Effects of Temperature on the Developmental and Reproductive Biology of North American Bean Thrips, Caliothrips fasciatus (Pergande) (Thysanoptera: Thripidae: Panchaetothripinae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of Thrips Colonies for Experiments
2.2. Preimaginal Development, Adult Longevity, and Female Fecundity across Nine Fluctuating Temperature Regimens
2.3. Statistical Analyses of Preimaginal Developmental, Female Fecundity, and Offspring Sex Ratio Data across Fluctuating Temperature Regimens
2.4. Fitting of Models to Temperature-Driven Caliothrips fasciatus Development Data
3. Results
3.1. Effects of Temperature on G1 Thrips Development Times
3.2. Effects of Temperature on G1 Thrips Longevity Times, G1 Female Fecundity Rates, and G2 Offspring Sex Ratios
3.3. Effects of Temperature on G2 Thrips Egg-to-Adult Development Times
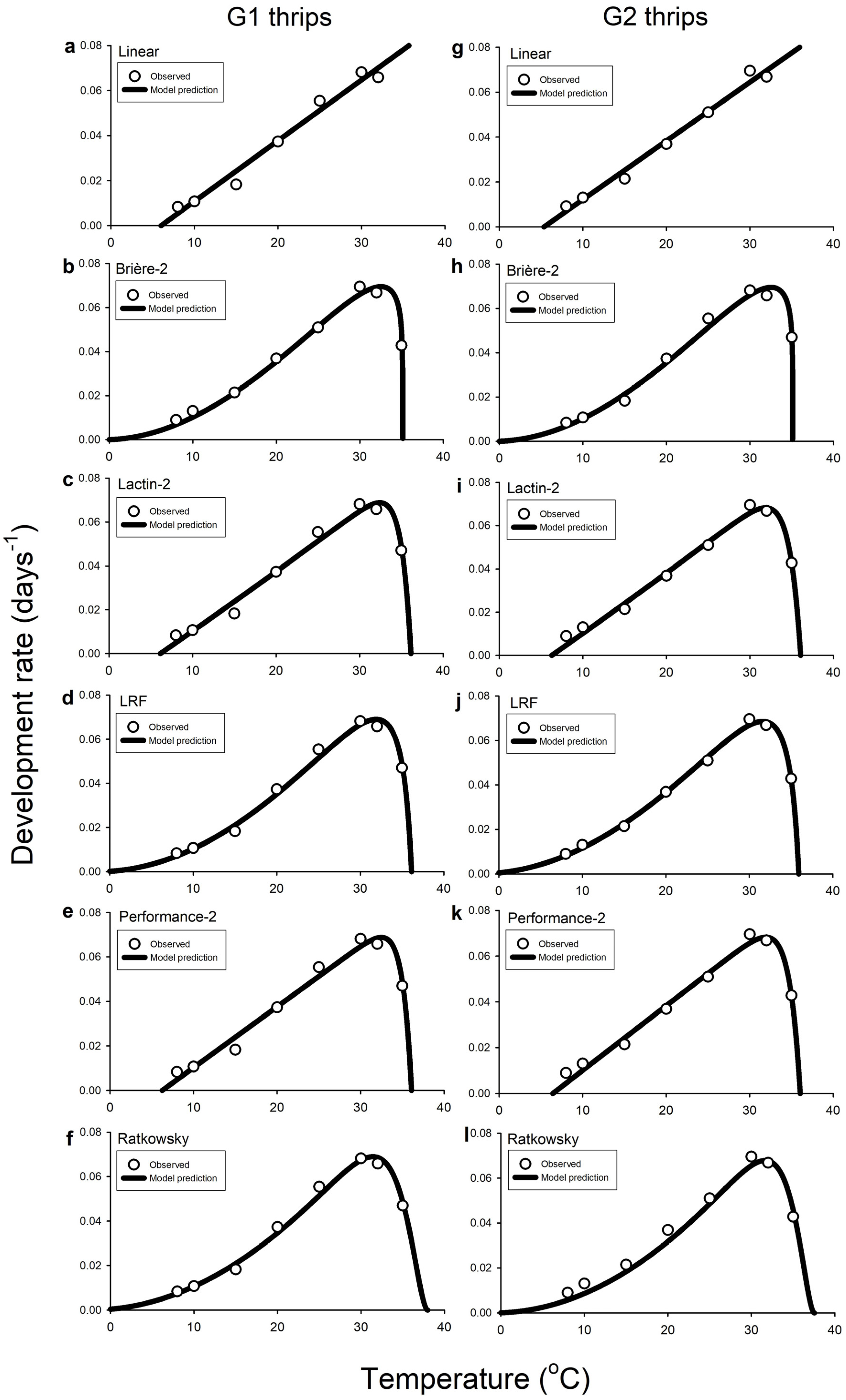
3.4. Fitting Models to Temperature-Driven Development Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mound, L.A.; Hoddle, M.S.; Hastings, A. Thysanoptera Californica—Thrips of California. Lucidcentral.org, Identic Pty Ltd., Queensland, Australia. 2019. Available online: https://keys.lucidcentral.org/keys/v3/thrips_of_california_2019//index.html (accessed on 5 June 2023).
- Hoddle, M.S. North American bean thrips—A perennial export problem for California citrus growers. Citrograph 2020, 11, 40–43. [Google Scholar]
- Mound, L.A.; Zhang, H.; Bei, Y. Caliothrips tongi n. sp. (Thysanoptera: Thripidae) from China, and a dubious record of North American bean thrips. Zootaxa 2011, 2736, 57–62. [Google Scholar] [CrossRef]
- Bailey, S.F. The biology of the bean thrips. Hilgardia 1933, 7, 467–522. [Google Scholar] [CrossRef]
- Bailey, S.F. The Bean Thrips. In Monograph Bulletin 609; University of California Experiment Station: Berkeley, CA, USA, 1937. [Google Scholar]
- Bailey, S.F. Thrips of economic importance in California. In Circular of the University of California Berkeley Agricultural Experiment Station 346; University of California Experiment Station: Berkeley, CA, USA, 1938. [Google Scholar]
- Hoddle, M.S.; Stosic, C.D.; Mound, L.A. Populations of North American bean thrips, Caliothrips fasciatus (Pergande) (Thysanoptera: Thripidae: Panchaetothripinae) not detected in Australia. Aust. J. Entomol. 2006, 45, 122–129. [Google Scholar] [CrossRef]
- Rugman-Jones, P.F.; Hoddle, M.S.; Amrich, R.; Heraty, J.M.; Stouthamer-Ingel, C.E.; Stouthamer, R. Phylogeographic structure, outbreeding depression, and reluctant virgin oviposition in bean thrips, Caliothrips fasciatus (Pergande) (Thysanoptera: Thripidae), in California. Bull. Entomol. Res. 2012, 102, 698–709. [Google Scholar] [CrossRef]
- Harman, J.A.; Mao, C.X.; Robinson, L.J.; Morse, J.G. Evaluation of two non-destructive sampling methods for bean thrips (Thysanoptera: Thripidae) detection in navel oranges. Crop Prot. 2007, 26, 1747–1754. [Google Scholar] [CrossRef]
- Harman, J.A.; Mao, C.X.; Morse, J.G. Selection of colour sticky traps for monitoring adult bean thrips, Caliothrips fasciatus (Thysanoptera: Thripidae). Pest Manag. Sci. 2007, 63, 201–216. [Google Scholar] [CrossRef]
- Bikoba, V.N.; Pupin, F.; Biasi, W.V.; Rutaganira, F.U.; Mitcham, E.J. Use of ethyl formate fumigation to control adult bean thrips in navel oranges. J. Econ. Entomol. 2019, 112, 591–596. [Google Scholar] [CrossRef]
- Walse, S.S.; Jimenez, L.R. Postharvest fumigation of fresh citrus with cylinderized phosphine to control bean thrips (Thysanoptera: Thripidae). Horticulturae 2021, 7, 134. [Google Scholar] [CrossRef]
- USDA-NAS. Citrus Fruits 2022 Summary. Available online: https://ccqc.org/wp-content/uploads/USDA-NASS-Citrus-Fruits-2022-Summary-090822.pdf (accessed on 5 June 2023).
- Anon. US Citrus Exports—Top Markets for Oranges. Available online: https://ccqc.org/wp-content/uploads/2021-Top-Export-Markets-for-Citrus-QTY-VAL.pdf (accessed on 5 June 2023).
- Williamson, M. Biological Invasions; Chapman and Hall: London, UK, 1997; p. 40. [Google Scholar]
- Seebens, H.; Briski, E.; Ghabooli, S.; Shiganova, T.; MacIsaac, H.J.; Blasius, B. Non-native species spread in a complex network: The interaction of global transport and local population dynamics determines invasion success. Proc. R. Soc. B 2019, 286, 20190036. [Google Scholar] [CrossRef]
- Cassey, P.; Delean, S.; Lockwood, J.L.; Sadowski, J.S.; Blackburn, T.M. Dissecting the null model for biological invasions: A meta-analysis of the propagule pressure effect. PLoS Biol. 2018, 16, e2005987. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.M.; Brockerhoff, E.G.; Bertelsmeier, C.; Blake, R.E.; Caton, B.; James, A.; MacLeod, A.; Nahrung, H.F.; Pawson, S.M.; Plank, M.J.; et al. Worldwide border interceptions provide a window into human-mediated global insect movement. Ecol. Appl. 2021, 31, e02412. [Google Scholar] [CrossRef] [PubMed]
- CDFA. California Citrus Acreage Report. 2022. Available online: https://www.nass.usda.gov/Statistics_by_State/California/Publications/Specialty_and_Other_Releases/Citrus/Acreage/202208citac.pdf (accessed on 7 March 2023).
- Munger, F. A method for rearing citrus thrips in the laboratory. J. Econ. Entomol. 1942, 35, 373–375. [Google Scholar] [CrossRef]
- Hoddle, M.S. Developmental and reproductive biology of Scirtothrips perseae (Thysanoptera: Thripidae): A new avocado pest in California. Bull. Entomol. Res. 2002, 92, 279–285. [Google Scholar] [CrossRef]
- [CIMIS] California Irrigation Management Information System. California Department of Water Resources’ California Irrigation Management Information System. State of California, Sacramento, CA. Available online: http://www.cimis.water.ca.gov (accessed on 5 June 2023).
- McCalla, K.A.; Keçeci, M.; Milosavljević, I.; Ratkowsky, D.A.; Hoddle, M.S. The influence of temperature variation on life history parameters and thermal performance curves of Tamarixia radiata (Hymenoptera: Eulophidae), a parasitoid of the Asian citrus psyllid (Hemiptera: Liviidae). J. Econ. Entomol. 2019, 112, 1560–1567. [Google Scholar] [CrossRef]
- Milosavljević, I.; McCalla, K.A.; Ratkowsky, D.A.; Hoddle, M.S. Effects of constant and fluctuating temperatures on development rates and longevity of Diaphorencyrtus aligarhensis (Hymenoptera: Eulophidae). J. Econ. Entomol. 2019, 112, 1062–1072. [Google Scholar] [CrossRef]
- SAS. Statistical Analysis Software, Users’ Guide Statistics Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2013.
- Streiner, D.L. Breaking up is hard to do: The heartbreak of dichotomizing continuous data. Can. J. Psychiatry 2002, 47, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Pasta, D. Learning when to be discrete: Continuous vs. categorical predictors. SAS Glob. Forum. Pap. 2009, 248, 1–10. [Google Scholar]
- Campbell, A.; Frazer, B.D.; Gilbert, N.; Gutierrez, A.P.; Mackauer, M. Temperature requirements of some aphids and their parasites. J. Appl. Ecol. 1974, 11, 431–438. [Google Scholar] [CrossRef]
- Yin, X.; Goudriaan, J.; Lantinga, E.A.; Vos, J.; Spiertz, H.J. A flexible sigmoid function of determinate growth. Ann. Bot. 2003, 91, 361–371. [Google Scholar] [CrossRef]
- Auzanneau, J.; Huyghe, C.; Escobar-Gutiérrez, A.J.; Julier, B.; Gastal, F.; Barre, P. Association study between the gibberellic acid insensitive gene and leaf length in a Lolium perenne L. synthetic variety. BMC Plant Biol. 2011, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Reddy, J.G.V.; Chen, L.; Ge, F. Comparison of thermal performance equations in describing temperature-dependent developmental rates of insects: (I) empirical models. Ann. Entomol. Soc. Am. 2015, 109, 211–215. [Google Scholar] [CrossRef]
- Brière, J.F.; Pracros, P.; Le Roux, A.Y.; Pierre, J.S. A novel rate model of temperature-dependent development for arthropods. Environ. Entomol. 1999, 28, 22–29. [Google Scholar] [CrossRef]
- Logan, J.A.; Wolkind, D.J.; Hoyt, S.C.; Tanigoshi, L.K. An analytic model for description of temperature dependent rate phenomena in arthropods. Environ. Entomol. 1976, 5, 1133–1140. [Google Scholar] [CrossRef]
- Lactin, D.J.; Holliday, N.J.; Johnson, D.L.; Craigen, R. Improved rate model of temperature-dependent development by arthropods. Environ. Entomol. 1995, 24, 68–75. [Google Scholar] [CrossRef]
- Lobry, J.R.; Rosso, L.; Flandrois, J.P. A FORTRAN subroutine for the determination of parameter confidence limits in non-linear models. Binary 1991, 3, 86–93. [Google Scholar]
- Rosso, L.; Lobry, J.R.; Flandrois, J.P. An unexpected correlation between cardinal temperatures of microbial growth highlighted by a new model. J. Theor. Biol. 1993, 162, 447–463. [Google Scholar] [CrossRef]
- Shi, P.; Ge, F.; Sun, Y.; Chen, C. A simple model for describing the effect of temperature on insect developmental rate. J. Asia Pac. Entomol. 2011, 14, 15–20. [Google Scholar] [CrossRef]
- Wang, L.; Shi, P.; Chen, C.; Xue, F. Effect of temperature on the development of Laodelphax striatellus (Homoptera: Delphacidae). J. Econ. Entomol. 2013, 106, 107–114. [Google Scholar] [CrossRef]
- Ratkowsky, D.A.; Lowry, R.K.; McMeekin, T.A.; Stokes, A.N.; Chandler, R. Model for bacterial culture growth rate throughout the entire biokinetic temperature range. J. Bacteriol. 1983, 154, 1222–1226. [Google Scholar] [CrossRef]
- Angilletta, M.J., Jr. Estimating and comparing thermal performance curves. J. Therm. Biol. 2006, 31, 541–545. [Google Scholar] [CrossRef]
- Bollen, K.A.; Jackman, R.W. Regression diagnostics: An expository treatment of outliers and influential cases. Mod. Methods Data Anal. 1990, 13, 257–291. [Google Scholar] [CrossRef]
- Shi, P.; Ge, F. A comparison of different thermal performance functions describing temperature-dependent development rates. J. Therm. Biol. 2010, 35, 225–231. [Google Scholar] [CrossRef]
- Ratkowsky, D.A.; Reddy, G.V. Empirical model with excellent statistical properties for describing temperature-dependent developmental rates of insects and mites. Ann. Entomol. Soc. Am. 2017, 110, 302–309. [Google Scholar] [CrossRef]
- Mirhosseini, M.; Fathipour, A.Y.; Reddy, G.V. Arthropod development’s response to temperature: A review and new software for modeling. Ann. Entomol. Soc. Am. 2017, 110, 507–520. [Google Scholar] [CrossRef]
- Milosavljević, I.; McCalla, K.A.; Morgan, D.J.W.; Hoddle, M.S. The effects of constant and fluctuating temperatures on development of Diaphorina citri (Hemiptera: Liviidae), the Asian citrus psyllid. J. Econ. Entomol. 2020, 113, 633–645. [Google Scholar] [CrossRef]
- Spiess, A.N.; Neumeyer, N. An evaluation of R2 as an inadequate measure for nonlinear models in pharmacological and biochemical research: A Monte Carlo approach. BMC Pharmacol. 2010, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Ratkowsky, D.A. Model Fitting and Uncertainty. In Modeling Microbial Responses in Food; McKellar, R.C., Lu, X., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 151–196. [Google Scholar]
- SigmaPlot, Version 12.3; Systat Software, Inc.: San Jose, CA, USA, 2013.
- Rebaudo, F.; Rabhi, V.-B. Modeling temperature-dependent development rate and phenology in insects: Review of major developments, challenges, and future directions. Entomol. Exp. Appl. 2018, 166, 607–617. [Google Scholar] [CrossRef]
- Prasad, T.V.; Nandogopal, V.; Gedia, M.V. Seasonal abundance of sesbania thrips, Caliothrips indicus Bagnall (sic) in groundnut. J. Agrometerol. 2008, 10, 211–214. [Google Scholar]
- Boica, A.L.; Costa, E.N.; Chiorato, A.F. Infestation of Caliothrips phaseoli (Thysanoptera: Thripidae) on bean cultivars grown in winter, rainy, and dry seasons in Brazil. Environ. Entomol. 2015, 44, 1108–1115. [Google Scholar]
- Tobin, P.C.; Robient, C. 2022. Advances in understanding and predicting the spread of invading insect populations. Curr. Opin. Insect Sci. 2022, 54, 100985. [Google Scholar] [CrossRef]
- Jarošik, V.; Kenis, M.; Honĕk, A.; Skuhrovec, J.; Pyšek, P. Invasive insects differ from non-invasive in their thermal requirements. PLoS ONE 2015, 10, e0131072. [Google Scholar] [CrossRef] [PubMed]
- Stacey, D.A.; Fellowes, M.D.E. Temperature and development rate of thrips: Evidence for a constraint on local adaptation? Eur. J. Entomol. 2002, 99, 399–404. [Google Scholar] [CrossRef]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Ann. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, S. Two new species of Caliothrips (Thysanoptera: Thripidae) and a key to Nearctic species. J. N. Y. Entomol. Soc. 1991, 99, 97–103. [Google Scholar]
- Wang, Z.; Mound, L.; Mao, R.; Tong, X. Two new synonyms among Panchaetothripinae (Thysanoptera: Thripidae) with three species newly recorded from China. Zootaxa 2022, 5190, 275–285. [Google Scholar] [CrossRef]
- Lu, G.; Zhao, Z.; Pan, X. Potential pest invasion risk posed by international sweet cherry trade. Food Energy Secur. 2021, 10, e257. [Google Scholar] [CrossRef]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Nat. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef]
| Hour | Mean Temperature (°C) | Photoperiod | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 10 | 15 | 20 | 25 | 30 | 32 | 35 | 37 | ||
| 0100 | 4 | 7 | 11 | 14 | 20 | 25 | 26 | 33 | 30 | dark |
| 0200 | 3 | 7 | 10 | 13 | 19 | 24 | 25 | 33 | 30 | |
| 0300 | 3 | 6 | 10 | 13 | 18 | 24 | 25 | 32 | 29 | |
| 0400 | 3 | 6 | 9 | 12 | 17 | 22 | 24 | 32 | 30 | |
| 0500 | 2 | 6 | 9 | 11 | 17 | 21 | 24 | 31 | 27 | |
| 0600 | 2 | 6 | 8 | 11 | 16 | 22 | 24 | 30 | 30 | light |
| 0700 | 1 | 6 | 8 | 13 | 18 | 24 | 26 | 32 | 34 | |
| 0800 | 2 | 6 | 12 | 17 | 21 | 26 | 29 | 34 | 36 | |
| 0900 | 5 | 8 | 15 | 19 | 23 | 28 | 31 | 36 | 37 | |
| 1000 | 8 | 10 | 17 | 21 | 25 | 30 | 33 | 37 | 40 | |
| 1100 | 11 | 12 | 18 | 22 | 27 | 32 | 35 | 39 | 42 | |
| 1200 | 13 | 13 | 20 | 24 | 29 | 34 | 37 | 40 | 43 | |
| 1300 | 14 | 14 | 21 | 26 | 31 | 35 | 38 | 40 | 44 | |
| 1400 | 15 | 15 | 22 | 27 | 33 | 36 | 40 | 41 | 45 | |
| 1500 | 16 | 15 | 23 | 28 | 33 | 37 | 40 | 41 | 45 | |
| 1600 | 16 | 15 | 23 | 29 | 34 | 38 | 40 | 40 | 45 | |
| 1700 | 15 | 14 | 23 | 29 | 34 | 38 | 40 | 40 | 43 | |
| 1800 | 13 | 13 | 21 | 28 | 33 | 37 | 39 | 38 | 42 | |
| 1900 | 11 | 11 | 18 | 25 | 30 | 36 | 37 | 36 | 40 | |
| 2000 | 9 | 10 | 16 | 23 | 27 | 33 | 34 | 33 | 37 | dark |
| 2100 | 8 | 10 | 14 | 21 | 26 | 31 | 32 | 32 | 35 | |
| 2200 | 7 | 9 | 12 | 19 | 24 | 29 | 31 | 31 | 35 | |
| 2300 | 6 | 9 | 11 | 18 | 23 | 27 | 30 | 30 | 35 | |
| 2400 | 6 | 8 | 11 | 16 | 21 | 26 | 28 | 29 | 34 | |
| Total Steps | 19 | 14 | 17 | 21 | 21 | 22 | 18 | 19 | 19 | |
| (A) Eggs (Development) | Num df | Den df | F | p |
| Sex (S) | 1 | 168 | 0.84 | 0.3612 |
| Temperature (T) | 7 | 168 | 187.21 | <0.0001 * |
| S × T | 7 | 168 | 0.31 | 0.9541 |
| (B) 1st Instar Larvae (Development) | Num df | Den df | F | p |
| Sex (S) | 1 | 168 | 0.17 | 0.6795 |
| Temperature (T) | 7 | 168 | 2.54 | 0.0164 * |
| S × T | 7 | 168 | 0.08 | 0.9992 |
| (C) 2nd Instar Larvae (Development) | Num df | Den df | F | p |
| Sex (S) | 1 | 168 | 0.01 | 0.9343 |
| Temperature (T) | 7 | 168 | 102.96 | <0.0001 * |
| S × T | 7 | 168 | 0.16 | 0.9922 |
| (D) Propupae (Development) | Num df | Den df | F | p |
| Sex (S) | 1 | 168 | 0.01 | 0.9152 |
| Temperature (T) | 7 | 168 | 59.36 | <0.0001 * |
| S × T | 7 | 168 | 0.48 | 0.8511 |
| (E) Pupae (Development) | Num df | Den df | F | p |
| Gender (S) | 1 | 168 | 0.01 | 0.9251 |
| Temperature (T) | 7 | 168 | 106.53 | <0.0001 * |
| S × T | 7 | 168 | 0.29 | 0.9557 |
| (F) Egg-to-Adult (Development) | Num df | Den df | F | p |
| Sex (G) | 1 | 168 | 0.31 | 0.5831 |
| Temperature (T) | 7 | 168 | 408.51 | <0.0001 * |
| S × T | 7 | 168 | 0.35 | 0.9288 |
| (G) Adult (Longevity) | Num df | Den df | F | p |
| Sex (S) | 1 | 168 | 0.04 | 0.8501 |
| Temperature (T) | 7 | 168 | 14.16 | <0.0001 * |
| S × T | 7 | 168 | 0.24 | 0.9746 |
| Temp. (°C) | Mean Development Times (Mean Days ± SE) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eggs | 1st Instar Larvae | 2nd Instar Larvae | Propupae | Pupae | Egg-to-Adult | Adults | ||||||||
| Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | |
| 8 | 55.33 ± 9.68 a (3) | 47.33 ± 10.34 a (3) | 2.67 ± 0.33 a (3) | 2.33 ± 0.33 a (3) | 30.33 ± 2.73 a (3) | 28.67 ± 2.69 a (3) | 9.33 ± 2.85 a (3) | 13.67 ± 3.53 a (3) | 28.33 ± 2.61 a (3) | 29.67 ± 2.67 a (3) | 125.33 ± 12.66 a (3) | 120.67 ± 14.31 a (3) | 2.67 ± 1.67 d (3) | 1.67 ± 0.67 d (3) |
| 10 | 52.57 ± 1.58 a (7) | 48.25 ± 1.44 a (4) | 2.28 ± 0.36 a (7) | 2.21 ± 0.18 a (4) | 21.29 ± 2.43 b (7) | 19.5 ± 3.95 b (4) | 5.43 ± 0.57 b (7) | 4.25 ± 0.85 b (4) | 10.57 ± 1.79 b (7) | 8.01 ± 1.58 b (4) | 92.14 ± 7.17 b (7) | 82.75 ± 10.41 b (4) | 28.14 ± 8.57 a (7) | 25.75 ± 5.98 a (4) |
| 15 | 23.92 ± 0.29 b (11) | 23.62 ± 0.67 b (10) | 2.25 ± 0.29 a (11) | 2.29 ± 0.27 a (10) | 7.42 ± 0.49 c (11) | 7.41 ± 0.87 c (10) | 11.25 ± 0.85 a (11) | 11.21 ± 1.12 a (10) | 9.23 ± 0.79 b (11) | 9.39 ± 0.93 b (10) | 53.17 ± 1.31 c (11) | 53.21 ± 2.11 c (10) | 23.58 ± 3.96 a (11) | 20.81 ± 3.71 a (10) |
| 20 | 16.23 ± 0.28 c (22) | 15.72 ± 0.31 c (14) | 1.36 ± 0.12 b (22) | 1.37 ± 0.11 b (14) | 4.65 ± 0.28 d (22) | 4.72 ± 0.41 d (14) | 1.63 ± 0.15 c (22) | 1.71 ± 0.17 c (14) | 3.68 ± 0.24 c (22) | 3.79 ± 0.35 c (14) | 27.54 ± 0.39 d (22) | 27.28 ± 0.51 d (14) | 13.18 ± 3.67 b (22) | 12.57 ± 4.04 b (14) |
| 25 | 11.13 ± 0.46 d (20) | 11.35 ± 0.85 d (16) | 1.33 ± 0.18 b (20) | 1.32 ± 0.42 b (16) | 3.89 ± 0.28 d (20) | 3.59 ± 0.24 d (16) | 1.45 ± 0.12 c (20) | 1.23 ± 0.1 c (16) | 2.09 ± 0.23 de (20) | 2.32 ± 0.33 de (16) | 20.19 ± 0.52 e (20) | 20.23 ± 0.79 e (16) | 9.12 ± 2.97 b (20) | 9.35 ± 1.96 b (16) |
| 30 | 7.09 ± 0.36 e (12) | 6.98 ± 0.45 e (11) | 1.28 ± 0.14 b (12) | 1.29 ± 0.15 b (11) | 2.36 ± 0.24 e (12) | 2.29 ± 0.21 e (11) | 1.25 ± 0.15 c (12) | 1.29 ± 0.16 c (11) | 1.25 ± 0.13 e (12) | 1.24 ± 0.14 e (11) | 14.25 ± 0.61 f (12) | 14.46 ± 0.83 f (11) | 5.08 ± 0.75 c (12) | 4.91 ± 0.86 c (11) |
| 32 | 7.82 ± 0.69 e (11) | 8.11 ± 0.75 e (10) | 1.17 ± 0.17 b (11) | 1.21 ± 0.23 b (10) | 2.09 ± 0.31 e (11) | 2.11 ± 0.45 e (10) | 1.21 ± 0.21 c (11) | 1.21 ± 0.22 c (10) | 1.75 ± 0.27 e (11) | 1.76 ± 0.35 e (10) | 14.73 ± 0.72 f (11) | 15.01 ± 0.92 f (10) | 3.73 ± 1.05 cd (11) | 3.82 ±1.73 cd (10) |
| 35 | 10.71 ± 0.58 d (20) | 10.03 ± 0.91 d (10) | 1.38 ± 0.23 b (20) | 1.31 ± 0.27 b (10) | 3.08 ± 0.32 d (20) | 3.32 ± 0.49 d (10) | 3.45 ± 0.39 b (20) | 3.4 ± 0.45 b (10) | 2.92 ± 0.32 cd (20) | 3.16 ± 0.57 cd (10) | 20.55 ± 0.39 e (20) | 20.51 ± 0.82 e (10) | 2.51 ± 0.41 d (20) | 2.23 ± 0.49 d (10) |
| p | <0.0001 | 0.0164 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| Temperature (°C) | Mean Lifetime Fecundity * of G1 Females and Sex of Progeny | ||
|---|---|---|---|
| Mean No. of G2 Larvae ± SE | Mean No. Adult G2 Thrips ± SE | ||
| Female | Male | ||
| 8 | 1.33 ± 0.88 d [3] {4} | 0.67 ± 0.33 [3] (2) | 0.67 ± 0.33 [3] (2) |
| 10 | 16.75 ± 8.52 bc [4] {67} | 11.75 ± 5.52 [4] (47) | 4.51 ± 2.12 [4] (18) |
| 15 | 24.89 ± 9.57 bc [11] {273} | 12.09 ± 4.57 [11] (122) | 12.54 ± 4.03 [11] (138) |
| 20 | 39.72 ± 11.76 ab [14] {556} | 20.71 ± 3.76 [14] (290) | 18.53 ± 6.14 [14] (259) |
| 25 | 50.82 ± 8.24 a [11] {559} | 27.91 ± 4.24 [11] (307) | 22.45 ± 2.57 [11] (247) |
| 30 | 19.25 ± 5.09 bc [12] {231} | 7.53 ± 3.09 [12] (143) | 4.53 ± 1.35 [12] (86) |
| 32 | 15.73 ± 7.12 bc [11] {173} | 9.68 ± 4.12 [11] (107) | 3.27 ± 1.99 [11] (36) |
| 35 | 13.53 ± 6.14 c [15] {203} | 8.23 ± 2.14 [15] (123) | 5.27 ± 1.09 [15] (78) |
| G2 Egg-to-Adult Development Times (Mean Days ± SE) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 8 °C | 10 °C | 15 °C | 20 °C | 25 °C | 30 °C | 32 °C | 35 °C | |
| Males | 107.50 ± 8.50 a (2) | 78.44 ± 7.82 b (18) | 47.82 ± 0.76 c (138) | 27.39 ± 0.19 d (259) | 19.81 ± 0.12 e (247) | 14.24 ± 0.35 e (86) | 14.72 ± 0.64 e (36) | 23.28 ± 0.21 d (78) |
| Females | 110.00 ± 0.50 a (2) | 74.77 ± 7.11 b (47) | 45.34 ± 0.43 c (122) | 26.79 ± 0.19 d (290) | 19.39 ± 0.31 e (307) | 14.51 ± 0.17 e (143) | 15.19 ± 0.23 e (112) | 23.41 ± 0.13 d (123) |
| Model | Model Equation † | Parameter | Parameter Estimate | References | |
|---|---|---|---|---|---|
| G1 Thrips | G2 Thrips | ||||
| Ordinary Linear ‡ | a | −0.0176 | −0.0139 | [28] | |
| b | 0.0027 | 0.0026 | |||
| K (degree days) ‡ | 370.37 | 384.61 | |||
| Tmin ‡ | 6.52 | 5.35 | |||
| R2adj | 0.9813 | 0.9849 | |||
| Lactin-2 (Logan-Lactin) | λ | −1.0161 | −1.0179 | [33,34] | |
| ρ | 0.0026 | 0.0027 | |||
| δ | 1.1561 | 1.3782 | |||
| Tmin * | 6.52 | 6.28 | |||
| Tu (Tmax *) | 36.14 | 37.63 | |||
| Topt * | 32.51 | 31.79 | |||
| RSS | 0.000016 | 0.000011 | |||
| Brière-2 | a | 0.000054 | 0.000051 | [32] | |
| b | 6.4394 | 5.8481 | |||
| Tmin | −1.37 | −2.74 | |||
| Tmax | 35.12 | 35.07 | |||
| Topt * | 32.52 | 31.92 | |||
| RSS | 0.000008 | 0.000002 | |||
| Lobry-Rosso-Flandrois (LRF) | μopt | 0.0689 | 0.0685 | [35,36] | |
| Tmin | −1.48 | −2.49 | |||
| Tmax | 36.13 | 35.92 | |||
| Topt | 31.89 | 31.59 | |||
| RSS | 0.000006 | 0.000005 | |||
| Performance-2 | Tmin | 6.52 | 6.23 | [37,38] | |
| Tmax | 36.09 | 36.05 | |||
| Topt * | 32.39 | 31.89 | |||
| b | 0.0027 | 0.0028 | |||
| c | 0.8807 | 0.7188 | |||
| RSS | 0.000014 | 0.000012 | |||
| Ratkowsky | b | 0.0083 | 0.0081 | [39] | |
| c | 0.4112 | 0.4417 | |||
| Tmin | −4.37 | −3.77 | |||
| Tmax | 37.98 | 37.49 | |||
| Topt * | 31.34 | 31.19 | |||
| RSS | 0.000005 | 0.000003 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoddle, M.S.; Milosavljević, I.; Amrich, R. Effects of Temperature on the Developmental and Reproductive Biology of North American Bean Thrips, Caliothrips fasciatus (Pergande) (Thysanoptera: Thripidae: Panchaetothripinae). Insects 2023, 14, 641. https://doi.org/10.3390/insects14070641
Hoddle MS, Milosavljević I, Amrich R. Effects of Temperature on the Developmental and Reproductive Biology of North American Bean Thrips, Caliothrips fasciatus (Pergande) (Thysanoptera: Thripidae: Panchaetothripinae). Insects. 2023; 14(7):641. https://doi.org/10.3390/insects14070641
Chicago/Turabian StyleHoddle, Mark S., Ivan Milosavljević, and Ruth Amrich. 2023. "Effects of Temperature on the Developmental and Reproductive Biology of North American Bean Thrips, Caliothrips fasciatus (Pergande) (Thysanoptera: Thripidae: Panchaetothripinae)" Insects 14, no. 7: 641. https://doi.org/10.3390/insects14070641
APA StyleHoddle, M. S., Milosavljević, I., & Amrich, R. (2023). Effects of Temperature on the Developmental and Reproductive Biology of North American Bean Thrips, Caliothrips fasciatus (Pergande) (Thysanoptera: Thripidae: Panchaetothripinae). Insects, 14(7), 641. https://doi.org/10.3390/insects14070641






