| ODONATA | ||||||
| Aeshnidae | - | - | - | - | ||
| Coenagrionidae | - | - | 10 | Subdominant | - | - |
| Biological Indicators/Class/Significance | Batuco | Carén | Chada |
|---|---|---|---|
| Family Biotic Index (FBI) | 4.94 | 5.10 | 3.27 |
| Class/Significance | III/Regular | III/Regular | I/Very good |
| Biological Monitoring Work Party (BMWP) | 4.41 | 6.44 | 5.00 |
| Class/Significance | V/Very bad | V/Very bad | V/Very bad |
| Stream Invertebrate Grade Number-Average Level (SIGNAL) | 2.54 | 3.35 | 3.19 |
| Class/Significance | V/Very bad | IV/Bad | V/Very bad |
| Friedman Ranking Test | ||||
|---|---|---|---|---|
| Biological Indices | n | gl | T Value | p Value |
| FBI/BMWP/SIGNAL | 41 | 2 | 390.85 | <0.0001 * |
| Wilcoxon signed-rank test | ||||
| n | Z value | p value | ||
| FBI/BMWP | 41 | −5.65 | 0.00 * | |
| FBI/SIGNAL | 41 | −5.64 | 0.00 * | |
| BMWP/SIGNAL | 41 | −2.80 | 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, S.; Huerta, A.; Palma, Á.; Vicencio, F.; Araya, J.E. Utilizing Insects as Bioindicators: An Approximation for Conservation in Urban Lentic Ecosystems in Central Chile. Insects 2024, 15, 831. https://doi.org/10.3390/insects15110831
Rodríguez S, Huerta A, Palma Á, Vicencio F, Araya JE. Utilizing Insects as Bioindicators: An Approximation for Conservation in Urban Lentic Ecosystems in Central Chile. Insects. 2024; 15(11):831. https://doi.org/10.3390/insects15110831
Chicago/Turabian StyleRodríguez, Sebastián, Amanda Huerta, Álvaro Palma, Francisco Vicencio, and Jaime E. Araya. 2024. "Utilizing Insects as Bioindicators: An Approximation for Conservation in Urban Lentic Ecosystems in Central Chile" Insects 15, no. 11: 831. https://doi.org/10.3390/insects15110831
APA StyleRodríguez, S., Huerta, A., Palma, Á., Vicencio, F., & Araya, J. E. (2024). Utilizing Insects as Bioindicators: An Approximation for Conservation in Urban Lentic Ecosystems in Central Chile. Insects, 15(11), 831. https://doi.org/10.3390/insects15110831





