Spontaneous Color Preferences and Associative Learning in Protaetia brevitarsis (Coleoptera: Scarabaeidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Stimuli
2.3. Experimental Arena
2.4. Test of Visual Preference for Specific Wavelengths
2.5. Test of Associative Color Learning
2.6. Selection Response to Visual Cues
2.7. Selection Response to Odors Combined with Visual Cues
2.8. Statistical Analysis
3. Results
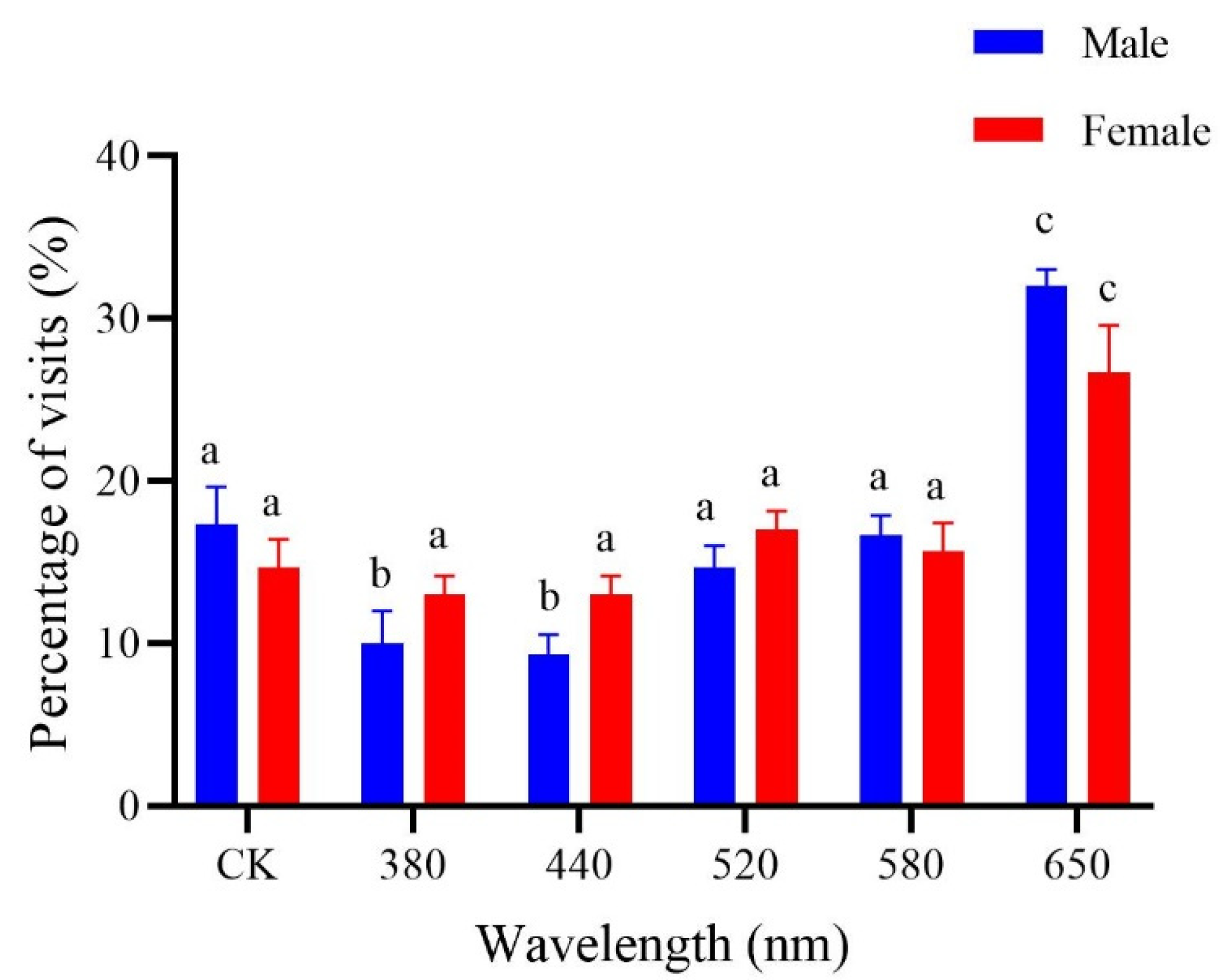
3.1. Visual Preferences of P. brevitarsis for Specific Spectra
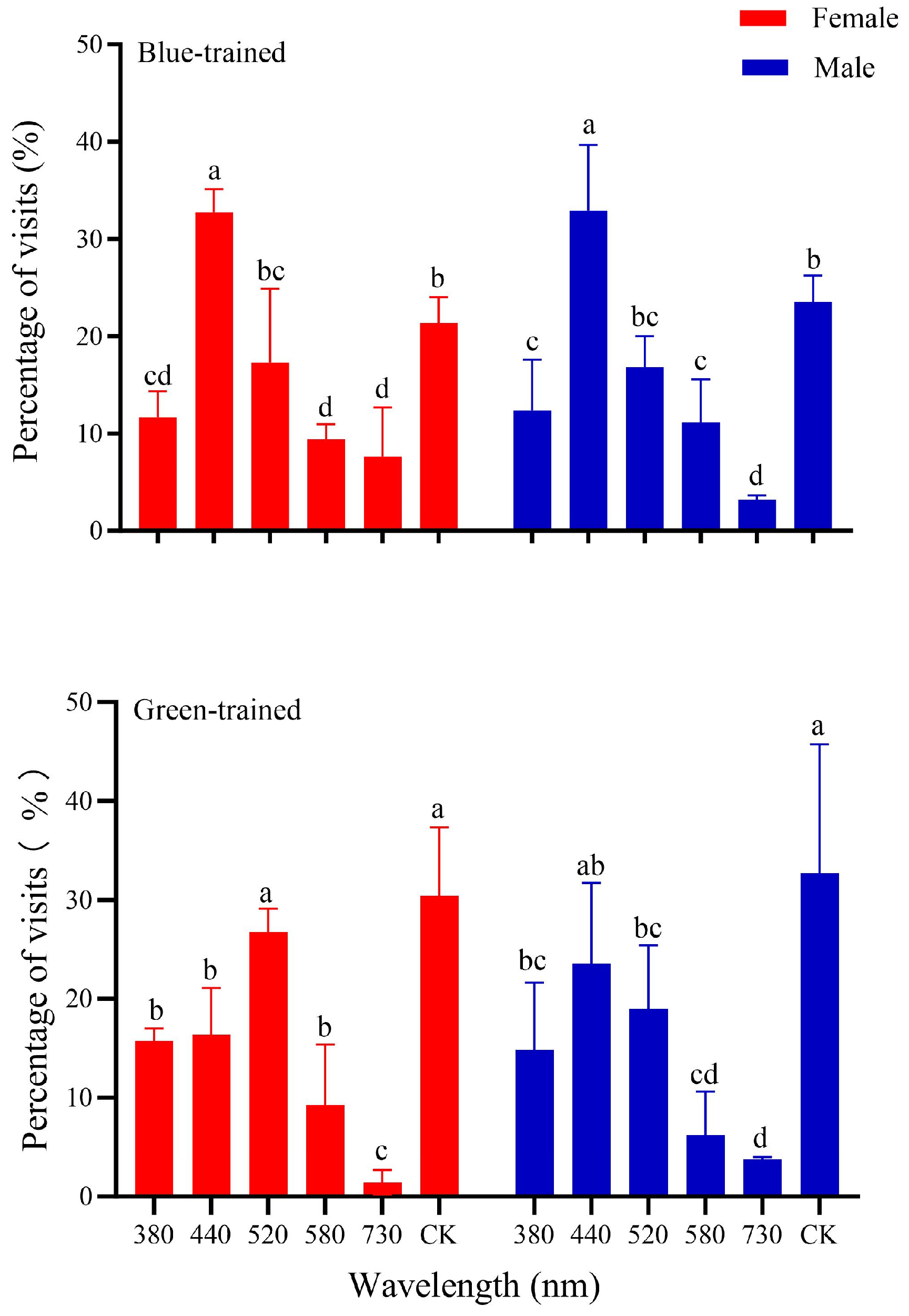
3.2. Associative Color Learning
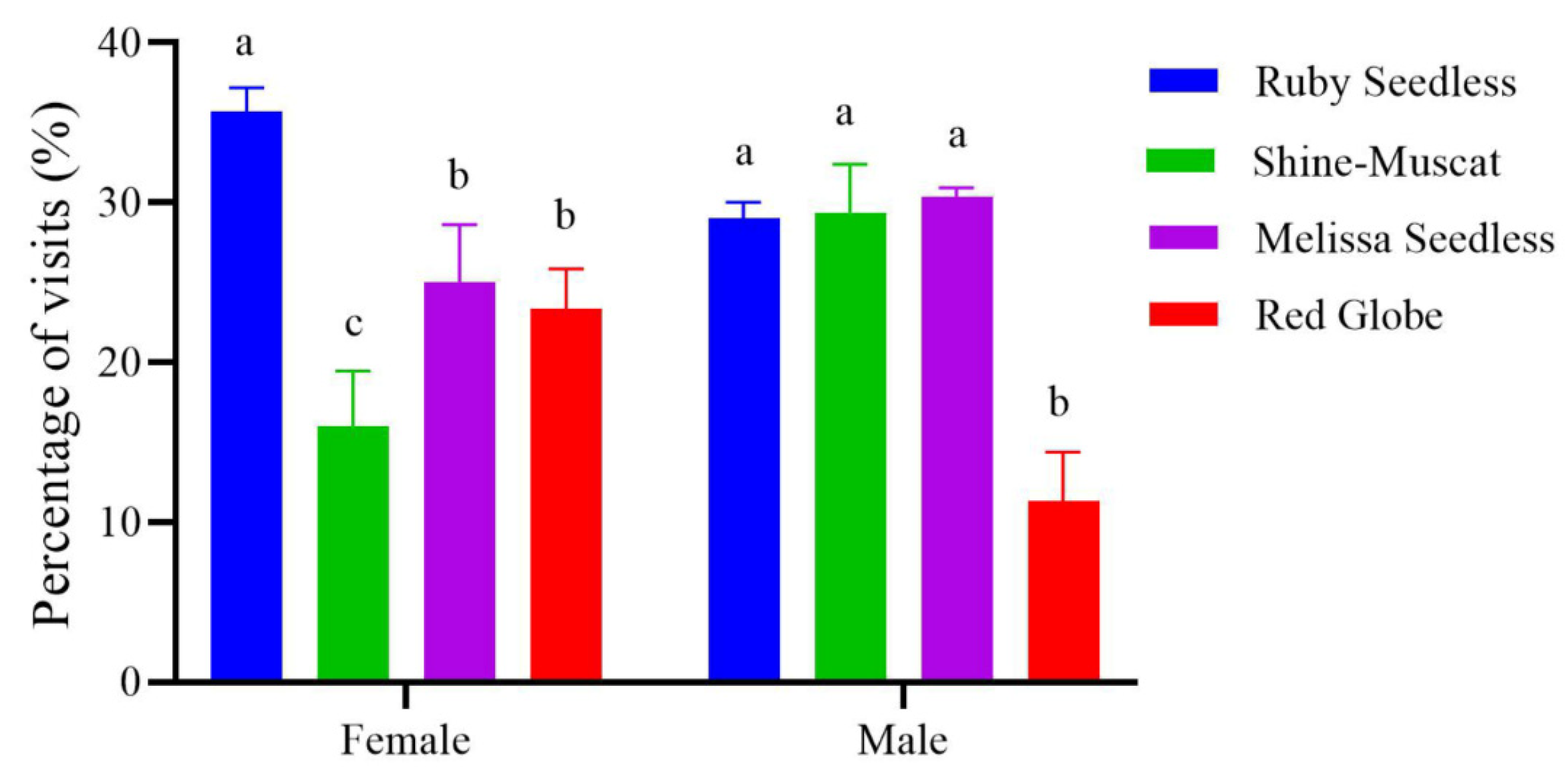
3.3. The Selection Preference for Grapes in the Absence of Odors
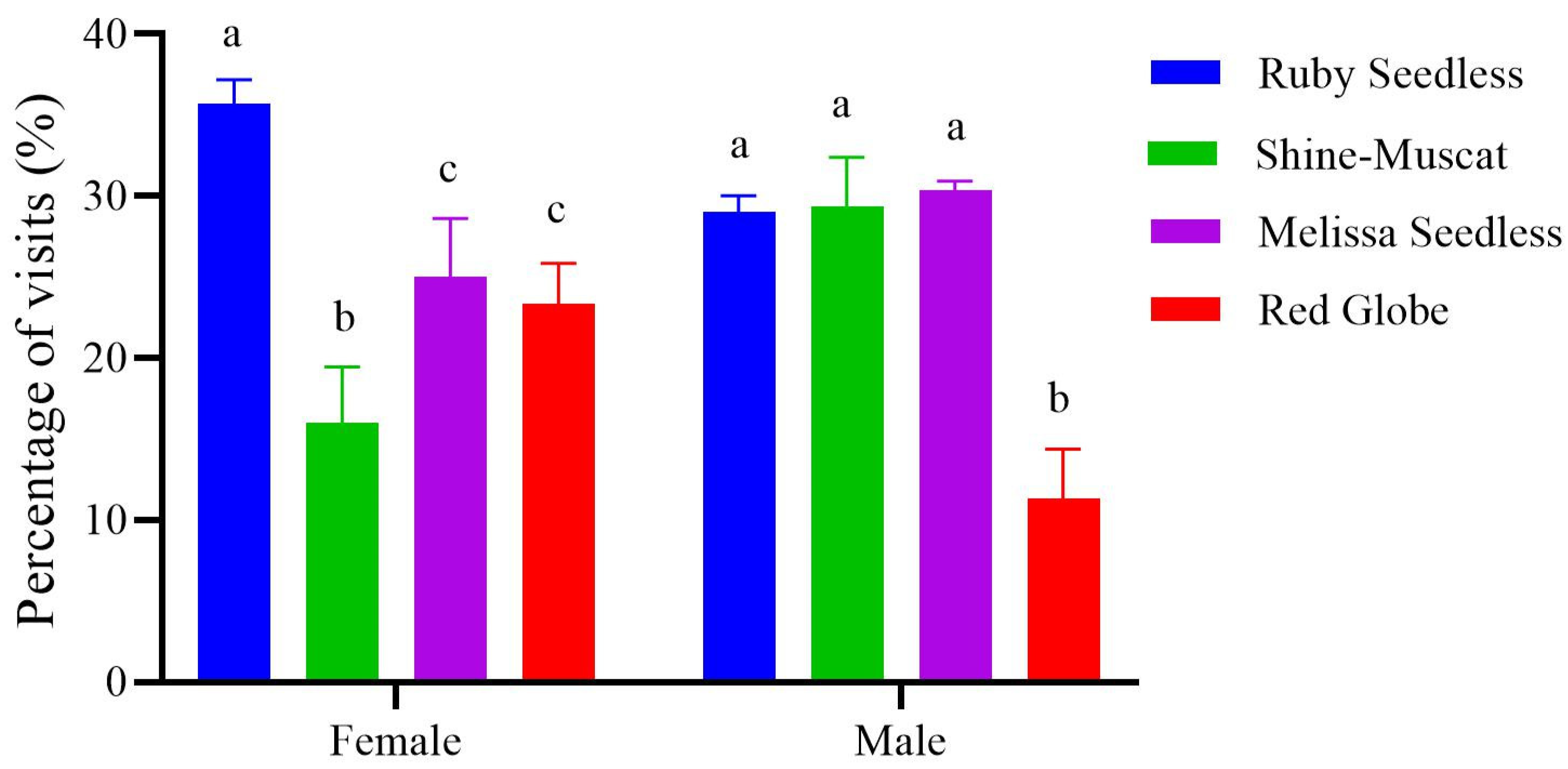
3.4. The Selection Preference for Grapes in the Presence of Odors
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dacke, M.; Byrne, M.J.; Scholtz, C.H.; Warrant, E.J. Lunar orientation in a beetle. Proc. R. Soc. B Biol. Sci. 2004, 271, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Pfeiffer, K.; Homberg, U. Spectral properties of identified polarized-light sensitive interneurons in the brain of the desert locust Schistocerca gregaria. J. Exp. Biol. 2007, 210, 1350–1361. [Google Scholar] [CrossRef]
- Brewer, M.J.; Elliott, N.C. Biological control of cereal aphids in north America and mediating effects of host plant and habitat manipulations. Annu. Rev. Entomol. 2004, 49, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, H.; Stewart, F.J.; Kinoshita, M. Swallowtail butterflies use multiple visual cues to select oviposition sites. Insects 2021, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, J.; Henze, M.J.; Svensson, G.P.; Lind, O.; Anderbrant, O. Visual cues of oviposition sites and spectral sensitivity of Cydia strobilella L. J. Insect Physiol. 2017, 101, 161–168. [Google Scholar] [CrossRef]
- Schoonhoven, L.M. Insect-plant relationships: The whole is more than the sum of its parts. Entomol. Exp. Appl. 2005, 115, 5–6. [Google Scholar] [CrossRef]
- Bernard, G.D.; Remington, C.L. Color vision in Lycaena butterflies: Spectral tuning of receptor arrays in relation to behavioral ecology. Proc. Natl. Acad. Sci. USA 1991, 88, 2783–2787. [Google Scholar] [CrossRef]
- Reeves, J.L. Vision should not be overlooked as an important sensory modality for finding host plants. Environ. Entomol. 2011, 40, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Van Der Kooi, C.J.; Stavenga, D.G.; Arikawa, K.; Belušič, G.; Kelber, A. Evolution of insect color vision: From spectral sensitivity to visual ecology. Annu. Rev. Entomol. 2021, 66, 435–461. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Arikawa, K.; Kinoshita, M. Color discrimination at the spatial resolution limit in a swallowtail butterfly, Papilio xuthus. J. Exp. Biol. 2006, 209, 2873–2879. [Google Scholar] [CrossRef]
- Ramírez, C.C.; Lavandero, B.; Archetti, M. Coevolution and the adaptive value of autumn tree colors: Color preference and growth rates of a southern beech aphid. J. Evol. Biol. 2008, 21, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Z.; Klein, M.G.; Sheng, C.F.; Li, Y.; Li, Q.Y. Male and female Popillia quadriguttata (Fabricius) and Protaetia brevitarsis (Lewis) (Coleoptera: Scarabaeidae) response to japanese beetle floral and pheromone lures. J. Asia Pac. Entomol. 2013, 16, 479–484. [Google Scholar] [CrossRef]
- Sharkey, C.R.; Fujimoto, M.S.; Lord, N.P.; Shin, S.; McKenna, D.D.; Suvorov, A.; Martin, G.J.; Bybee, S.M. Overcoming the loss of blue sensitivity through opsin duplication in the largest animal group, beetles. Sci. Rep. 2017, 7, 8. [Google Scholar] [CrossRef]
- Lord, N.P.; Plimpton, R.L.; Sharkey, C.R.; Suvorov, A.; Lelito, J.P.; Willardson, B.M.; Bybee, S.M. A cure for the blues: Opsin duplication and subfunctionalization for short-wavelength sensitivity in jewel beetles (Coleoptera: Buprestidae). BMC Evol. Biol. 2016, 16, 107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pengsakul, T.; Tukayo, M.; Yu, L.; Fang, W.; Luo, D. Host-location behavior of the tea green leafhopper Empoasca Vitis Göthe (Hemiptera: Cicadellidae): Olfactory and visual effects on their orientation. Bull. Entomol. Res. 2018, 108, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.O.; Rose, S.; Malsch, P. The role of vision in the host plant-finding behavior of the hessian fly. Physiol. Entomol. 1993, 18, 31–42. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Ericson, L. Visual cues override olfactory cues in the host-finding process of the monophagous leaf beetle Altica engstroemi. Entomol. Exp. Appl. 2007, 125, 81–88. [Google Scholar] [CrossRef]
- Sun, F.; Bao, C.; Jing, T.Z. Locating host plants via orientation to standing visual targets has dispersal benefits for the monophagous leaf beetle Ambrostoma quadriimpressum. Entomol. Exp. Appl. 2016, 158, 229–235. [Google Scholar] [CrossRef]
- Finch, S.; Collier, R.H. Host-plant selection by insects—A theory based on appropriate/inappropriate landings by pest insects of cruciferous plants. Entomol. Exp. Appl. 2000, 96, 91–102. [Google Scholar] [CrossRef]
- He, S.; Zhou, Z.R.; Wu, Z.P.; Wang, C.Y.; Han, X.X.; Ye, X.Y.; Zhao, Z.L.; Wang, Y.A. Study on the occurrence of Postosia brevitarsis Leiwis and its prevention and control technology. Chin. Agric. Sci. Bull. 2006, 22, 314–316. [Google Scholar] [CrossRef]
- Cai, H.H. Movements Among Hosts and Control Technology of Potosia brevitarsis Lewis. Master’s Thesis, Shihezi University, Shihezi, China, 2020. [Google Scholar]
- Cai, H.; Zhang, T.; Su, Y.; Wang, Z.; Zhang, X.; Wang, S.; Liu, Y. Influence of trap color, type, and placement on capture efficacy for Protaetia brevitarsis (Coleoptera: Scarabaeidae). J. Econ. Entomol. 2021, 114, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Dimeglio, A.S.; Kuhar, T.P.; Weber, D.C. Color preference of harlequin bug (Heteroptera: Pentatomidae). J. Econ. Entomol. 2017, 110, 2275–2277. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Dyer, A.G.; Rössler, W.; Spaethe, J. Innate color preference, individual learning and memory retention in the ant Camponotus blandus. J. Exp. Biol. 2017, 220, 3315–3326. [Google Scholar] [CrossRef]
- An, L.; Neimann, A.; Eberling, E.; Algora, H.; Brings, S.; Lunau, K. The yellow specialist: Dronefly Eristalis tenax prefers different yellow colors for landing and proboscis extension. J. Exp. Biol. 2018, 221, jeb184788. [Google Scholar] [CrossRef]
- Wu, L.H.; Chen, R.C.; Han, R.C.; Wei, H.Y.; Zheng, L.X. Study on the preference of Diaphorina citri Kuwayama to different colors. J. Fruit Sci. 2018, 35, 1509–1515. [Google Scholar] [CrossRef]
- Arikawa, K.; Wakakuwa, M.; Qiu, X.; Kurasawa, M.; Stavenga, D.G. Sexual dimorphism of short-wavelength photoreceptors in the small white butterfly, Pieris rapae crucivora. J. Neurosci. 2005, 25, 5935–5942. [Google Scholar] [CrossRef] [PubMed]
- Long, X.Z.; Wei, D.W.; Gao, X.Y.; Jiang, X.D.; Yv, Y.H.; Zeng, X.R. Trapping effects of various colored sticky traps and led lights with different wavelengths on Procontarinia fructiculi adults. China Plant Prot. 2021, 41, 53–58. [Google Scholar] [CrossRef]
- Imafuku, M.; Shimizu, I.; Imai, H.; Shichida, Y. Sexual difference in color sense in a lycaenid butterfly, Narathura japonica. Zool. Sci. 2007, 24, 611–613. [Google Scholar] [CrossRef]
- Stephens, D.W. Learning and behavioral ecology: Incomplete information and environmental predictability. In Insect Learning: Ecology and Evolutionary Perspectives; Springer: Boston, MA, USA, 1993; pp. 195–218. [Google Scholar] [CrossRef]
- Blackiston, D.J.; Casey, E.S.; Weiss, M.R. Retention of memory through metamorphosis: Can a moth remember what it learned as a caterpillar? PLoS ONE 2008, 3, e1736. [Google Scholar] [CrossRef]
- Costa, A.; Ricard, I.; Davison, A.C.; Turlings, T.C.J. effects of rewarding and unrewarding experiences on the response to host-induced plant odors of the generalist parasitoid Cotesia marginiventris (Hymenoptera: Braconidae). J. Insect Behav. 2010, 23, 303–318. [Google Scholar] [CrossRef][Green Version]
- Blackiston, D.; Briscoe, A.D.; Weiss, M.R. Color vision and learning in the monarch butterfly, Danaus plexippus (Nymphalidae). J. Exp. Biol. 2011, 214, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Kinoshita, M.; Arikawa, K. Innate preference and learning of color in the male cotton bollworm moth, Helicoverpa armigera. J. Exp. Biol. 2016, 219, 3857–3860. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, L.; Brockmann, A.; Spaethe, J. Learning of monochromatic stimuli in Apis cerana and Apis mellifera by means of PER conditioning. J. Insect Physiol. 2019, 114, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.D.; Han, Y.; Wu, J.H.; Li, P.; Fu, B.L.; Qiu, H.Y.; Liu, K. Preference of Megalurothrips usitatus (Thysanoptera:Thripidae) to different colors and light-waves in lab. Plant Prot. 2015, 41, 169–172. [Google Scholar] [CrossRef]
- Brace, N.; Kemp, R.; Snelgar, R. SPSS for Psychologists: A Guide to Data Analysis Using SPSS for Windows, 3rd ed.; Lawrence Erlbaum Associates Publishers: New York, NY, USA, 2006. [Google Scholar]
- Xu, Y.L.; Pan, H.S.; Liang, G.M.; Yang, Y.Z.; Lu, Y.H. Field evaluation of light—Emitting diodes with different wavelengths as traps of Anomala corpulenta and Holotrichia parallela. Xinjiang Agric. Sci. 2020, 57, 2028–2033. [Google Scholar]
- Zhang, Q.H.; Ma, J.H.; Yang, Q.Q.; Byers, J.A.; Klein, M.G.; Zhao, F.Y.; Luo, Y.Q. Olfactory and visual responses of the longlegged chafer Hoplia spectabilis Medvedev (Coleoptera: Scarabaeidae) in Qinghai Province, China. Pest Manag. Sci. 2011, 67, 162–169. [Google Scholar] [CrossRef]
- Martínez-Harms, J.; Vorobyev, M.; Schorn, J.; Shmida, A.; Keasar, T.; Homberg, U.; Schmeling, F.; Menzel, R. Evidence of red sensitive photoreceptors in Pygopleurus israelitus (Glaphyridae: Coleoptera) and its implications for beetle pollination in the southeast Mediterranean. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2012, 198, 451–463. [Google Scholar] [CrossRef]
- Balamurali, G.S.; Edison, A.; Somanathan, H.; Kodandaramaiah, U. Spontaneous color preferences and color learning in the fruit-feeding butterfly, Mycalesis mineus. Behav. Ecol. Sociobiol. 2019, 73, 39. [Google Scholar] [CrossRef]
- Tang, Y.H.; Bi, S.Y.; Wang, X.D.; Ji, S.X.; Huang, C.; Zhang, G.F.; Guo, J.Y.; Yang, N.W.; Ma, D.F.; Wan, F.H.; et al. Opsin mutants alter host plant selection by color vision in the nocturnal invasive pest Tuta absoluta. Int. J. Biol. Macromol. 2024, 265, 130636. [Google Scholar] [CrossRef]
- Xue, H.W.; Wu, W.J. Preferences of Bactrocera cucurbitae (Diptera: Tephritidae) to different colors: A quantitative investigation using virtual wavelength. Acta Entomol. Sin. 2013, 56, 161–166. [Google Scholar]
- Wen, C.; Ma, T.; Wang, C.; Wen, J.B.; Ji, Y.C.; Wen, X.J. Progress in research on the compound eye structure and visual navigation of insects. Chin. J. Appl. Entomol. 2019, 56, 28–36. [Google Scholar] [CrossRef]



| Wavelength (nm) | R | G | B | Illumination (lx) |
|---|---|---|---|---|
| 380 | 97 | 0 | 97 | 1400 |
| 440 | 0 | 0 | 255 | 2360 |
| 520 | 54 | 255 | 0 | 2100 |
| 580 | 255 | 255 | 0 | 2430 |
| 650 | 255 | 0 | 0 | 600 |
| CK (white) | 255 | 255 | 255 | 4500 |
| Wavelength (nm) | R | G | B | Illumination (lx) |
|---|---|---|---|---|
| 625 | 255 | 78 | 0 | 1230 |
| 635 | 255 | 39 | 0 | 900 |
| 645–700 | 255 | 0 | 0 | 600 |
| 710 | 233 | 0 | 0 | 530 |
| 720 | 210 | 0 | 0 | 500 |
| 730 | 188 | 0 | 0 | 460 |
| Wavelength (nm) | R | G | B | Illumination (lx) |
|---|---|---|---|---|
| 380 | 97 | 0 | 97 | 1400 |
| 440 | 0 | 0 | 255 | 2360 |
| 520 | 54 | 255 | 0 | 2100 |
| 580 | 255 | 255 | 0 | 2430 |
| 730 | 188 | 0 | 0 | 460 |
| CK (white) | 255 | 255 | 255 | 4500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Cui, Z.; Huang, X.; Dhiloo, K.H.; Kong, F.; Wang, Z.; Liu, Y. Spontaneous Color Preferences and Associative Learning in Protaetia brevitarsis (Coleoptera: Scarabaeidae). Insects 2024, 15, 780. https://doi.org/10.3390/insects15100780
Wu H, Cui Z, Huang X, Dhiloo KH, Kong F, Wang Z, Liu Y. Spontaneous Color Preferences and Associative Learning in Protaetia brevitarsis (Coleoptera: Scarabaeidae). Insects. 2024; 15(10):780. https://doi.org/10.3390/insects15100780
Chicago/Turabian StyleWu, Hui, Zhuangzhi Cui, Xiaoqing Huang, Khalid Hussain Dhiloo, Fanfang Kong, Zhongyue Wang, and Yongqiang Liu. 2024. "Spontaneous Color Preferences and Associative Learning in Protaetia brevitarsis (Coleoptera: Scarabaeidae)" Insects 15, no. 10: 780. https://doi.org/10.3390/insects15100780
APA StyleWu, H., Cui, Z., Huang, X., Dhiloo, K. H., Kong, F., Wang, Z., & Liu, Y. (2024). Spontaneous Color Preferences and Associative Learning in Protaetia brevitarsis (Coleoptera: Scarabaeidae). Insects, 15(10), 780. https://doi.org/10.3390/insects15100780







