1. Introduction
Mosquitoes are the deadliest animals on the planet given the number of particular disease pathogens they are capable of transmitting [
1]. Their ability to carry and spread diseases to humans is responsible for millions of deaths every year. The main diseases are malaria, lymphatic filariasis, dengue, Japanese encephalitis, chikungunya, and Zika.
Aedes aegypti (L.) and
Aedes albopictus (Skuse) are the primary vectors of dengue and chikungunya viruses in Thailand.
Anopheles minimus (Theobald) is one of the most important malarial vectors in forested areas of Thailand and other countries in the Greater Mekong Subregion.
Culex quinquefasciatus Say is the most common vector across urban and semiurban areas and transmits
Wuchereria bancrofti,
Plasmodium (avian malaria), myxomatosis virus, encephalitis viruses, and other disease agents across the world [
2]. Since vaccines are not currently available for most of these diseases, one of the most efficient methods of disease control is to rely on vector control approaches, as promoted by the World Health Organization (WHO), to reduce disease transmission [
3].
Multiple measures, such as vaccination, preventive medications, and vector control, may be employed alone or in combination to limit the spread of diseases transmitted by vectors. Among these tools, vector control is an approach widely used to prevent or control outbreaks for most vector-borne diseases [
4]. In general, mosquito control using chemical insecticides remains the most feasible technique for the control of many vectors. Unfortunately, many important mosquito species have developed resistance to different insecticides, especially
Ae. aegypti, which has been reported to be resistant to many active ingredients across much of Thailand [
5,
6]. Therefore, one alternative method to combat blood-sucking insects is to use insect repellents as a form of personal protection to prevent transmission [
7].
Mosquito repellents play a major role in disrupting disease transmission by reducing human–vector contact. DEET, previously called
N,
N-diethyl-m-toluamide or now
N,
N-3-methylbenzamide, was first registered in the USA in 1957, for use by military personnel in insect-infested areas. At present, DEET remains the gold standard insect repellent, a compound that is effective against most biting insects and other arthropod pests (such as mosquitoes, biting flies, ticks, fleas, and chigger mites) [
7]. DEET has a remarkable safety profile after 66 years of extensive use, but toxic reactions have been reported in highly sensitive people or whenever the product is misapplied or misused [
8].
Essential oils are complex mixtures of the volatile organic compounds present in plants. The repellent properties of several essential oils appear to be associated with specific compounds. Commercial applications of vetiver grass (
Vetiveria zizanioides (L.) Nash:syn.
Chrysopogon zizanioides (L.) Roberty) mostly involve the production of vetiver oil (VO) using root distillation. VO consists of a complex mixture of more than 300 compounds, with the major ones being vetiverol, vetivene, alpha- and beta-vetivone, khusimol, elemol, vetiselinenol, beta-eudesmol, terpenes, zizanoic acid, vanillin, hydrocarbons, sesquiterpenes, alcohols, and ketones [
9]. Vetiver grass has been reported to have insect-repellent properties against ants, ticks, cockroaches, termites, mosquitoes, weevils, and beetles [
10,
11,
12,
13]. In addition to essential oils, a pure compound named β-caryophyllene oxide (BCO) is a bicyclic sesquiterpene, a representative of an epoxide derived from the olefin of (E)-caryophyllene. This compound is a common sesquiterpene present in many well-known aromatic-repellent plants, such as cloves, basil, cinnamon, and citrus [
14]. A more recent study showed that BCO is an efficient mosquito repellent [
15], and other studies showed that vetiver oil exhibits irritant and repellent activities against mosquitoes [
16]. Furthermore, the phototoxicity and genotoxicity of BCO and VO were investigated by Nararak et al. in 2020 and 2022 [
17,
18]. According to the findings on phototoxic and genotoxic dangers, BCO and VO have no phototoxic potential and no substantial genotoxic response. As a result, these plant-based derivatives with repellent properties offer a viable alternative to synthetically created active compounds.
An excito-repellency (ER) test system has been used to evaluate the avoidance behavior of mosquitoes of test compounds [
19,
20,
21]. This ER system allows the insecticide avoidance behavior of female mosquitoes to be studied by testing the contact irritancy and noncontact repellency of specific compounds. Contact irritability refers to direct tarsal contact with an insecticide that can cause a mosquito to escape from the test chamber. On the other hand, noncontact repellency results in insects detecting chemicals from a distance and escaping from the treated area without making physical contact with the insecticide.
Plant-based candidates, VO and BCO, were initially selected based on our previous studies [
15,
16,
17,
18]. A more recent study reported that BCO at 1% showed spatial repellency against
Ae. aegypti (29.9%),
Ae. albopictus (25.45%),
An. dirus (31.67%), and
An. minimus (86.9%), as well as high contact irritancy rates for
Ae. aegypti (59.3%),
Ae. albopictus (56.36%),
An. dirus (32.73%), and
An. minimus (92.2%) [
15,
17]. On the other hand, essential oil from VO at 2.5–5% elicited great repellency responses in
Ae. albopictus (63.7%) and
An. minimus (66.05%) [
18,
22]. Thus, VO and its two constituents (valencene and vetiverol) could be considered as safe repellents, and effective against mosquitoes [
22]. Based on the spatial repellent activities previously shown by VO and BCO, the current study used both compounds to compare the behavioral responses of
Ae. aegypti,
Ae. albopictus,
An. minimus, and
Cx. quinquefasciatus to single and combined mixtures of VO and BCO using ER test chambers.
3. Results
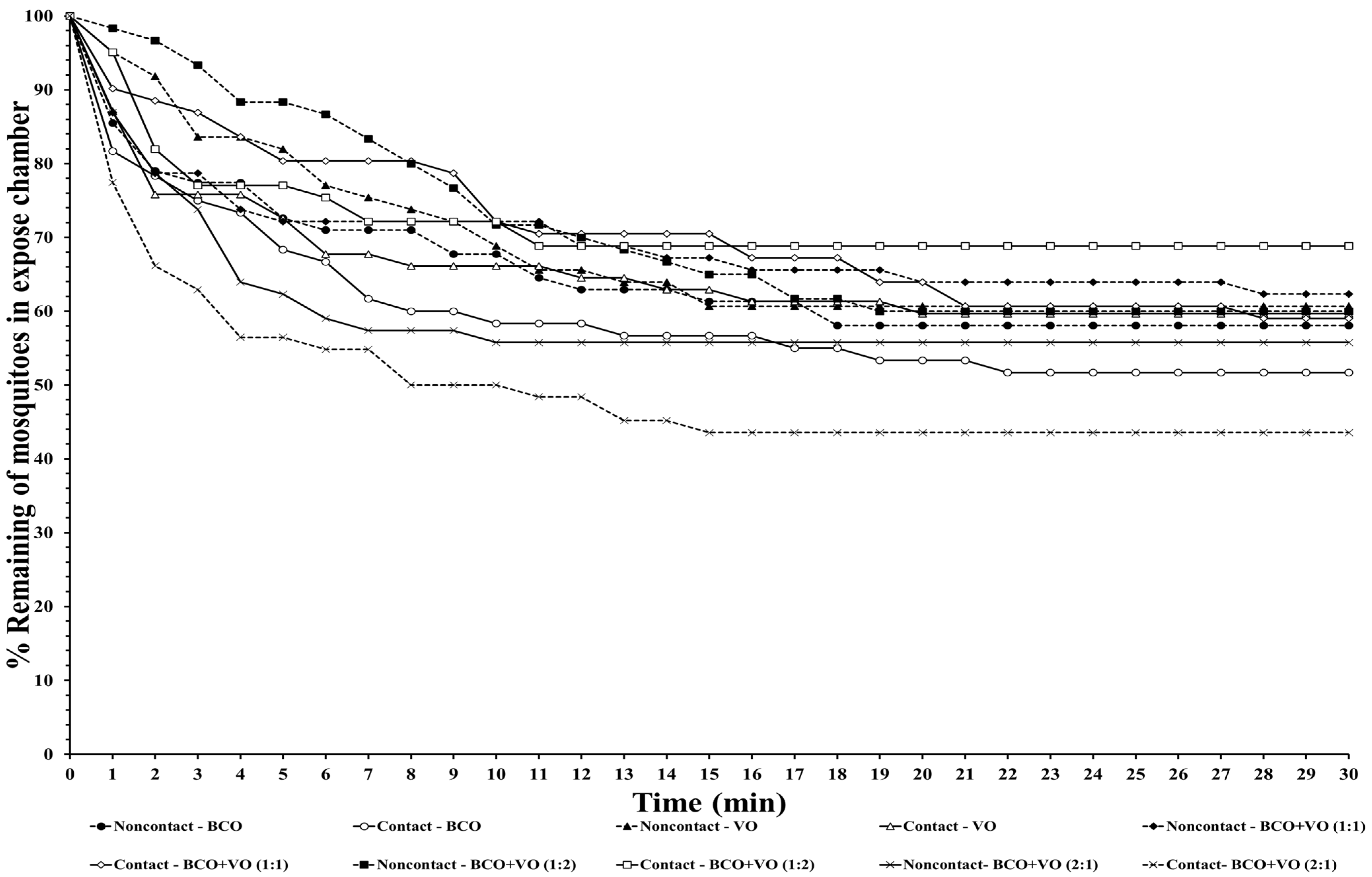
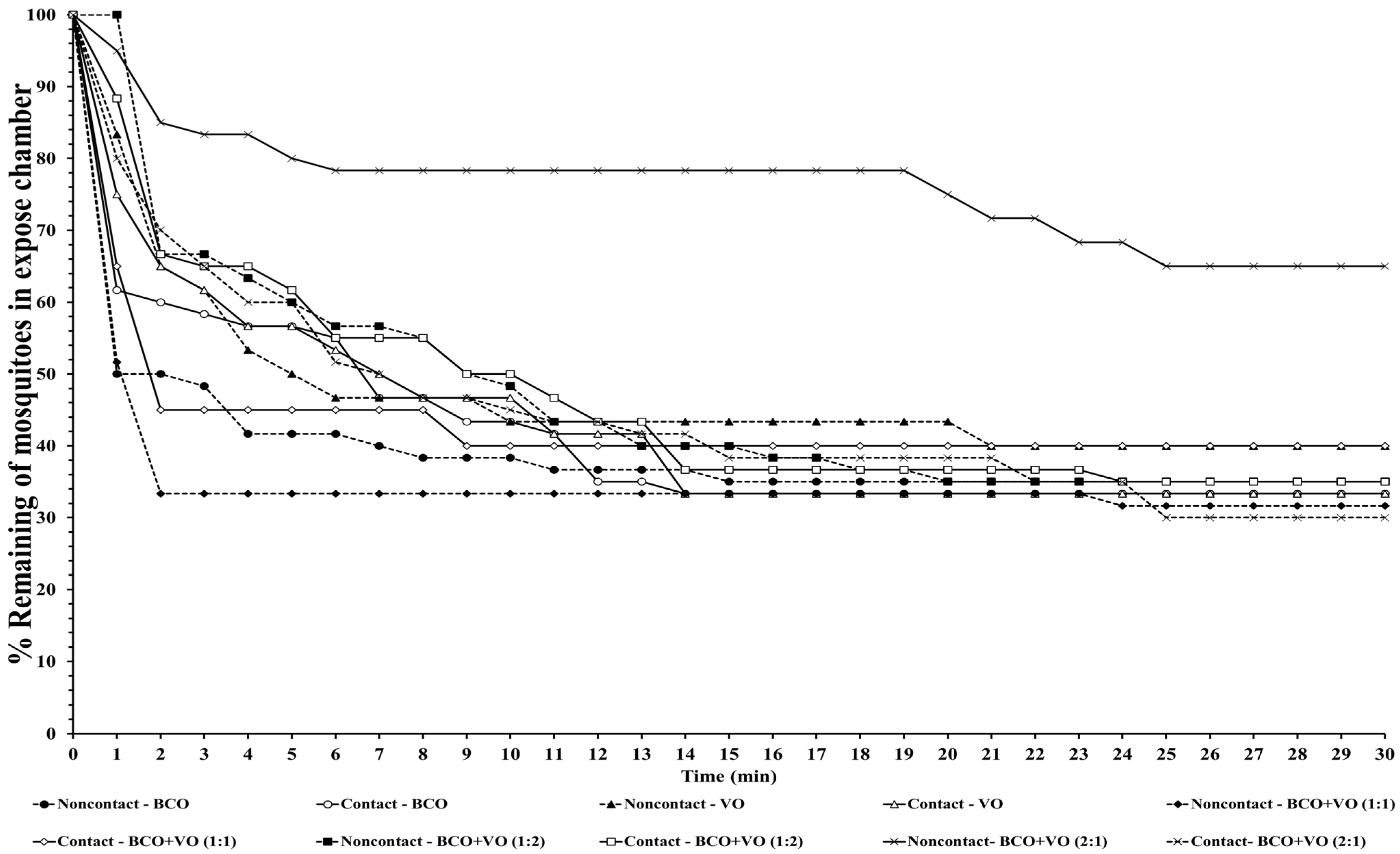
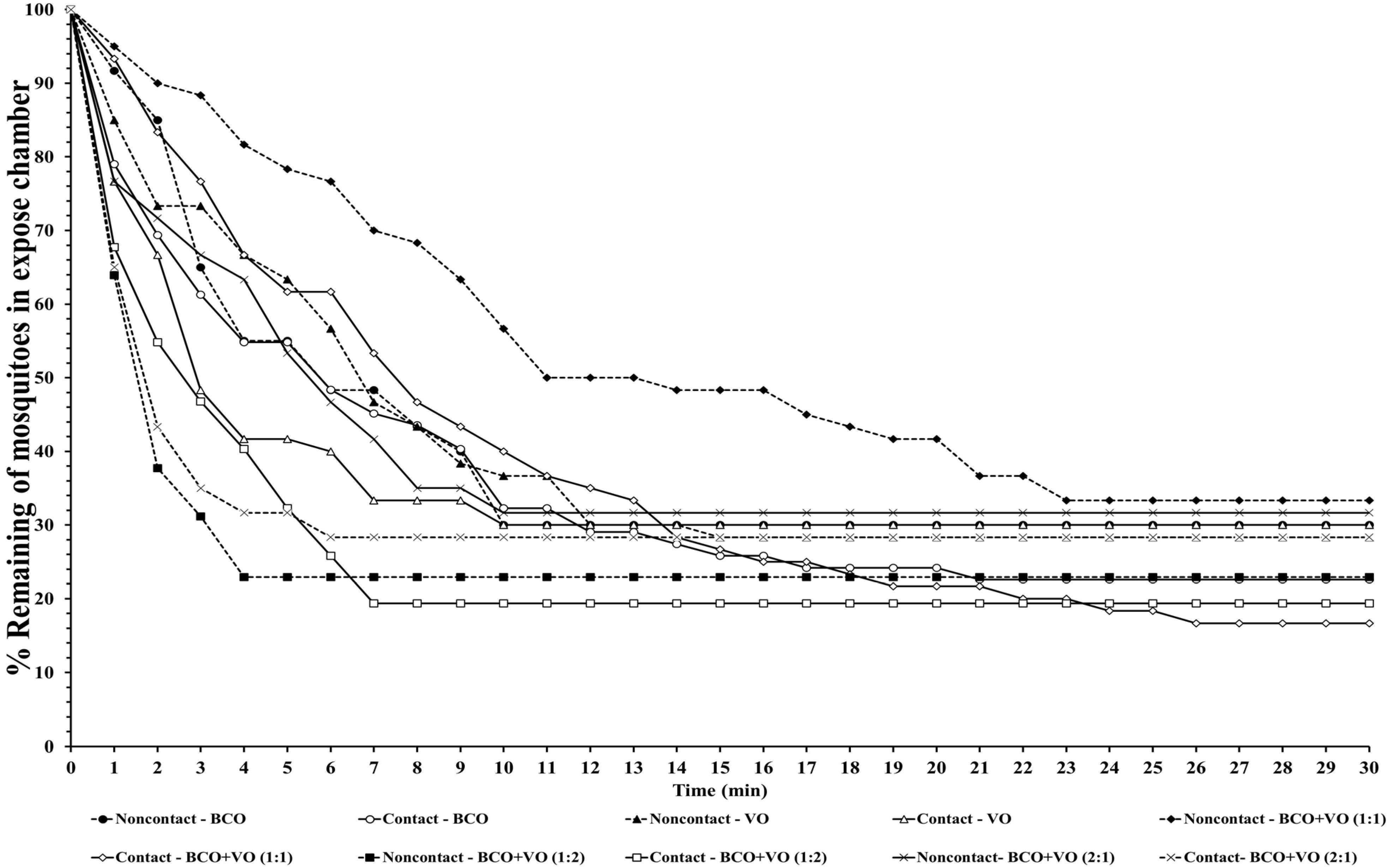
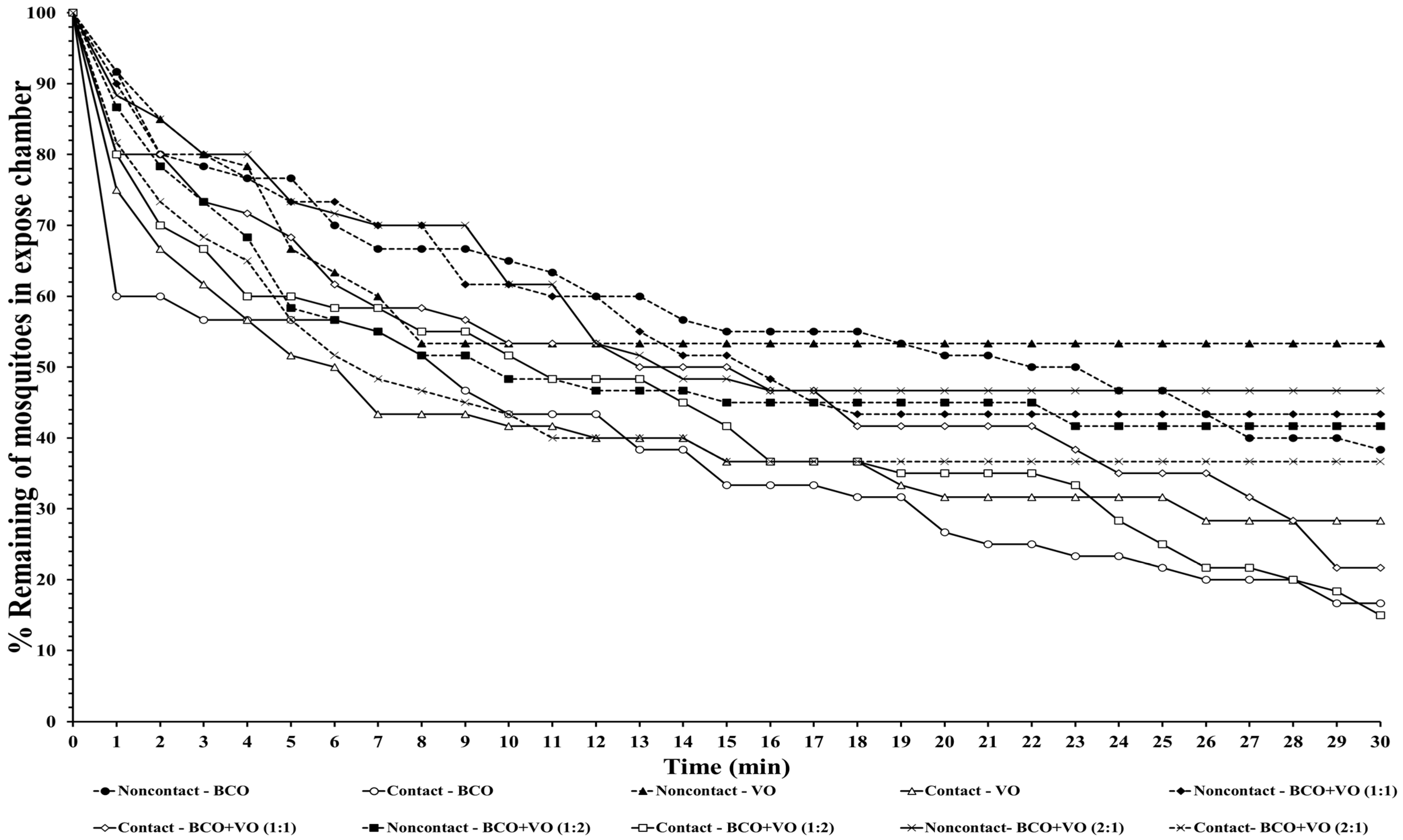
The escape responses of the four mosquito species were tested using exposure to a single chemical or dual mixture of chemical repellents, specifically BCO 1% and VO 2.5% (
Table 1). The escape patterns during the 30-min exposure period for the four mosquito species are given in
Figure 1,
Figure 2,
Figure 3 and
Figure 4. The escape rates represent the probabilities for mosquitoes escaping from a chamber for a particular chemical or mixture of chemicals and concentration. The percentages of the populations of the four species that escaped within a 30 min period of exposure to either an individual or a combination of BCO and VO in the contact and noncontact trials are presented in
Table 2. The percentage represents the probability of mosquitoes escaping from the chamber with a given chemical repellent and formulation. Strong escape responses were observed with mixtures of BCO + VO (1:1) in both the noncontact and contact trials for
An. minimus (61.54–80.39%) and
Cx. quinquefasciatus (51.85–75.47%), as well as for BCO + VO (1:2) for
An. minimus (74.07–78.18%) and
Cx. quinquefasciatus (55.36–83.64%) (
Table 2,
Figure 3 and
Figure 4). For
Aedes species, a higher percentage of escaped mosquitoes was observed with BCO + VO (2:1) in the contact and noncontact trials for
Ae. aegypti (40.35–53.70%) and for contact only for
Ae. albopictus (67.27%). For BCO + VO (1:1), the latter species had high escape rates in the contact and noncontacts trials (53.85–62.75%), as shown in
Table 2 and
Figure 1 and
Figure 2. Overall,
An. minimus and
Cx. quinquefasciatus demonstrated more robust escape responses than
Ae. aegypti and
Ae. albopictus. With
Ae. albopictus, a weak noncontact escape pattern was found for BCO + VO (2:1), as shown in
Table 2 and
Figure 3 and
Figure 4.
The knockdown of escaped and nonescaped mosquitoes was observed during the exposure period (30 min), as shown in
Table 3. The percentage knockdown of nonescaped
Ae. albopictus specimens in the contact trials for BCO + VO (1:1) was 33.33%, 23.8% for BCO + VO (1:2), and 22.22% for BCO + VO (2:1). For
An. minimus and
Cx. quinquefasciatus, the percentage knockdown of nonescaped specimens in the contact trials for BCO + VO (2:1) were 29.41% and 18.18%, respectively (
Table 3). No knockdown specimens were observed for
Ae. aegypti. Overall, there was no mortality in any of the test populations when exposed to either plant-based repellent (
Table 3).
The multiple log-rank comparisons between two species exposed to repellent compounds in either the contact or noncontact trials are shown in
Table 4. For the contact trials, there were significant differences (
p < 0.05) in the escape responses when
Ae. aegypti was compared with the other species at the two concentrations of BCO + VO (1:1 and 1:2) but not for BCO + VO (2:1). For the noncontact trials, significant differences (
p < 0.05) in the escape responses between two species were found for most pairs for BCO + VO (1:2) (
Table 4).
Table 5 presents the log-rank comparisons of the mosquito escape responses between paired concentrations of BCO and VO in the contact and noncontact trials. There were no significant differences in either paired contact or noncontact trials in
Cx. quinquefasciatus for all comparisons (
p > 0.05). For
Ae. aegypti, significant differences were found for BCO + VO (2:1) vs. BCO + VO (1:2) and BCO + VO (2:1) vs. BCO + VO (1:1) in contact trials. Significant differences were also found in four cases of
Ae. albopictus in a noncontact trial (BCO vs. BCO + VO (2:1), VO vs. BCO + VO (2:1), BCO + VO (2:1) vs. BCO + VO (1:2), and BCO + VO (2:1) vs. BCO + VO (1:1)). For
An. minimus, differences were significant in three cases, including BCO vs. BCO + VO (1:2), VO vs. BCO + VO (1:2), and VO vs. BCO + VO (1:2) in noncontact trials. The BCO + VO (1:2) vs. BCO + VO (1:1) comparison was significant in both contact and noncontact trials with
An. minimus, respectively (
Table 5). Multiple log-rank comparisons were conducted between the contact and noncontact trials for each single compound or mixture (
Table 6). Interestingly, no significant differences in escape patterns for
Ae. aegypti were observed in the paired comparisons between the contact and noncontact trials. For
Ae. albopictus, no significant differences in escape patterns were apparent in the paired comparisons between the contact and noncontact trials, except for BCO + VO (2:1). Likewise, marked differences in the escape responses were found in the paired contact and noncontact trials (
p < 0.05), with BCO + VO (1:1) for
An. minimus. Escape responses were also significantly different between the contact and noncontact trials when
Cx. quinquefasciatus was tested against BCO, VO, and BCO + VO (1:2), as shown in
Table 6.
The escape patterns of the mosquitoes from chambers treated with test compounds were defined as escape times for 25% (ET
25), 50% (ET
50), and 75% (ET
75) of the test populations to leave the treated chambers within 30 min (
Table 7).
Aedes albopictus had a faster response to all single or combinations of BCO and VO compounds with ET
25 values of 1–2 min in both contact and noncontact trials.
Aedes aegypti had delayed escape responses to all combinations with ET
25 values of 3–10 min in both contact and noncontact trials, except for BCO + VO (2:1), for which lower values of 2–3 min were recorded, respectively. For ET
75, no response was observed in either contact or noncontact trails for
Ae. aegypti and
Ae. albopictus for all test compounds.
Anopheles minimus had fast escape responses of 1–3 min at ET
25–ET
50 to BCO + VO (1:2) in both contact and noncontact trials and at ET
25 for all compounds, except for BCO + VO (1:1).
Culex quinquefasciatus displayed ET
25 values of 1–3 min in contact trials for all compounds tested but showed a delayed response of 3–6 min in noncontact trials (
Table 7).
4. Discussion
Currently, insecticide resistance poses a serious threat to the success of vector control programs and has added to the pressure on scientists to develop new and enhanced vector control tools [
28]. Globally, numerous studies, including the present investigation, have clearly identified that traditionally used plant-based insect repellents are promising and could control arthropod pests and vectors transmitting disease agents [
29]. Repellents play an important role in preventing or reducing the incidence of vector-borne diseases by preventing human–vector contact. Synthetic repellents are a conventional means of personal protection against most biting insects and pests, with the worldwide market for personal insect repellents estimated at more than USD 2 billion annually [
30]. One of the most effective and widely used insect repellents is
N, N-diethyl-meta-toluamide (DEET) [
31]. There are more than 200 different DEET products available commercially, ranging from concentrations of 5 to 100% [
14]. However, critical concerns have been raised over the safety of DEET in other studies [
32,
33,
34].
In general, plant-based repellents are known to be safer and environmentally friendlier alternative sources compared with chemical insect repellents. The plant products that are in use include a wide range of substances, from crude plant extracts to essential oils and isolated compounds. In the present study, the combination of BCO and VO showed repellent efficacy against laboratory-colonized mosquito populations. VO has been reported to show a high repellent efficacy against mosquitoes [
16,
22]. The repellent properties of some plant-based repellents against many arthropods are based on their aromatic constituents [
35]. BCO is widely found in plant-based essential oils and presents the capacity to repel mosquitoes [
36,
37,
38,
39,
40,
41,
42,
43]. More recent studies have reported that
Ae. aegypti,
Ae. albopictus,
An. minimus, and
An. dirus had high avoidance response rates to 1% concentrations of BCO compared with DEET at the same concentration [
15,
20], showing the high repellency potential of this compound.
Deploying synergistic combinations aims to reduce the dose of the substances and to help with reducing the risk of developing physiological resistance by any insect population. To support this approach, we mixed two compounds with confirmed mosquito repellent activity to increase repellent efficacy—three binary mixture forms of BCO (1%) and VO (2.5%) were prepared to test the excito-repellency activity on
Ae. aegypti,
Ae. albopictus,
An. minimus, and
Cx. quinquefasciatus using an ER repellency test system. The combinations of BCO and VO (2:1) produced higher escape percentages in contact trials with
Aedes mosquitoes than a single compound. Similar to the preceding investigation, Panthawong et al. [
44] documented the observation of robust escape behavior in contact trials involving VO and
Andrographis paniculata (AP). This escape pattern was consistently observed across all combinations, with escape percentages ranging from 56% to 73%. Notably, the combination ratio of 1:4 yielded the highest escape rate at 73.33% for VO: AP in the contact trial. Furthermore, Boonyuan et al. [
45] reported that the most potent contact irritant effect was achieved with a VO: AP ratio of 1:1 (
v:
v) against
Cx. quinquefasciatus mosquitoes, resulting in an impressive 96.67% escape rate, surpassing the effectiveness of DEET, which achieved an 88.67% escape rate.
Tisgratog et al. [
46] conducted a comprehensive review, revealing that
An. minimus and
Cx. quinquefasciatus mosquitoes exhibited a heightened avoidance response to various essential oils, including
Cymbopogon nardus,
Citrus hystrix,
Nepeta cataria,
Syzygium aromaticum, and
Ocimum americanum, when compared with
Aedes mosquitoes. It is worth noting that the escape percentage observed could have been influenced by the specific mosquito species under investigation.
Anopheles and
Culex mosquitoes demonstrated a greater sensitivity to repellents than
Aedes mosquitoes, as the latter are known to possess a higher tolerance to repellent substances [
47]. In an additional study conducted Noosidum et al. (2014) [
48], a mixture of
Litsea cubeba (LC) and
Litsea salicifolia (LS) at 0.075% against
Ae. aegypti showed the highest synergistic action (65.5% escaped) compared with that with unmixed oil alone at the same concentration (LC = 20% and LS = 32.2%). In addition, mixtures of LC and LS at 0.075% demonstrated the highest noncontact repellency (62.7%), which was significantly better than the use of LC (20%) or LS (20.3%) alone. In a study of the protection period against mosquito bites,
Litsea (
L. cubeba) + rosewood (
Aniba rosaeodora) in a ratio of 1:1 (
v/
v) at a 10% concentration showed 86% repellency for 4 h against
Ae. aegypti using human-skin test methods (K & D module) [
49]. However, some combinations produced a reduction (antagonistic effect) compared with the pure compound. Pavela (2014) [
50] reported that L-carvone and gallic acid created an antagonistic effect with the other aromatic compounds to
Spodoptera littoralis Boisd., a lepidopteran pest of crops. This suggested that the synergistic or additive effects of dual mixtures and the activity could be species-specific; therefore, no generalization to other insect species could be drawn from this study [
51].
The combinations of BCO and VO were effective against the four tested mosquito vector species, especially
Cx. quinquefasciatus and
An. minimus, but some combinations were less effective when tested against
Ae. aegypti and
Ae. albopictus. However, the BCO and VO mixture provided better protection against mosquito bites than when applying a repellent alone.
Culex quinquefasciatus exhibited much stronger escape responses against the mixtures of BCO: VO than other species. Phasomkusolsil and Soonwera (2011) [
52] found that
An. minimus and
Cx. quinquefasciatus were more sensitive to several different oils compared with
Ae. aegypti. Sathantriphop et al. (2014) [
21] reported that
Cx. quinquefasciatus and
An. minimus had much stronger behavioral responses to VO than
Ae. aegypti and
Ae. albopictus. One study examining the effect of a repellent on the olfactory system of
Culex mosquito antennal sensilla neurons showed that β-caryophyllene and (-)-caryophyllene oxide produced a strong response on short, blunt-tipped type I (SBT-I-B) sensilla [
53].
In terms of toxicity, binary mixtures of BCO and VO produced a percentage knockdown at 1 h in a nonescape chamber. Notably, BCO + VO (2:1) produced knockdown activity with
Ae. albopictus,
An. minimus, and
Cx. quinquefasciatus. Noosidum et al. (2014) [
48] also tested ER with
L. cubeba 0.175% and
L. salicifolia 0.175% against
Ae. aegypti. The synergist effect produced 67.8% knockdown. Another study presented a comparison of the synergistic effects of
Rosmarinus officinalis L. essential oil constituents against the larvae and an ovarian cell line of the cabbage looper,
Trichoplusia ni (Lepidoptera: Noctuidae). The insecticidal activity of rosemary oil appeared to be a consequence of the synergistic reaction between 1,8-cineole and (±) camphor [
54].