Evaluation of the Stability of a 1,8-Cineole Nanoemulsion and Its Fumigant Toxicity Effect against the Pests Tetranychus urticae, Rhopalosiphum maidis and Bemisia tabaci
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation of Nanoemulsion
2.2. Characterization of Nanoemulsion
2.3. Acaricidal Activity
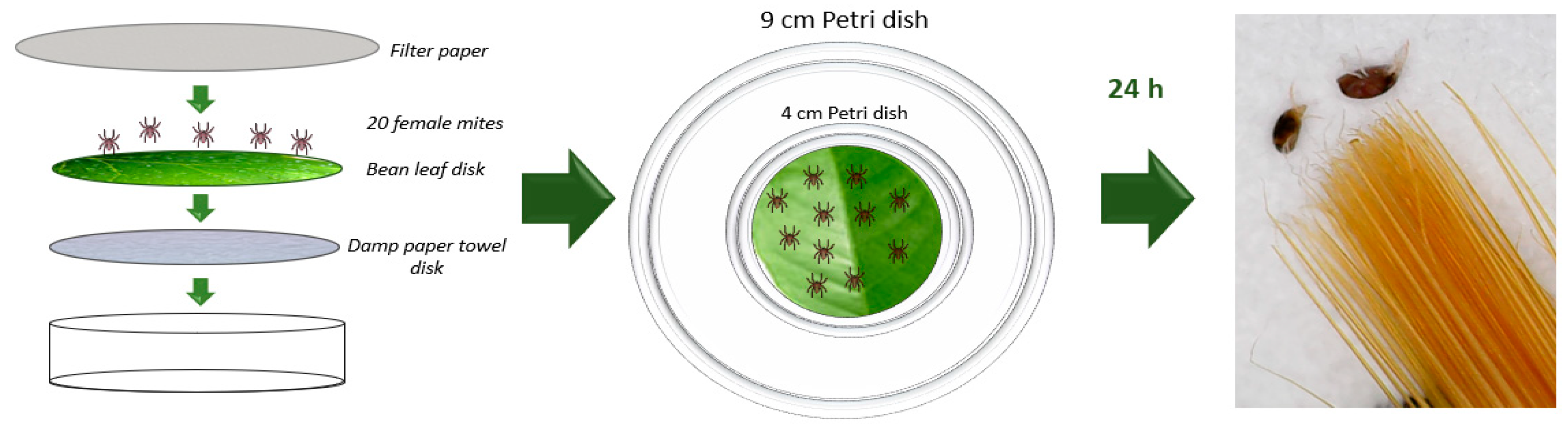
2.3.1. Tested Animals
2.3.2. Fumigant Toxicity against Adults
2.4. Aphicidal Activity
2.4.1. Tested Animals
2.4.2. Fumigant Toxicity against Wingless Adults
2.5. Insecticidal Activity
2.5.1. Tested Animals
2.5.2. Fumigant Toxicity against Adults
2.6. Data Analysis
3. Results and Discussion
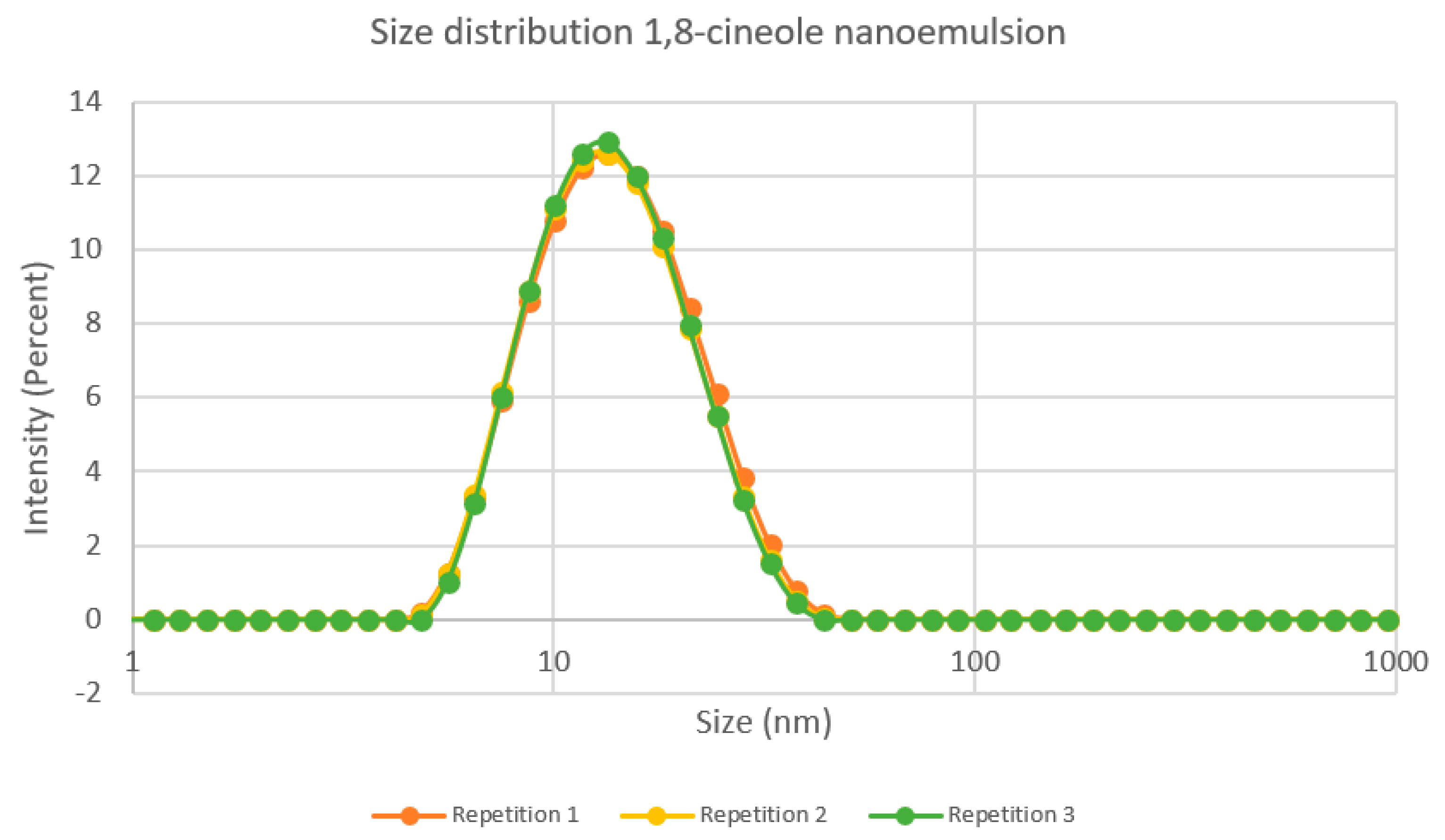
3.1. Characterization of Nanoemulsion
3.1.1. Visual Appearance and Droplet Size
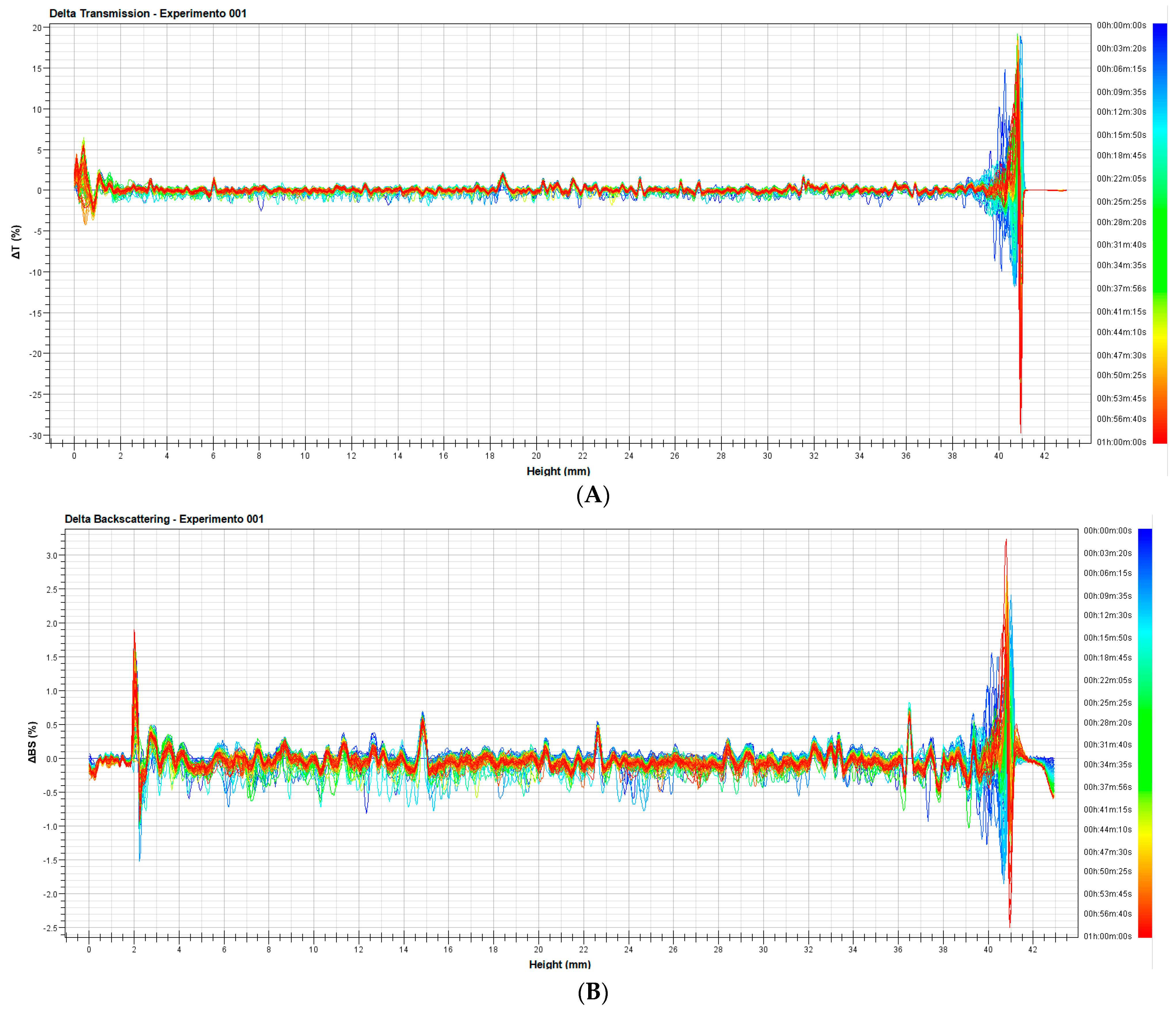
3.1.2. Evaluation of Stability of the Nanoemulsion
3.1.3. Analysis of Stability of the Nanoemulsion with Turbiscan LAB®
3.2. Evaluation of Sealed 1, 8-Cineole against Arthropod Pests T. urticae, and R. maidis
3.2.1. Acaricidal Activity in Petri Dishes
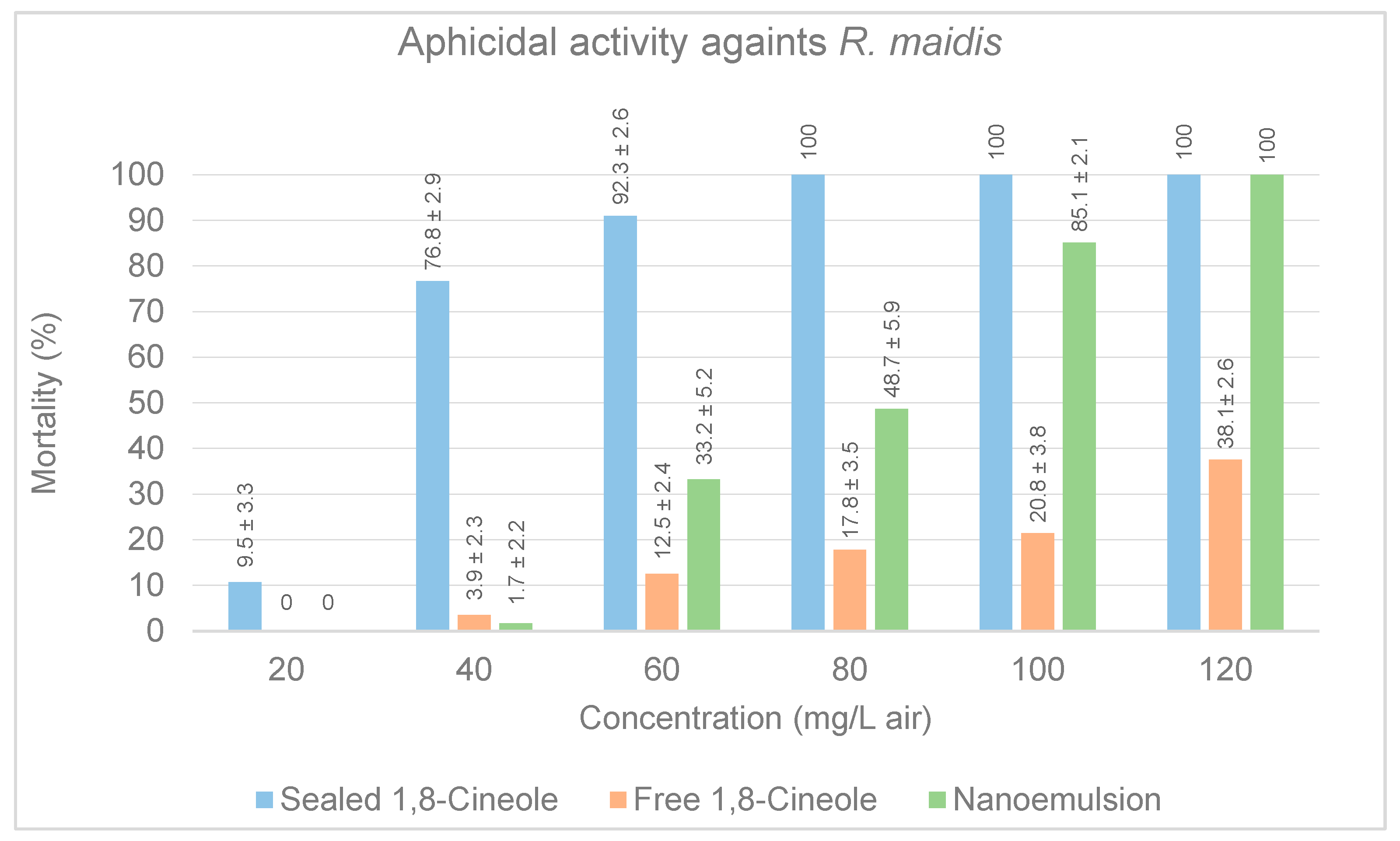
3.2.2. Aphicidal Activity in Petri Dishes
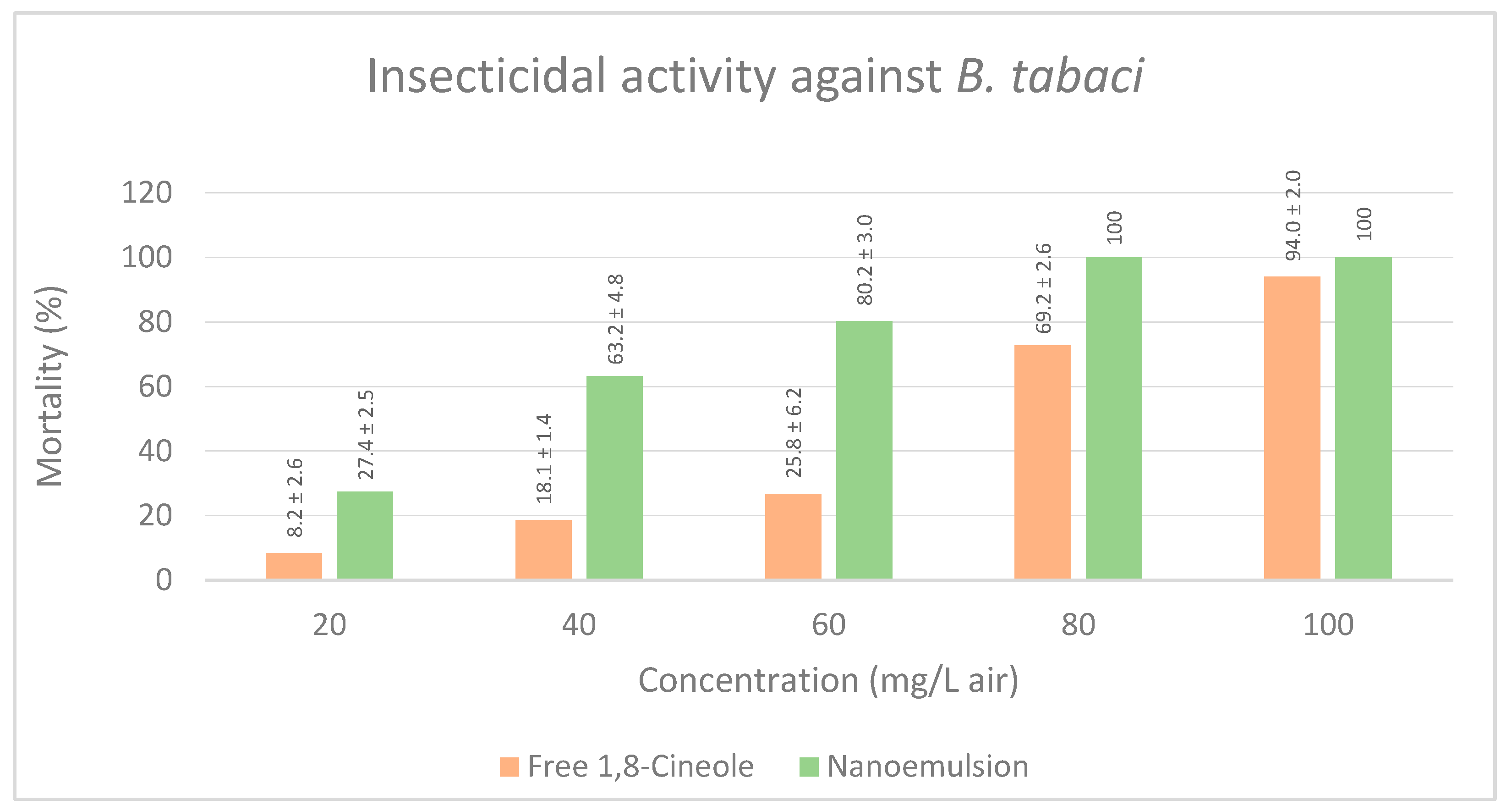
3.2.3. Insecticidal Activity against B. tabaci
3.2.4. Evaluation of Free Compound and Nanoemulsion Three against Arthropod Pests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, X.; Ma, Q.; Yang, F.; Zhang, C.; Zhao, X.; Chang, F.; Han, L. Crop pest image recognition based on the improved ViT method. Inf. Process. Agric. 2023; in press. [Google Scholar] [CrossRef]
- Agut, B.; Pastor, V.; Jaques, J.A.; Flors, V. Can Plant Defence Mechanisms Provide New Approaches for the Sustainable Control of the Two-Spotted Spider Mite Tetranychus urticae? Int. J. Mol. Sci. 2018, 19, 614. [Google Scholar] [CrossRef]
- Maldonado-Michel, M.A.; Muñiz-Valencia, R.; Peraza-Campos, A.L.; Parra-Delgado, H.; Chan-Cupul, W. Acaricidal, ovicidal and fagoinhibition activities of seed extracts from Swietenia humilis against Tetranychus urticae under laboratory conditions. Ind. Crop. Prod. 2022, 177, 114494. [Google Scholar] [CrossRef]
- Taghizadeh, R.; Chi, H. Demography of Tetranychus urticae (Acari: Tetranychidae) under different nitrogen regimes with estimations of confidence intervals. Crop. Prot. 2022, 155, 105920. [Google Scholar] [CrossRef]
- Harris, A.L.; Ullah, R.; Fountain, M.T. The evaluation of extraction techniques for Tetranychus urticae (Acari: Tetranychidae) from apple (Malus domestica) and cherry (Prunus avium) leaves. Exp. Appl. Acarol. 2017, 72, 367–377. [Google Scholar] [CrossRef]
- Jonckheere, W.; Dermauw, W.; Zhurov, V.V.; Wybouw, N.; Van Den Bulcke, J.; Villarroel, C.A.; Greenhalgh, R.; Grbic, M.; Schuurink, R.C.; Tirry, L.; et al. The Salivary Protein Repertoire of the Polyphagous Spider Mite Tetranychus urticae: A Quest for Effectors. Mol. Cell Proteom. 2016, 15, 3594–3613. [Google Scholar] [CrossRef]
- Khajehali, J.; Van Nieuwenhuyse, P.; Demaeght, P.; Tirry, L.; Van Leeuwen, T. Acaricide resistance and resistance mechanisms in Tetranychus urticae populations from rose greenhouses in the Netherlands. Pest Manag. Sci. 2011, 67, 1424–1433. [Google Scholar] [CrossRef]
- Adesanya, A.W.; Lavine, M.D.; Moural, T.W.; Lavine, L.C.; Zhu, F.; Walsh, D.B. Mechanisms and management of acaricide resistance for Tetranychus urticae in agroecosystems. J. Pest Sci. 2021, 94, 639–663. [Google Scholar] [CrossRef]
- Al-Shammery, K.A.; Al-Khalaf, A.A. Effect of host preference and micro habitats on the survival of Tetranychus urticae Koch (Acari: Tetranychidae) in Saudi Arabia. J. King Saud Univ. -Sci. 2022, 34, 102030. [Google Scholar] [CrossRef]
- Sun, J.; Li, C.; Jiang, J.; Song, C.; Wang, C.; Feng, K.; Wei, P.; He, L. Cross resistance, inheritance and fitness advantage of cyetpyrafen resistance in two-spotted spider mite, Tetranychus urticae. Pestic. Biochem. Phys. 2022, 183, 105062. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Capinera, J.L. Order Homoptera—Aphids, Leaf- and Planthoppers, Psyllids and Whiteflies. In Handbook of Vegetable Pests; Capinera, J.L., Ed.; Academic Press: Cambridge, MA, USA, 2001; Volume 1, pp. 279–346. [Google Scholar]
- Alam, M.J.; Ahmed, K.S.; Mollah, M.R.A.; Tareq, M.Z.; Chowdhury, M.M.I. Effect of spacing on mustard yield at Shibganj and Sadar upazila of Bogra district. Bangladesh J. Environ. Sci. 2015, 28, 133–136. [Google Scholar]
- Straub, R.H.; Emmett, B.A. Pests of Monocotyledon Crops. In Vegetable Crop Pests, 1st ed.; McKinlay, R.G., Ed.; Palgrave Macmillan London: London, UK, 1992; pp. 213–262. [Google Scholar]
- Fuchsberg, J.D.; Yong, T.; Losey, J.E.; Carter, M.; Hoffmann, M.R. Evaluation of corn leaf aphid (Rhopalosiphum maidis; Homoptera: Aphididae) honeydew as a food source for the egg parasitoid Trichogramma ostriniae (Hymenoptera: Trichogrammatidae). Biol. Control. 2007, 40, 230–236. [Google Scholar] [CrossRef]
- Edde, P.A. Arthropod pests of maize Zea mays (L.). In Field Crop Arthropod Pests of Economic Importance; Edde, P.A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 410–465. [Google Scholar]
- Carena, M.J.; Glogoza, P. Resistance of maize to the corn leaf aphid: A review. Maydica 2004, 49, 241–254. [Google Scholar]
- Guo, L.; Lv, H.; Tan, D.; Liang, N.; Guo, C.; Chu, D. Resistance to insecticides in the field and baseline susceptibility to cyclaniliprole of whitefly Bemisia tabaci (Gennadius) in China. Crop. Prot. 2020, 130, 105065. [Google Scholar] [CrossRef]
- Johnston, N.; Paris, T.; Paret, M.L.; Freeman, J.; Martini, X. Repelling whitefly (Bemisia tabaci) using limonene-scented kaolin: A novel pest management strategy. Crop. Prot. 2020, 154, 105905. [Google Scholar] [CrossRef]
- Shah, R.A.; Al-Sadi, A.M.; Scott, I.A.; Al-Raeesi, A.; Al-Jahdhami, A. Insecticide resistance monitoring in whitefly (Bemisia tabaci) (Hemiptera: Aleyrodidae) in Oman. J. Asia-Pac. Entomol. 2020, 23, 1248–1254. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, F.; Tong, H.; Hu, Y.; Zhang, X.; Tian, T.; Zhang, Y.; Su, Q. Plant flavonoids enhance the tolerance to thiamethoxam and flupyradifurone in whitefly Bemisia tabaci (Hemiptera: Aleyrodidae). Pestic. Biochem. Phys. 2021, 171, 104744. [Google Scholar] [CrossRef]
- Li, Y.; Mbata, G.N.; Simmons, A.M.; Punnuri, S. Susceptibility of snap bean cultivars to the sweetpotato whitefly, Bemisia tabaci, in the southern United States. Crop. Prot. 2022, 159, 106022. [Google Scholar] [CrossRef]
- Onstad, D.W. IPM and Insect Resistance Management. In Insect Resistance Management, Biology, Economics, and Prediction, 2nd ed.; Onstad, D.W., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 515–532. [Google Scholar]
- Roy, D.; Biswas, S.; Biswas, A.; Chakraborty, G.; Sarkar, P.K. Can insecticide mixtures be considered to surmount neonicotinoid resistance in Bemisia tabaci? J. Asia-Pac. Entomol. 2022, 25, 101901. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Ammar, E.-D.; Whitefield, A.E.; Redinaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef]
- Czosnek, H.; Gorovits, R.; Ghanim, M. Factors controlling the fate of tomato yellow leaf curl virus (TYLCV) in its vector, the whitefly vector Bemisia tabaci. In Plant Virus-Host Interaction, 2nd ed.; Gaur, R.K., Khurana, S.M.P., Sharma, P., Hohn, T., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 231–266. [Google Scholar]
- Padmaja, P.G. Insect Pest Resistance in Sorghum. In Biotic Stress Resistance in Millets; Das, I.K., Padmaja, P.G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 105–145. [Google Scholar]
- Tehri, K.; Gulati, R.; Geroh, M. Host plant responses, biotic stress and management strategies for the control of Tetranychus urticae Koch (Acarina: Tetranychidae). Agric. Rev. 2014, 35, 250. [Google Scholar] [CrossRef]
- Nauen, R.; Denholm, I. Resistance of insect pests to neonico-tinoid insecticides: Current status and future prospects. Arch. Insect Biochem. Physiol. 2005, 58, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Konanz, S. Spiromesifen as a new chemical option for resistance management in whiteflies and spider mites. Pfanzenschutz-Nachr. Bayer. 2005, 58, 485–502. [Google Scholar]
- Satar, G.; Ulusoy, M.R.; Nauen, R.; Dong, K. Neonicotinoid insecticide resistance among populations of Bemisia tabaci in the Mediterranean region of Turkey. B Insectol. 2018, 71, 171–177. [Google Scholar]
- Alam, M.; Mukta, L.; Nahar, N.; Haque, M.; Razib, S. Management practices of aphid (Rhopalosiphum maidis) in infested maize field. Bangladesh J. Environ. Sci. 2020, 38, 23–28. [Google Scholar]
- Toledo, P.F.; Viteri Jumbo, L.O.; Rezende, S.M.; Haddi, K.; Silva, B.A.; Mello, T.S.; Della Lucia, T.M.; Aguiar, R.W.; Smagghe, G.; Oliveira, E.E. Disentangling the ecotoxicological selectivity of clove essential oil against aphids and non-target ladybeetles. Sci. Total Environ. 2020, 718, 137328. [Google Scholar] [CrossRef] [PubMed]
- Rabbi, A.; Uddin, M.N.; Alim, M.A.; Al Bachchu, M.A.; Bhuyain, M.M.H.; Akter, S. Efficacy of some pesticides against Tetranychus urticae Koch (Acari: Tetranychidae) and their residual effects on Coccinella septempunctata (L.) (Coleoptera: Coccinellidae). Int. J. Trop. Insect Sci. 2021, 42, 615–626. [Google Scholar] [CrossRef]
- Hallahan, D. Monoterpenoid biosynthesis in glandular trichomes of labiate plants. Adv. Bot. Res. 2020, 31, 77–120. [Google Scholar]
- Isman, M.B. Plant essential oils for pest and disease management. Crop. Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Grodnitzky, J.A.; Coats, J.R. QSAR Evaluation of Monoterpenoids’ Insecticidal Activity. J. Agric. Food Chem. 2002, 50, 4576–4580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, T.; Zhang, Y.; Wang, L.; Xie, Y. Fumigant toxicity of monoterpenes against fruitfly, Drosophila melanogaster. Ind. Crop. Prod. 2016, 81, 147–151. [Google Scholar] [CrossRef]
- Umpiérrez, M.L.; Paullier, J.; Porrini, M.P.; Garrido, M.G.; Santos, E.; Rossini, C. Potential botanical pesticides from Asteraceae essential oils for tomato production: Activity against whiteflies, plants and bees. Ind. Crop. Prod. 2017, 109, 686–692. [Google Scholar] [CrossRef]
- Bibiano, C.S.; Alves, D.S.; Freire, B.C.; Bertolucci, S.K.V.; Carvalho, G.A. Toxicity of essential oils and pure compounds of Lamiaceae species against Spodoptera frugiperda (Lepidoptera: Noctuidae) and their safety for the nontarget organism Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Crop. Prot. 2022, 158, 106011. [Google Scholar] [CrossRef]
- De Sena Filho, J.G.; Soares de Almeida, A.; Pinto-Zevallos, D.; Barreto, I.C.; Cabral de Holanda Cavalcanti, S.; Nunes, R.; Teodoro, A.V.; Xavier, H.S.; Barbosa Filho, J.M.; Guan, L.; et al. From plant scent defense to biopesticide discovery: Evaluation of toxicity and acetylcholinesterase docking properties for Lamiaceae monoterpenes. Crop. Prot. 2023, 164, 106126. [Google Scholar] [CrossRef]
- Sánchez-Ramos, I.; Castañera, P. Acaricidal activity of natural monoterpenes on Tyrophagus putrescentiae (Schrank), a mite of stored food. J. Stored Prod. Res. 2000, 37, 93–101. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Arami, S.A.; Abdelgaleil, S.M. Acaricidal and quantitative structure activity relationship of monoterpenes against the two-spotted spider mite, Tetranychus urticae. Exp. Appl. Acarol. 2010, 52, 261–274. [Google Scholar] [CrossRef]
- Zahran, H.E.D.M.; Abdelgaleil, S.A. Insecticidal and developmental inhibitory properties of monoterpenes on Culex pipiens L. (Diptera: Culicidae). J. Asia-Pac. Entomol. 2011, 14, 46–51. [Google Scholar] [CrossRef]
- Afify, A.E.M.; Ali, F.; Turky, A.F. Control of Tetranychus urticae Koch by extracts of three essential oils of chamomile, marjoram and Eucalyptus. Asian Pac. J. Trop. Biomed. 2012, 2, 24–30. [Google Scholar] [CrossRef]
- Roh, H.S.; Lee, B.S.; Park, C.G. Acaricidal and repellent effects of myrtacean essential oils and their major constituents against Tetranychus urticae (Tetranychidae). J. Asia-Pac. Entomol. 2013, 16, 245–249. [Google Scholar] [CrossRef]
- Laborda, R.; Manzano, I.S.H.; Gamón, M.; Gavidia, I.; Pérez-Bermúdez, P.; Boluda, R. Effects of Rosmarinus officinalis and Salvia officinalis essential oils on Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crop. Prod. 2013, 48, 106–110. [Google Scholar] [CrossRef]
- Zandi-Sohani, N.; Ramezani, L. Evaluation of five essential oils as botanical acaricides against the strawberry spider mite Tetranychus turkestani Ugarov and Nikolskii. Int. Biodeter. Biodegr. 2015, 98, 101–106. [Google Scholar] [CrossRef]
- Pavela, R.; Stepanycheva, E.A.; Shchenikova, A.V.; Chermenskaya, T.D.; Petrova, M.I. Essential oils as prospective fumigants against Tetranychus urticae Koch. Ind. Crop. Prod. 2016, 94, 755–761. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Badawy, M.E.I.; Mahmoud, N.F.; Marei, A.E.M. Acaricidal activity, biochemical effects and molecular docking of some monoterpenes against two-spotted spider mite (Tetranychus urticae Koch). Pestic. Biochem. Phys. 2019, 156, 105–115. [Google Scholar] [CrossRef]
- Kordali, S.; Kesdek, M.; Cakir, A. Toxicity of monoterpenes against larvae and adults of Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Ind. Crop. Prod. 2007, 26, 278–297. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, Z.; Yin, Z.; Jia, R.; Song, X.; Li, L.; Zou, Y.; Liang, X.; Li, L.; He, C.; et al. In vitro acaricidal activity of 1,8-cineole against Sarcoptes scabiei var. cuniculi and regulating effects on enzyme activity. Parasitol. Res. 2015, 114, 2959–2967. [Google Scholar] [CrossRef]
- Gharib, R.; Jemâa, J.M.B.; Charcosset, C.; Fourmentin, S.; Greige-Gerges, H. Retention of Eucalyptol, a Natural Volatile Insecticide, in Delivery Systems Based on Hydroxypropyl-β-Cyclodextrin and Liposomes. Eur. J. Lipid Sci. Technol. 2020, 122, 1900402. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Morais, J.M.; Burgess, D.J. Micro- and Nanoemulsions (Controlled Release Parenteral Drug Delivery Systems). In Long Acting Injections and Implants; Springer: Berlin/Heidelberg, Germany, 2011; pp. 221–238, in press. [Google Scholar]
- Ghosh, V.; Ranjha, R.; Gupta, A.K. Polymeric encapsulation of anti-larval essential oil nanoemulsion for controlled release of bioactive compounds. Inorg. Chem. Commun. 2023, 150, 110507. [Google Scholar] [CrossRef]
- Ostertag, F.; Weiss, J.; McClements, D.J. Low-energy formation of edible nanoemulsions: Factors influencing droplet size produced by emulsion phase inversion. J. Colloid Interface Sci. 2012, 388, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.G.; Wang, S.; Dou, T.T.; Liu, S.; Li, M.Y.; Hua, R.M.; Li, S.G.; Lin, H.F. Aphicidal Activity of Illicium verum Fruit Extracts and Their Effects on the Acetylcholinesterase and Glutathione S-transferases Activities in Myzus persicae (Hemiptera: Aphididae). J. Insect Sci. 2016, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Belay, D.K.; Huckaba, R.M.; Ramirez, A.M.; Rodrigues, J.C.V.; Foster, J.E. Insecticidal Control of Bemisia tabaci (Hemiptera: Aleyrodidae) Transmitting Carlavirus on Soybeans and Detection of the Virus in Alternate Hosts. Crop. Prot. 2012, 35, 53–57. [Google Scholar] [CrossRef]
- Wagan, T.A.; Cai, W.; Hua, H. Repellency, toxicity, and anti-oviposition of essential oil of Gardenia jasminoides and its four major chemical components against whiteflies and mites. Sci. Rep. 2018, 8, 9375. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: New York, NY, USA, 1971; p. 333. [Google Scholar]
- Ren, G.; Sun, Z.; Wang, Z.; Zheng, X.; Xu, Z.; Sun, D. Nanoemulsion formation by the phase inversion temperature method using polyoxypropylene surfactants. J. Colloid Interface Sci. 2019, 540, 177–184. [Google Scholar] [CrossRef]
- Miastkowska, M.; Śliwa, P. Influence of Terpene Type on the Release from an O/W Nanoemulsion: Experimental and Theoretical Studies. Molecules 2020, 25, 2747. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Abdelgaleil, S.A.M.; Mahmoud, N.F.; Marei, A.E.S.M. Preparation and characterizations of essential oil and monoterpene nanoemulsions and acaricidal activity against two-spotted spider mite (Tetranychus urticae Koch). Int. J. Acarol. 2018, 44, 330–340. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason. Sonochem. 2013, 20, 338–344. [Google Scholar] [CrossRef]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan Lab® Expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloid Surf. B 2009, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Cristiano, M.C.; Fresta, M.; Torella, D.; Paolino, D. Positively Charged Lipid as Potential Tool to Influence the Fate of Ethosomes. Appl. Sci. 2021, 11, 7060. [Google Scholar] [CrossRef]
- Perrucci, S. Acaricidal Activity of Some Essential Oils and Their Constituents against Tyrophagus longior, a Mite of Stored Food. J. Food Prot. 1995, 58, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Benddine, H.; Zaid, R.; Babaali, D.; Daoudi-Hacini, S. Biological activity of essential oils of Myrtus communis (Myrtaceae, Family) and Foeniculum vulgare (Apiaceae, Family) on open fields conditions against corn aphids Rhopalosiphum maidis (Fitch, 1856) in western Algeria. J. Saudi Soc. Agric. Sci. 2022, 22, 78–88. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Chrzanowski, G.; Sprawka, I.; Sytykiewicz, H. Aphicidal activity of selected Asteraceae essential oils and their effect on enzyme activities of the green peach aphid, Myzus persicae (Sulzer). Pestic. Biochem. Phys. 2018, 145, 84–92. [Google Scholar] [CrossRef]
- Yang, N.; Li, A.; Wan, F.; Liu, W.; Johnson, D.L. Effects of plant essential oils on immature and adult sweetpotato whitefly, Bemisia tabaci biotype B. Crop. Prot. 2010, 29, 1200–1207. [Google Scholar] [CrossRef]
- Liu, X.C.; Hu, J.F.; Zhou, L.; Liu, Z.L. Evaluation of fumigant toxicity of essential oils of Chinese medicinal herbs against Bemisia tabaci. J. Entomol. Zool. Stud. 2014, 2, 164–169. [Google Scholar]
- Liu, J.; Hua, J.; Qu, B.; Guo, X.; Wang, Y.; Meini, S.; Luo, S. Insecticidal Terpenes From the Essential Oils of Artemisia nakaii and Their Inhibitory Effects on Acetylcholinesterase. Front. Plant Sci. 2021, 12, 720816. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.S.; Sequin, C.J.; Foti, N.; Campos-Soldini, M.P. Insecticidal, fungicidal, phytotoxic activity and chemical composition of Lavandula dentata essential oil. Biocatal. Agric. Biotechnol. 2021, 35, 102092. [Google Scholar] [CrossRef]
- Almadiy, A.A. Insecticidal and acetylcholinesterase inhibitory activities of Achillea biebersteinii essential oil and its nanoemulsion and major monoterpenes against Tribolium castaneum. J. Asia-Pac. Entomol. 2021, 24, 1170–1178. [Google Scholar] [CrossRef]
- Suresh, U.; Murugan, K.; Zengin, G.; Aziz, A.T.; Cianfaglione, K.; Wang, L.; Maggi, F. Encapsulation of sea fennel (Crithmum maritimum) essential oil in nanoemulsion and SiO2 nanoparticles for treatment of the crop pest Spodoptera litura and the dengue vector Aedes aegypti. Ind. Crop. Prod. 2020, 158, 113033. [Google Scholar] [CrossRef]
- Almadiy, A.A.; Nenaah, G.E.; Albogami, B. Bioactivity of Deverra tortuosa essential oil, its nanoemulsion, and phenylpropanoids against the cowpea weevil, a stored grain pest with eco-toxicological evaluations. Environ. Sci. Pollut. Res. 2022, 29, 65112–65127. [Google Scholar] [CrossRef] [PubMed]
- Gharsan, F.N.; Kamel, W.M.; Alghamdi, T.M.; Alghamdi, A.A.; Althagafi, A.O.; Aljassim, F.; Al-Ghamdi, S.N. Toxicity of citronella essential oil and its nanoemulsion against the sawtoothed grain beetle Oryzaephilus surinamensis (Coleoptera: Silvanidae). Ind. Crop. Prod. 2022, 184, 115024. [Google Scholar] [CrossRef]
- Salazar, A.M.; Arismendi, N.; López, M.G.V.; Vargas, M.; Schoebitz, M.; Palacio, D.A.; Becerra, J.; Cedeño, B.; Zapata, N. Stability of the oil-based nanoemulsion of Laureliopsis philippiana (Looser) and its insecticidal activity against tomato borer (Tuta absoluta Meyrick). Ind. Crop. Prod. 2022, 188, 115635. [Google Scholar] [CrossRef]


| 1,8-Cineole | N 1 | LC50 (CI) 2 mg/L Air | LC95 (CI) 2 mg/L Air | Slope ± SE | Χ2(df) | p-Value |
|---|---|---|---|---|---|---|
| Sealed | 1060 | 19.5 (16.43–22.70) a 3 | 32.34 (26.72–50.14) a | 7.484 (0.387) | 40.62 (4) | 0.000 |
| Free | 1080 | 137.46 (106.89–317.92) c | 329.49 (189.43–2283.43) bc | 4.333 (0.593) | 8.67 (4) | 0.070 |
| Nanoemulsión | 1440 | 55.23 (48.16–63.59) b | 140.90 (108.11–233.64) b | 4.044 (0.218) | 38.65 (6) | 0.000 |
| 1,8-Cineole | N 1 | LC50 (CI) 2 mg/L Air | LC95 (CI) 2 mg/L Air | Slope ± SE | X2(df) | p-Value |
|---|---|---|---|---|---|---|
| Sealed | 720 | 31.98 (23.89–39.53) a 3 | 59.18 (46.56–101.24) a | 6.152 (0.367) | 7.09 (2) | 0.029 |
| Free | 900 | 140.27 (118.44–207.68) c | 240.36 (185.12–430.57) bc | 0.016 (0.002) | 6.659 (3) | 0.086 |
| Nanoemulsion | 720 | 73.05 (58.96–86.50) b | 118.01 (96.53–212.25) ab | 7.897 (0.445) | 28.88 (3) | 0.000 |
| 1,8-Cineole (Sealed) | N 1 | LC50 (CI) 2 mg/L Air | LC95 (CI) 2 mg/L Air | Slope ± SE | X2(df) | p-Value |
|---|---|---|---|---|---|---|
| Free | 1200 | 61.308 (33.34–100.32) ab 3 | 152.93 (95.97–4689.22) ab | 4.144 (0.239) | 96.42 (4) | 0.000 |
| Nanoemulsion | 1350 | 30.98 (21.69–38.51) a | 80.44 (62.43–131.25) a | 3.969 (0.201) | 33.93 (4) | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayllón-Gutiérrez, R.; López-Maldonado, E.A.; Macías-Alonso, M.; González Marrero, J.; Díaz-Rubio, L.; Córdova-Guerrero, I. Evaluation of the Stability of a 1,8-Cineole Nanoemulsion and Its Fumigant Toxicity Effect against the Pests Tetranychus urticae, Rhopalosiphum maidis and Bemisia tabaci. Insects 2023, 14, 663. https://doi.org/10.3390/insects14070663
Ayllón-Gutiérrez R, López-Maldonado EA, Macías-Alonso M, González Marrero J, Díaz-Rubio L, Córdova-Guerrero I. Evaluation of the Stability of a 1,8-Cineole Nanoemulsion and Its Fumigant Toxicity Effect against the Pests Tetranychus urticae, Rhopalosiphum maidis and Bemisia tabaci. Insects. 2023; 14(7):663. https://doi.org/10.3390/insects14070663
Chicago/Turabian StyleAyllón-Gutiérrez, Rocío, Eduardo Alberto López-Maldonado, Mariana Macías-Alonso, Joaquín González Marrero, Laura Díaz-Rubio, and Iván Córdova-Guerrero. 2023. "Evaluation of the Stability of a 1,8-Cineole Nanoemulsion and Its Fumigant Toxicity Effect against the Pests Tetranychus urticae, Rhopalosiphum maidis and Bemisia tabaci" Insects 14, no. 7: 663. https://doi.org/10.3390/insects14070663
APA StyleAyllón-Gutiérrez, R., López-Maldonado, E. A., Macías-Alonso, M., González Marrero, J., Díaz-Rubio, L., & Córdova-Guerrero, I. (2023). Evaluation of the Stability of a 1,8-Cineole Nanoemulsion and Its Fumigant Toxicity Effect against the Pests Tetranychus urticae, Rhopalosiphum maidis and Bemisia tabaci. Insects, 14(7), 663. https://doi.org/10.3390/insects14070663







