GIS-Enhanced Survey of Potential Aedes aegypti and Aedes albopictus Artificial Oviposition Containers Distributed across Communities in Trinidad, West Indies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
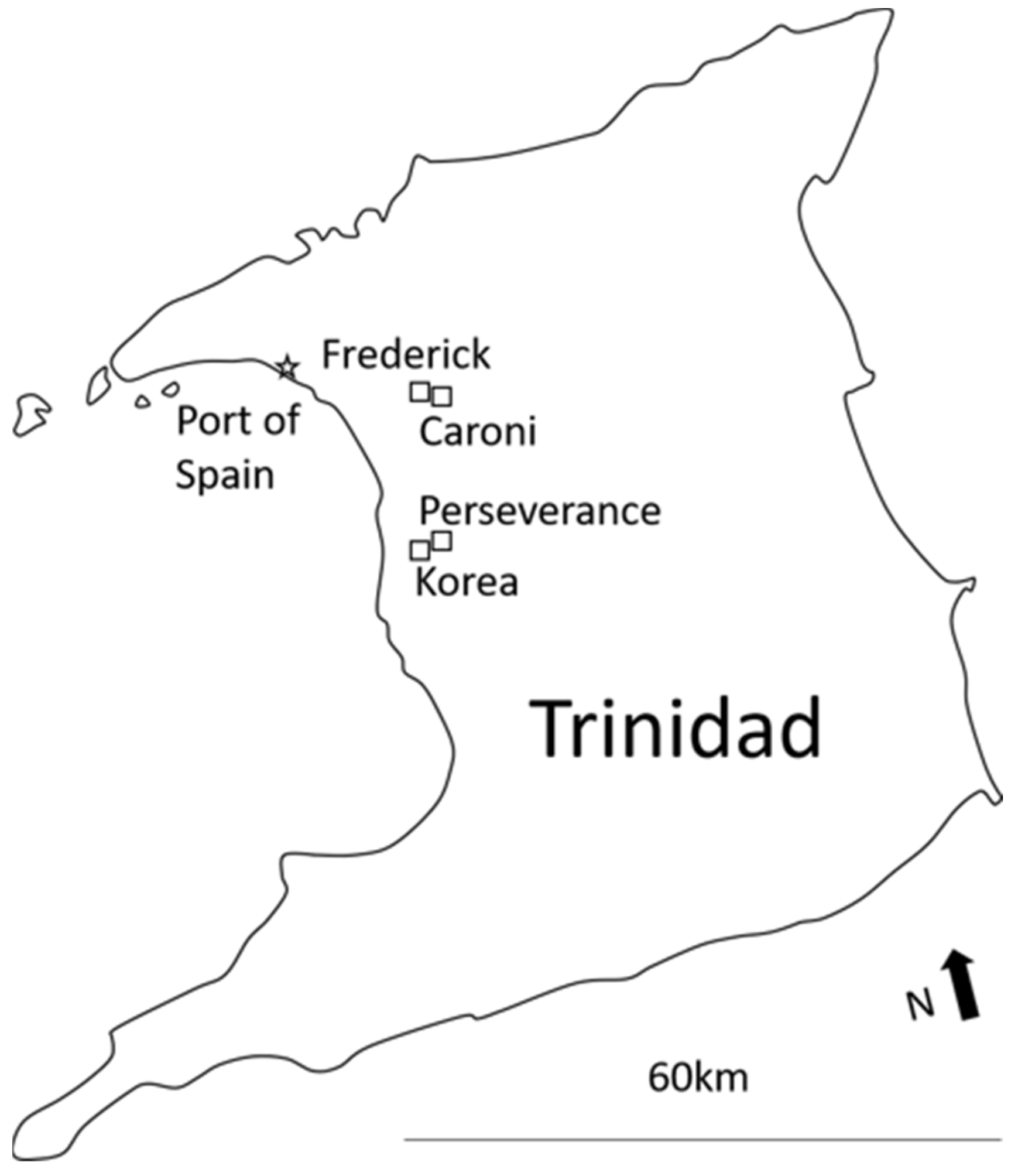
2.1. Study Sites
2.2. Survey Methods
2.3. Entomological Indices
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Dengue—Global Situation. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON518 (accessed on 19 August 2024).
- Silva, J.P.; Fernandez-Sesma, A. Challenges on the development of a dengue vaccine: A comprehensive review of the state of the art. J. Gen. Virol. 2023, 104, 001831. [Google Scholar]
- Medina-Magues, L.G.; Gergen, J.; Jasny, E.; Petsch, B.; Lopera-Madrid, J.; Medina-Magues, E.S.; Salas-Quinchucua, C.; Osorio, J.E. mRNA vaccine protects against Zika virus. Vaccines 2021, 9, 1464. [Google Scholar] [CrossRef] [PubMed]
- Rezza, G.; Weaver, S.C. Chikungunya as a paradigm for emerging viral diseases: Evaluating disease impact and hurdles to vaccine development. PLoS Negl. Trop. Dis. 2019, 13, e0006919. [Google Scholar] [CrossRef] [PubMed]
- Chadee, D.D. Key premises, a guide to Aedes aegypti (Diptera: Culicidae) surveillance and control. Bull. Entomol. Res. 2004, 94, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Chadee, D.D. Oviposition strategies adopted by gravid Aedes aegypti (L.) (Diptera: Culicidae) as detected by ovitraps in Trinidad, West Indies (2002–2006). Acta Trop. 2009, 111, 279–283. [Google Scholar] [CrossRef]
- Chadee, D.D.; Fat, F.H.; Persad, R.C. First record of Aedes albopictus from Trinidad, West Indies. J. Am. Mosq. Control Assoc. 2003, 19, 438–439. [Google Scholar]
- James, L.D.; Winter, N.; Stewart, A.T.M.; Feng, R.S.; Nandram, N.; Mohammed, A.; Duman-Scheel, M.; Romero-Severson, E.; Severson, D.W. Field trials reveal the complexities of deploying and evaluating the impacts of yeast-baited ovitraps on Aedes mosquito densities in Trinidad, West Indies. Sci. Rep. 2022, 12, 4047. [Google Scholar] [CrossRef]
- Hapairai, L.K.; Mysore, K.; James, L.D.; Scheel, N.D.; Realey, J.S.; Sun, L.; Gerber, L.E.; Feng, R.S.; Romero-Severson, E.; Mohammed, A.; et al. Evaluation of large volume yeast interfering RNA lure-and-kill ovitraps for attraction and control of Aedes mosquitoes. Med. Vet. Entomol. 2021, 35, 361–370. [Google Scholar] [CrossRef]
- Chadee, D.D.; Corbet, P.S. Seasonal incidence and diel patterns of oviposition in the field of the mosquito, Aedes aegypti (L.) (Diptera: Culicidae) in Trinidad, West Indies: A preliminary study. Ann. Trop. Med. Parasit. 1987, 81, 151–161. [Google Scholar] [CrossRef]
- Gonzalez-Escobar, G.; Churaman, C.; Rampersadd, C.; Singh, R.; Nathaniel, S. Mayaro virus detection in patients from rural and urban areas of Trinidad and Tobago during the chikungunya and Zika virus outbreaks. Pathog. Glob. Health 2021, 115, 188–195. [Google Scholar] [CrossRef]
- Christie, C.D.C.; Lue, A.M.; Melbourne-Chambers, R.H. Dengue, chikungunya and zika arbovirus infections in Caribbean children. Curr. Opin. Pediatr. 2023, 35, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Barrera, R.; Avila, J.; Gonzalez-Tellez, S. Unreliable supply of potable water and elevated Aedes aegypti larval indices: A causal relationship? J. Am. Mosq. Control Assoc. 1993, 2, 189–195. [Google Scholar]
- Chadee, D.D.; Doon, R.; Severson, D.W. Surveillance of dengue fever cases using a novel Aedes aegypti population sampling method in Trinidad, West Indies: The cardinal points approach. Acta Trop. 2007, 104, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chadee, D.D.; Rahaman, A. Use of water drums by humans and Aedes aegypti in Trinidad. J. Vector Ecol. 2000, 25, 28. [Google Scholar] [PubMed]
- Lozano-Fuentes, S.; Elizondo-Quiroga, D.; Farfan-Ale, J.A.; Loroño-Pino, M.A.; Garcia-Rejon, J.; Gomez-Carro, S.; Lira-Zumbardo, V.; Najera-Vazquez, R.; Fernandez-Salas, I.; Calderson-Martinez, J.; et al. Use of Google EarthTM to strengthen public health capacity and facilitate management of vector-borne diseases in resource-poor environments. Bull. World Health Org. 2008, 86, 718–725. [Google Scholar]
- Eisen, L.; Lozano-Fuentes, S.S. Use of mapping and spatial and space-time modeling approaches in operational control of Aedes aegypti and dengue. PLoS Negl. Trop. Dis. 2009, 3, e411. [Google Scholar] [CrossRef]
- Ai-Leen, G.T.; Jin Song, R. The use of GIS in ovitrap monitoring for dengue control in Singapore. Dengue Bull. 2000, 24, 110–116. [Google Scholar]
- Chansang, C.; Kittayapong, P. Application of mosquito sampling count and geospatial methods to improve dengue vector surveillance. Am. J. Trop. Med. Hyg. 2007, 77, 897–902. [Google Scholar] [CrossRef]
- Morales-Perez, A.; Nava-Aguilera, E.; Hernandez-Alvarez, C.; Alvarado-Castro, V.M.; Arostegui, J.; Legorreta-Soberanis, J.; Flores-Moreno, M.; Morales-Nava, L.; Harris, E.; Ledogar, R.J.; et al. Utility of entomological indices for predicting transmission of dengue virus: Secondary analysis of data from the Camino Verde trial in Mexico and Nicaragua. PLoS Negl. Trop. Dis. 2020, 14, e0008768. [Google Scholar] [CrossRef]
- Chadee, D.D. Seasonal incidence and horizontal distribution patterns of oviposition by Aedes aegypti in an urban environment in Trinidad, West Indies. J. Am. Mosq. Control Assoc. 1992, 8, 281–284. [Google Scholar]
- Focks, D.A.; Chadee, D.D. Pupal survey: An epidemiologically significant surveillance method for Aedes aegypti: An example using data from Trinidad. Am. J. Trop. Med. Hyg. 1997, 56, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Clemons, A.; Mori, A.; Haugen, M.; Severson, D.; Duman-Scheel, M. Aedes aegypti: Culturing and egg collection. Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5507. [Google Scholar] [CrossRef] [PubMed]
- Darsie, R.F.; Ward, R.A. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. Mosq. Syst. Supp. 1981, 1, 1–398. [Google Scholar] [CrossRef]
- Webb, C.E.; Russell, R.C. A laboratory investigation of the mosquito control potential of the monomolecular film Aquatain® mosquito formula against immature stages of Aedes aegypti and Culex quinquefasciatus. J. Am. Mosq. Control Assoc. 2009, 25, 106–110. [Google Scholar] [CrossRef]
- WHO. Guidelines for Dengue Surveillance and Mosquito Control, 2nd ed.; WHO Regional Office for the Western Pacific: Manila, Philippines, 2003. [Google Scholar]
- Morrison, A.C.; Getis, A.; Santiago, M.; Rigau-Perez, J.G.; Rieter, P. Exploratory space-time analysis of reported dengue cases during an outbreak in Florida, Puerto Rico, 1991–1992. Am. J. Trop. Med. Hyg. 1998, 59, 287–298. [Google Scholar] [CrossRef]
- Reiner, R.C.; Stoddard, S.T.; Scott, T.W. Socially structured human movement shapes dengue transmission despite the diffusive effect of mosquito dispersal. Epidemics 2014, 6, 30–36. [Google Scholar] [CrossRef]
- Stodddard, S.T.; Forshey, B.M.; Morrison, A.C.; Paz-Soldan, V.A.; Vazquez-Prolopec, G.M.; Astete, H.; Reiner, R.C., Jr.; Vilcarromero, S.; Elder, J.P.; Halsey, E.S.; et al. House-to-house human movement drives dengue virus transmission. Proc. Natl. Acad. Sci. USA 2013, 15, 994–999. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Golding, N.; Moritz, U.G.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Camargo, C.; Alfonso-Parra, C.; Diaz, S.; Rincon, D.F.; Ramirez-Sanchez, L.F.; Agudelo, J. Spatial and temporal population dynmics of male and female Aedes albopictus at a local scale in Medellin, Colombia. Parasit. Vec. 2021, 14, 312. [Google Scholar] [CrossRef]
- Ferreira-de-Lima, V.H.; Camara, D.C.P.; Honorio, N.A.; Lima-Camara, T.N. The Asian tiger mosquito in Brazil: Observations on biology and ecological interactions since first detection in 1986. Acta Trop. 2020, 205, 105386. [Google Scholar] [CrossRef] [PubMed]
- Heinisch, M.R.S.; Diaz-Quijano, F.A.; Chiaravalloti-Neto, F.; Pancetti, F.G.M.; Coelho, R.R.; Dos Santos Andrade, P. Seasonal and spatial distribution of Aedes aegypti and Aedes albopictus in a municipal urban park in Sao Paulo, SP, Brazil. Acta Trop. 2019, 189, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Reiskind, M.H.; Lounibos, L.P. Spatial and temporal patterns of abundance of Aedes aegypti L. (Stegomyia aegypti) and Aedes albopictus (Skuse) [Stegomyia albopictus (Skuse)] in southern Florida. Med. Vet. Entomol. 2013, 27, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Chadee, D.D. Effects of ‘closed’ houses on the Aedes aegypti eradication programme in Trinidad. Med. Vet. Entomol. 1988, 2, 193–198. [Google Scholar] [CrossRef]
- Stewart, A.T.M.; Winter, N.; Igiede, J.; Hapairai, L.K.; James, L.D.; Feng, R.S.; Mohammed, A.; Severson, D.W.; Duman-Scheel, M. Community acceptance of yeast interfering RNA larvicide technology for control of Aedes mosquitoes in Trinidad. PLoS ONE 2020, 15, e0237675. [Google Scholar] [CrossRef]
- Winter, N.; Stewart, A.T.M.; Igiede, J.; Wiltshire, R.M.; Hapairai, L.K.; James, L.D.; Mohammed, A.; Severson, D.W.; Duman-Scheel, M. Assessment of Trinidad community stakeholder perspectives on the use of yeast interfering RNA-baited ovitraps for biorational control of Aedes mosquitoes. PLoS ONE 2021, 16, e0252997. [Google Scholar] [CrossRef]
- Barrera, R.; Amador, M.; Munoz, J.; Acevedo, V. Integrated vector control of Aedes aegypti mosquitoes around target houses. Parasit. Vec. 2018, 11, 88. [Google Scholar] [CrossRef]
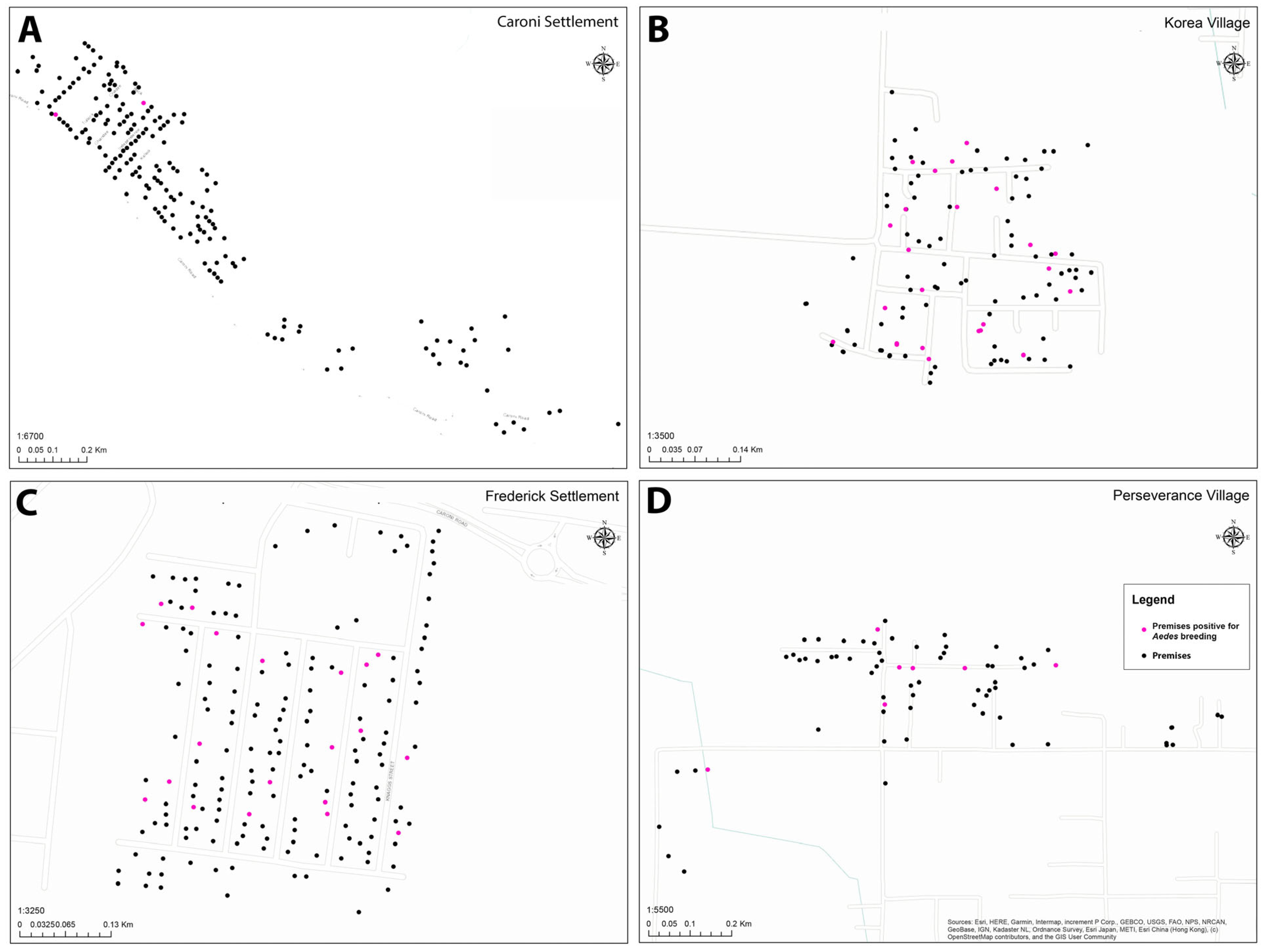
| Location | Total No. Premises | No. Premises Inspected | Percent Premises Inspected |
|---|---|---|---|
| Caroni Settlement | 385 | 187 | 48.6% |
| Frederick Settlement | 329 | 175 | 53.2% |
| Perseverance Village | 135 | 71 | 52.6% |
| Korea Village | 162 | 112 | 69.1% |
| Total | 1011 | 545 | 53.9% |
| Container Type | Caroni Settlement | Frederick Settlement | Perseverance Village | Korea Village | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | + | # | + | # | + | # | + | # | #% | + | +% | |
| Tanks | 359 | 0 | 313 | 4 | 136 | 1 | 135 | 1 | 943 | 8.60 | 6 | 0.64 |
| Drums | 174 | 1 | 148 | 9 | 50 | 3 | 132 | 12 | 504 | 4.60 | 25 | 4.96 |
| Tubs/Basins/Buckets | 928 | 0 | 869 | 14 | 425 | 0 | 647 | 13 | 2869 | 26.18 | 27 | 0.94 |
| Tires | 163 | 1 | 73 | 2 | 63 | 2 | 156 | 8 | 455 | 4.15 | 13 | 2.86 |
| Plant Saucers/Vases | 89 | 0 | 82 | 12 | 95 | 0 | 90 | 0 | 356 | 3.25 | 12 | 3.37 |
| Small Misc. | 1883 | 0 | 1782 | 14 | 758 | 9 | 1410 | 14 | 5833 | 53.17 | 37 | 0.63 |
| Total | 3596 | 2 | 3267 | 55 | 1527 | 15 | 2570 | 48 | 10,960 | 120 | ||
| Location | HI a | BI b | HIkey c | BIkey d | Premises with No Key Containers (%) | Premises with No Misc. Containers (%) |
|---|---|---|---|---|---|---|
| Caroni Settlement | 1.1 | 1.1 | 0.5 | 0.5 | 2.7 | 3.7 |
| Frederick Settlement | 14.3 | 31.4 | 8.0 | 10.9 | 2.3 | 5.1 |
| Perseverance Village | 9.9 | 21.1 | 4.2 | 5.6 | 1.4 | 2.8 |
| Korea Village | 21.4 | 42.9 | 13.4 | 23.3 | 2.7 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hapairai, L.K.; Seeramsingh, R.; James, L.D.; Feng, R.S.; Nandram, N.; Mohammed, A.; Duman-Scheel, M.; Severson, D.W. GIS-Enhanced Survey of Potential Aedes aegypti and Aedes albopictus Artificial Oviposition Containers Distributed across Communities in Trinidad, West Indies. Insects 2024, 15, 779. https://doi.org/10.3390/insects15100779
Hapairai LK, Seeramsingh R, James LD, Feng RS, Nandram N, Mohammed A, Duman-Scheel M, Severson DW. GIS-Enhanced Survey of Potential Aedes aegypti and Aedes albopictus Artificial Oviposition Containers Distributed across Communities in Trinidad, West Indies. Insects. 2024; 15(10):779. https://doi.org/10.3390/insects15100779
Chicago/Turabian StyleHapairai, Limb K., Roshan Seeramsingh, Lester D. James, Rachel S. Feng, Naresh Nandram, Azad Mohammed, Molly Duman-Scheel, and David W. Severson. 2024. "GIS-Enhanced Survey of Potential Aedes aegypti and Aedes albopictus Artificial Oviposition Containers Distributed across Communities in Trinidad, West Indies" Insects 15, no. 10: 779. https://doi.org/10.3390/insects15100779
APA StyleHapairai, L. K., Seeramsingh, R., James, L. D., Feng, R. S., Nandram, N., Mohammed, A., Duman-Scheel, M., & Severson, D. W. (2024). GIS-Enhanced Survey of Potential Aedes aegypti and Aedes albopictus Artificial Oviposition Containers Distributed across Communities in Trinidad, West Indies. Insects, 15(10), 779. https://doi.org/10.3390/insects15100779





