The Spatiotemporal Distribution, Abundance, and Seasonal Dynamics of Cotton-Infesting Aphids in the Southern U.S.
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Aphid Monitoring
2.2. Statistical Analyses
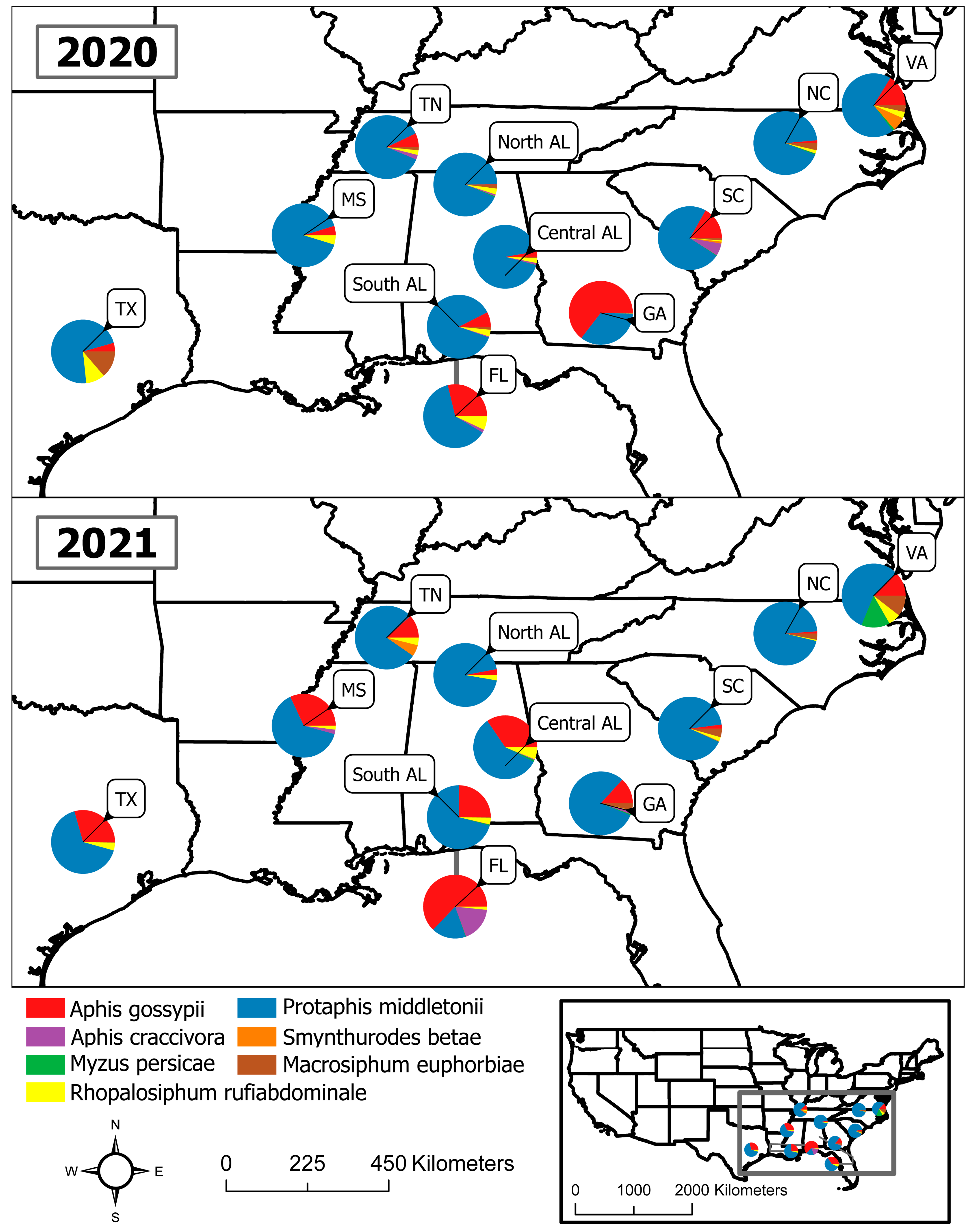
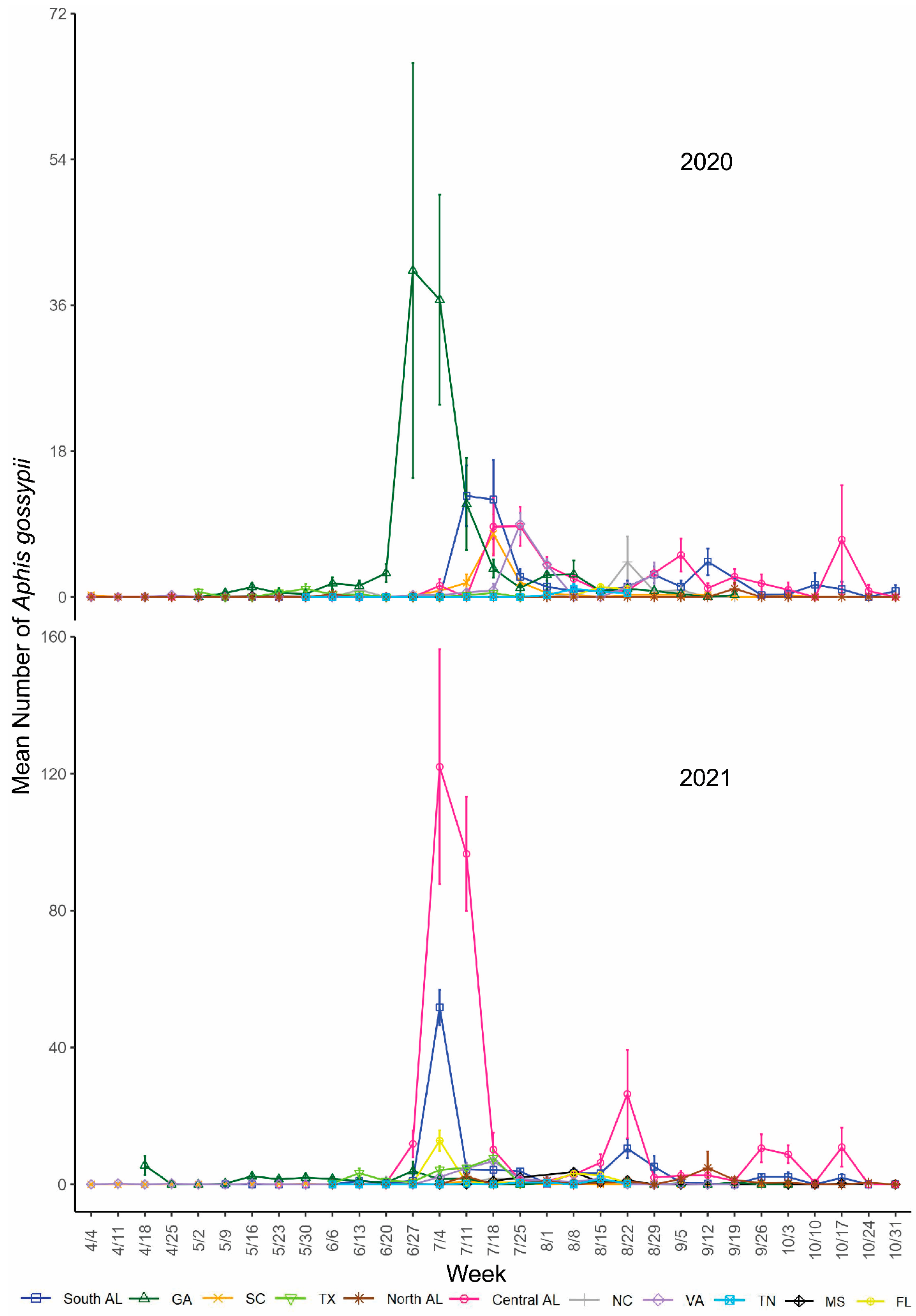
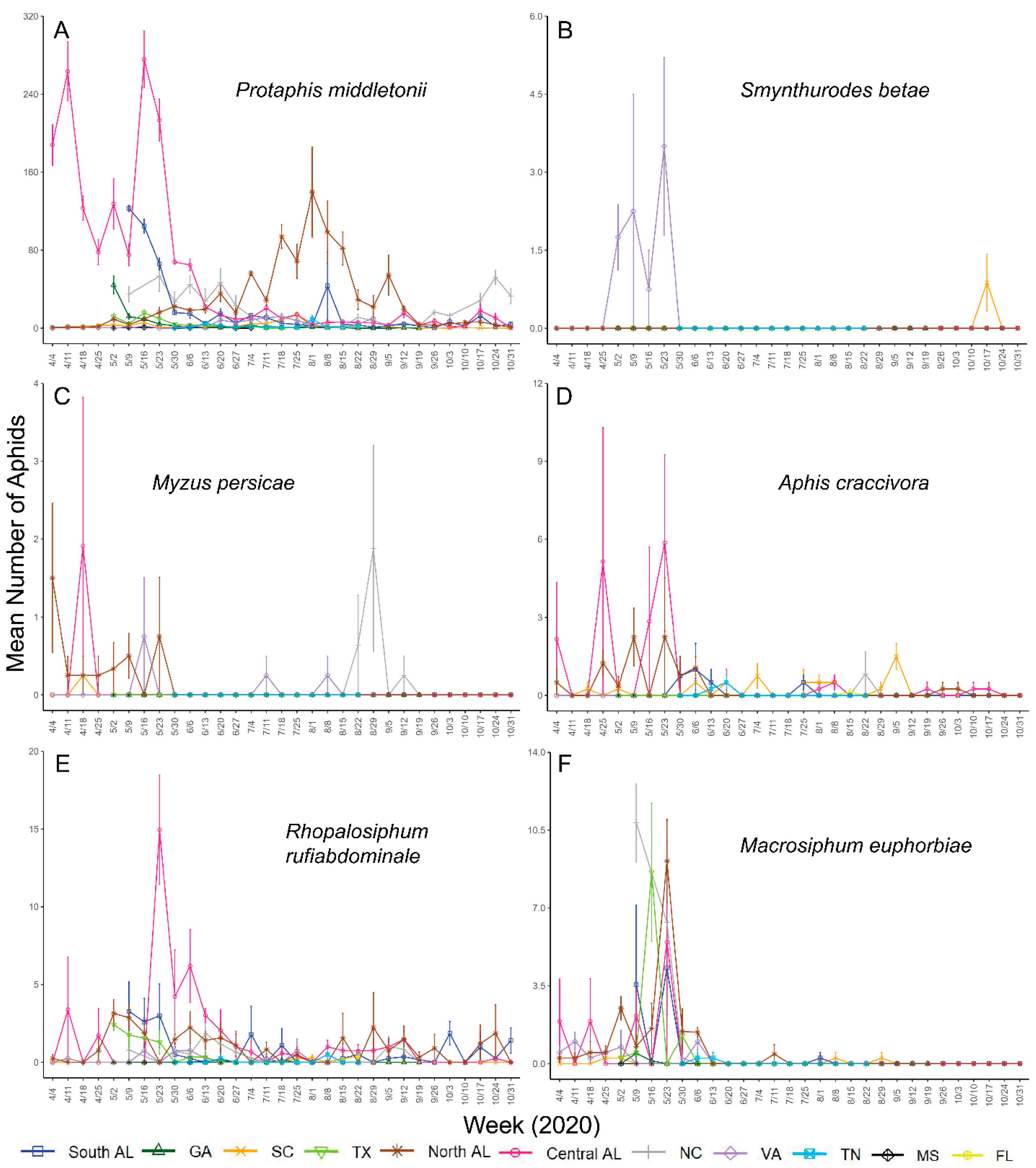
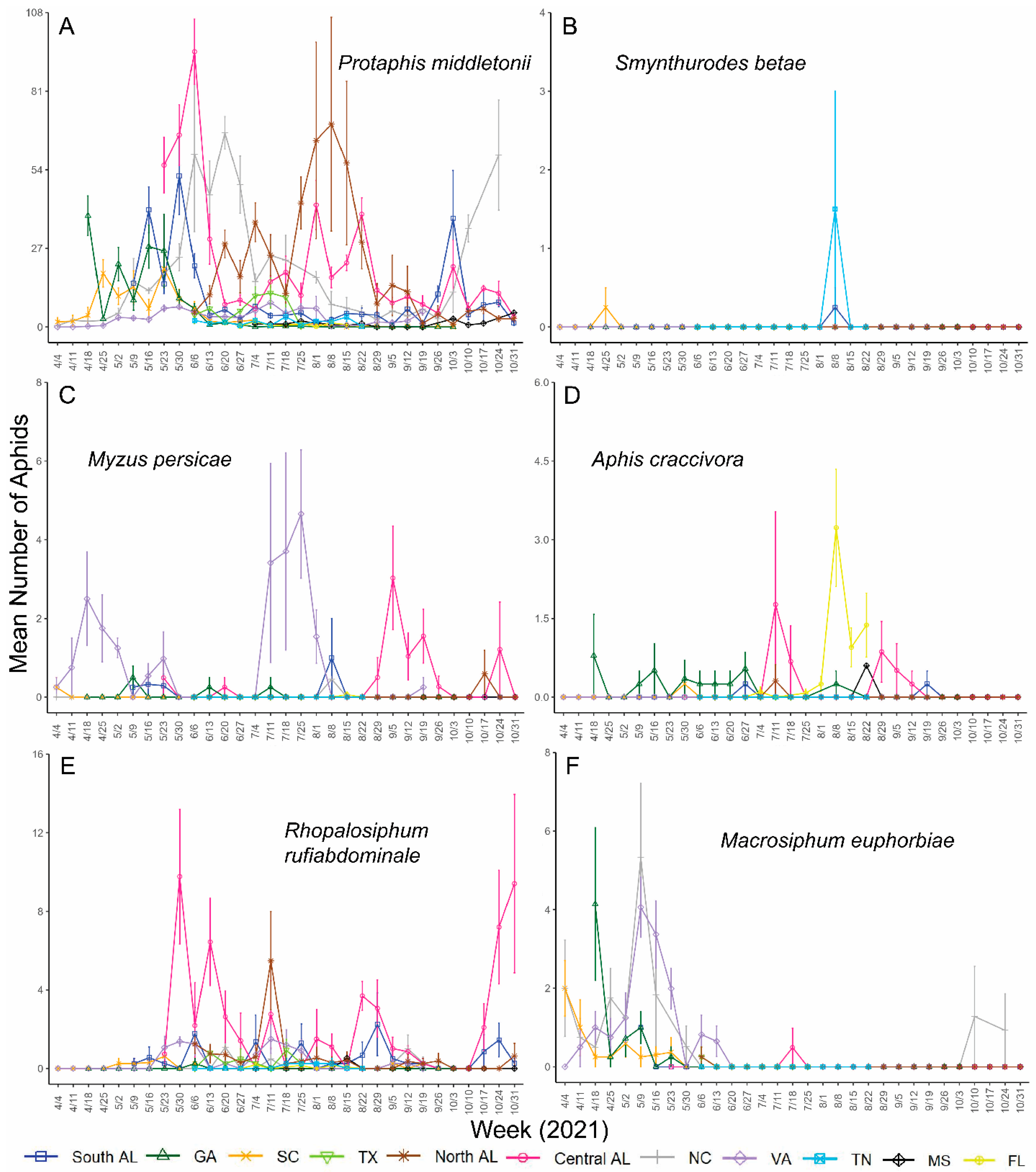
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Claflin, S.B.; Jones, L.E.; Thaler, J.S.; Power, A.G. Crop-dominated landscapes have higher vector-borne plant virus prevalence. J. Appl. Ecol. 2017, 54, 1190–1198. [Google Scholar] [CrossRef]
- Claflin, S.B.; Hernandez, N.; Groves, R.; Thaler, J.S.; Power, A.G. Intra-annual variation and landscape composition interactively affect aphid community composition. Ecosphere 2019, 10, e02710. [Google Scholar] [CrossRef]
- Carrière, Y.; Degain, B.; Hartfield, K.A.; Nolte, K.D.; Marsh, S.E.; Ellers-Kirk, C.; Leeuwen, W.J.D.V.; Liesner, L.; Dutilleul, P.; Palumbo, J.C. Assessing transmission of crop diseases by insect vectors in a landscape context. J. Econ. Entomol. 2014, 107, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Orta, G.; Albajes, R.; Achon, M.A. Early planting, management of edges and non-crop habitats reduce potyvirus infection in maize. Agron. Sustain. Dev. 2020, 40, 21. [Google Scholar] [CrossRef]
- Angelella, G.M.; Holland, J.D.; Kaplan, I. Landscape composition is more important than local management for crop virus–insect vector interactions. Agric. Ecosyst. Environ. 2016, 233, 253–261. [Google Scholar] [CrossRef]
- Hadi, B.A.R.; Flanders, K.L.; Bowen, K.I.; Murphy, J.F.; Halbert, S.E. Species composition of aphid vectors (Hemiptera: Aphididae) of barley yellow dwarf virus and cereal yellow dwarf virus in Alabama and Western Florida. J. Econ. Entomol. 2011, 104, 1167–1173. [Google Scholar] [CrossRef]
- Wallis, C.M.; Fleischer, S.J.; Luster, D.; Gildow, F.E. Aphid (Hemiptera: Aphididae) species composition and potential aphid vectors of plum pox virus in Pennsylvania peach orchards. J. Econ. Entomol. 2005, 98, 1441–1450. [Google Scholar] [CrossRef]
- Groves, R.L.; Walgenbach, J.F.; Moyer, J.W.; Kennedy, G.G. The role of weed hosts and tobacco thrips, Frankliniella fusca, in the epidemiology of tomato spotted wilt virus. Plant Dis. 2002, 86, 573–582. [Google Scholar] [CrossRef]
- Groves, R.L.; Walgenbach, J.F.; Moyer, J.W.; Kennedy, G.G. Seasonal dispersal patterns of Frankliniella fusca (Thysanoptera: Thripidae) and tomato spotted wilt virus occurrence in central and eastern North Carolina. J. Econ. Entomol. 2003, 96, 1–11. [Google Scholar] [CrossRef]
- Hsu, C.L.; Hoepting, C.A.; Fuchs, M.; Shelton, A.M.; Nault, B.A. Temporal dynamics of iris yellow spot virus and its vector, thrips tabaci (Thysanoptera: Thripidae), in seeded and transplanted onion fields. Environ. Entomol. 2010, 39, 266–277. [Google Scholar] [CrossRef]
- Jeger, M.J.; Holt, J.; Van Den Bosch, F.; Madden, L.V. Epidemiology of insect-transmitted plant viruses: Modelling disease dynamics and control interventions. Physiol. Entomol. 2004, 29, 291–304. [Google Scholar] [CrossRef]
- Chitturi, A.; Conner, K.; Sikora, E.J.; Jacobson, A.L. Monitoring seasonal distribution of thrips vectors of soybean vein necrosis virus in Alabama soybeans. J. Econ. Entomol. 2018, 111, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Swenson, K.G. Role of aphids in the ecology of plant viruses. Annu. Rev. Phytopathol. 1968, 6, 351–374. [Google Scholar] [CrossRef]
- Irwin, M.E.; Thresh, J.M. Epidemiology of barley yellow dwarf: A study in ecological complexity. Annu. Rev. Phytopathol. 1990, 28, 393–424. [Google Scholar] [CrossRef]
- Silva, T.F.; Corrêa, R.L.; Castilho, Y.; Silvie, P.; Bélot, J.L.; Vaslin, M.F.S. Widespread distribution and a new recombinant species of Brazilian virus associated with cotton blue disease. Virol. J. 2008, 5, 123. [Google Scholar] [CrossRef]
- Sharman, M.; Lapbanjob, S.; Sebunruang, P.; Belot, J.L.; Galbieri, R.; Giband, M.; Suassuna, N. First report of cotton leafroll dwarf virus in Thailand using a species-specific PCR validated with isolates from Brazil. Australas. Plant Dis. Notes 2015, 10, 3513–3516. [Google Scholar] [CrossRef]
- Corrêa, R.L.; Silva, T.F.; Simões-Araújo, J.L.; Barroso, P.A.V.; Vidal, M.S.; Vaslin, M.F.S. Molecular characterization of a virus from the family Luteoviridae associated with cotton blue disease. Arch. Virol. 2005, 150, 1357–1367. [Google Scholar] [CrossRef]
- Cauquil, J.; Vaissayre, M. La “maladie bleue” du cotonnier en Afrique: Transmission de cotonnier à cotonnier par Aphis gossypii Glover. Cot. Fib. Trop. 1971, 26, 463–466. [Google Scholar]
- Ray, J.D.; Sharman, M.; Quintao, V.; Rossel, B.; Westaway, J.; Gambley, C. Cotton leafroll dwarf virus detected in Timor-Leste. Australas. Plant Dis. Notes 2016, 11, 5–7. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Mukherjee, P.K.; Kranthi, S. Genetic similarity between cotton leafroll dwarf virus and chickpea stunt disease associated virus in India. Plant Pathol. J. 2016, 32, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Agrofoglio, Y.C.; Delfosse, V.C.; Casse, M.F.; Hopp, H.E.; Kresic, I.B.; Distéfano, A.J. Identification of a new cotton disease caused by an atypical cotton leafroll dwarf virus in Argentina. Phytopathology 2017, 107, 369–376. [Google Scholar] [CrossRef]
- Aboughanem-Sabanadzovic, N.; Allen, T.W.; Wilkerson, T.H.; Conner, K.N.; Sikora, E.J.; Nichols, R.L.; Sabanadzovic, S. First report of cotton leafroll dwarf virus in upland cotton (Gossypium hirsutum L.) in Mississippi. Plant Dis. 2019, 103, 1798. [Google Scholar] [CrossRef]
- Alabi, O.J.; Isakeit, T.; Vaughn, R.; Stelly, D.; Conner, K.N.; Gaytán, B.C.; Villegas, C.; Hitzelberger, C.; De Santiago, L.; Monclova-Santana, C.; et al. First report of cotton leafroll dwarf virus infecting upland cotton (Gossypium hirsutum) in Texas. Plant Dis. 2020, 104, 998. [Google Scholar] [CrossRef]
- Iriarte, F.B.; Dey, K.K.; Small, I.M.; Conner, K.N.; O’Brien, G.K.; Johnson, L.; Savery, C.; Carter, E.; Sprague, D.; Nichols, R.L.; et al. First report of cotton leafroll dwarf virus in Florida. Plant Dis. 2020, 104, 2744. [Google Scholar] [CrossRef]
- Price, T.; Valverde, R.; Singh, R.; Davis, J.; Brown, S.; Jones, H. First report of cotton leafroll dwarf virus in Louisiana. Plant Health Prog. 2020, 21, 142–143. [Google Scholar] [CrossRef]
- Tabassum, A.; Bag, S.; Roberts, P.; Suassuna, N.; Chee, P.; Whitaker, J.R.; Conner, K.N.; Brown, J.; Nichols, R.L.; Kemerait, R.C. First report of cotton leafroll dwarf virus infecting cotton in Georgia, USA. Plant Dis. 2019, 103, 1803. [Google Scholar] [CrossRef]
- Thiessen, L.D.; Schappe, T.; Zaccaron, M.; Conner, K.; Koebernick, J.; Jacobson, A.; Huseth, A. First report of cotton leafroll dwarf virus in cotton plants affected by cotton leafroll dwarf disease in North Carolina. Plant Dis. 2020, 104, 3275. [Google Scholar] [CrossRef]
- Wang, H.; Greene, J.; Mueller, J.; Conner, K.; Jacobson, A. First report of cotton leafroll dwarf virus in cotton fields of South Carolina. Plant Dis. 2020, 104, 2532. [Google Scholar] [CrossRef]
- Ali, A.; Mokhtari, S. First report of cotton leafroll dwarf virus infecting cotton (Gossypium hirsutum L.) in Kansas. Plant Dis. 2020, 104, 1080. [Google Scholar] [CrossRef]
- Faske, T.R.; Stainton, D.; Aboughanem-Sabanadzovic, N.; Allen, T.W. First report of cotton leafroll dwarf virus from upland cotton (Gossypium hirsutum) in Arkansas. Plant Dis. 2020, 104, 2742. [Google Scholar] [CrossRef]
- Ali, A.; Mokhtari, S.; Ferguson, C. First report of cotton leafroll dwarf virus from cotton (Gossypium hirsutum) in Oklahoma. Plant Dis. 2020, 104, 2531. [Google Scholar] [CrossRef]
- Brown, S.; Conner, K.; Hagan, A.; Jacobson, A.; Koebernick, J.; Lawrence, K.; Bag, S.; Kemerait, B.; Peng, C.; Allen, T.; et al. Report of a Researh Review and Planning Meeting on Cotton Leafroll Dwarf Virus; Cotton Incorporated: Orange Beach, AL, USA, 2019; Volume 104, p. 1880. [Google Scholar]
- Zaccaron, M.; Koebernick, J.; Roark, D.; Taylor, S.; Huseth, A.S.; Conner, K.; Strayer-Scherer, A.; Hagan, A.; Bowen, K.; Greene, J.; et al. Results from the 2021 CLRDV sentinel plots in AL, FL, GA, SC, NC, VA, MS, AR, LA, TN, and TX and resistance screening efforts at auburn university. In Proceedings of the 2022 Beltwide Cotton Conferences, San Antonio, TX, USA, 4–6 January 2022; pp. 342–343. [Google Scholar]
- Avelar, S.; Schrimsher, D.W.; Lawrence, K.; Brown, J.K. First report of cotton leafroll dwarf virus associated with cotton blue disease symptoms in Alabama. Plant Dis. 2019, 103, 592. [Google Scholar] [CrossRef]
- Mahas, J.W.; Fredericka, H.B.; Roberts, P.M.; Ray, C.H.; Miller, G.L.; Sharman, M.; Conner, K.; Kesheimer, A.; Bag, S.; Blythe, E.K.; et al. Investigating the effects of planting date and Aphis gossypii management on reducing the final incidence of cotton leafroll dwarf virus. Crop Prot. 2022, 158, 106005. [Google Scholar] [CrossRef]
- Sedhain, N.P.; Bag, S.; Morgan, K.; Carter, R.; Triana, P.; Whitaker, J.; Kemerait, R.C.; Roberts, P.M. Natural host range, incidence on overwintering cotton and diversity of cotton leafroll dwarf virus in Georgia USA. Crop Prot. 2021, 144, 105604. [Google Scholar] [CrossRef]
- Pandey, S.; Bag, S.; Roberts, P.; Conner, K.; Balkcom, K.S.; Price, A.J.; Jacobson, A.L.; Srinivasan, R. Prospective alternate hosts of an emerging polerovirus in cotton landscapes in the southeastern United States. Viruses 2022, 14, 2249. [Google Scholar] [CrossRef] [PubMed]
- Chappell, T.M.; Beaudoin, A.L.P.; Kennedy, G.G. Interacting virus abundance and transmission intensity underlie tomato spotted wilt virus incidence: An example weather-based model for cultivated tobacco. PLoS ONE 2013, 8, e73321. [Google Scholar] [CrossRef]
- Stoetzel, M.B.; Miller, G.L.; O’Brien, P.J.; Graves, J.B. Aphids (Homoptera: Aphididae) colonizing cotton in the United States. Fla. Entomol. 1996, 79, 193. [Google Scholar] [CrossRef]
- Heilsnis, B.; McLaughlin, A.; Conner, K.; Koebernick, J.; Jacobson, A.L. Vector competency of Aphis gossypii and Bemisia tabaci to transmit cotton leafroll dwarf virus. J. Cotton Sci. 2022, 26, 23–30. [Google Scholar] [CrossRef]
- Michelotto, M.D.; Busoli, A.C. Caracterização da transmissão do vírus do mosaico-das-nervuras do algodoeiro pelo pulgão Aphis gossypii com relação à persistência e ao tempo necessário para inoculação. Bragantia 2007, 66, 441–447. [Google Scholar] [CrossRef]
- Michelotto, M.D.; Busoli, A.C. Eficiência de ninfas e adultos de Aphis gossypii glov. Na transmissão do vírus do mosaico das nervuras do algodoeiro. Bragantia 2003, 62, 255–259. [Google Scholar] [CrossRef]
- Heilsnis, B.; Mahas, J.B.; Conner, K.; Pandey, S.; Clark, W.; Koebernick, J.; Srinivasan, R.; Martin, K.; Jacobson, A.L. Characterizing the vector competence of Aphis gossypii, Myzus persicae and Aphis craccivora (Hemiptera: Aphididae) to transmit cotton leafroll dwarf virus to cotton in the United States. J. Econ. Entomol. 2023, 116, 719–725. [Google Scholar] [CrossRef]
- Weathersbee, A.A.I.I.; Hardee, D.D.; Meredith Jnr, W.R. Effects of cotton genotype on seasonal abundance of cotton aphid (Homoptera: Aphididae). J. Agric. Entomol. 1994, 11, 29–37. [Google Scholar]
- Abney, M.R.; Ruberson, J.R.; Herzog, G.A.; Kring, T.J.; Steinkraus, D.C.; Roberts, P.M. Rise and fall of cotton aphid (Hemiptera: Aphididae) populations in southeastern cotton production systems. J. Econ. Entomol. 2008, 101, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Marti, O.G.; Olson, D.M. Neozygites fresenii (Entomophthorales: Neozygitaceae) prevalence in Aphis gossypii (Homoptera: Aphididae) in three south central Georgia cotton fields. J. Econ. Entomol. 2006, 41, 22–32. [Google Scholar] [CrossRef]
- Marti, O.G.; Olson, D.M. Effect of tillage on cotton aphids (Homoptera: Aphididae), pathogenic fungi, and predators in south central Georgia cotton fields. J. Entomol. Sci. 2007, 42, 354–367. [Google Scholar] [CrossRef]
- Sanchez-Peña, S.R. Entomogenous fungi associated with the cotton aphid in the Texas high plains. Southwest. Entomol. 1993, 18, 69–71. [Google Scholar]
- Chan, C.K.; Forbes, A.R.; Raworth, D.A. Aphid-transmitted viruses and their vectors of the world. Agric. Can. Res. Branch Tech. Bull. 1991, 216, 2–90. [Google Scholar]
- Reddy, S.V.; Lava Kumar, P. Transmission and properties of a new luteovirus associated with chickpea stunt disease in India. Curr. Sci. 2004, 86, 1157–1161. [Google Scholar]
- Morsello, S.C.; Groves, R.L.; Nault, B.A.; Kennedy, G.G. Temperature and precipitation affect seasonal patterns of dispersing tobacco thrips, Frankliniella fusca, and onion thrips, Thrips tabaci (Thysanoptera: Thripidae) caught on sticky traps. Environ. Entomol. 2008, 37, 79–86. [Google Scholar] [CrossRef]
- Mahas, J.B.; Ray, C.; Kesheimer, A.; Conner, K.; Jacobson, A.L. Seasonal dynamics of aphid flights and cotton leafroll dwarf virus spread in Alabama. Insects 2023, 14, 604. [Google Scholar] [CrossRef]
- Stewart, S.D. Observations about some unusual cotton pests in Tennessee during 2003. In Proceedings of the 2004 Beltwide Cotton Conferences, San Antonio, TX, USA, 5–9 January 2004; pp. 1564–1567. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide, 2nd ed.; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Taylor, L.R. Assessing and interpreting the spatial distributions of insect populations. Annu. Rev. Entomol. 1984, 29, 321–357. [Google Scholar] [CrossRef]
- Hutchins, L.W. The bases for temperature zonation in geographical distribution. Ecol. Soc. Am. 1947, 17, 325–335. [Google Scholar] [CrossRef]
- Smith, J.R.; Letten, A.D.; Ke, P.J.; Anderson, C.B.; Hendershot, J.N.; Dhami, M.K.; Dlott, G.A.; Grainger, T.N.; Howard, M.E.; Morrison, B.M.L.; et al. A global test of ecoregions. Nat. Ecol. Evol. 2018, 2, 1889–1896. [Google Scholar] [CrossRef]
- Simon, J.C.; Rispe, C.; Sunnucks, P. Ecology and evolution of sex in aphids. Trends Ecol. Evol. 2002, 17, 34–39. [Google Scholar] [CrossRef]
- Campbell, A.; Frazer, B.D.; Gilbert, N.; Gutierrez, A.P.; Mackauer, M. Temperature requirements of some aphids and their parasites. Br. Ecol. Soc. 1974, 11, 431–438. [Google Scholar] [CrossRef]
- Pons, X.; Comas, J.; Albajes, R. Overwintering of cereal aphids (Homoptera: Aphididae) on durum wheat in a Mediterranean climate. Environ. Entomol. 1993, 22, 381–387. [Google Scholar] [CrossRef]
- Bell, J.R.; Alderson, L.; Izera, D.; Kruger, T.; Parker, S.; Pickup, J.; Shortall, C.R.; Taylor, M.S.; Verrier, P.; Harrington, R. Long-term phenological trends, species accumulation rates, aphid traits and climate: Five decades of change in migrating aphids. J. Anim. Ecol. 2015, 84, 21–34. [Google Scholar] [CrossRef]
- Hullé, M.; Coeur d’Acier, A.; Bankhead-Dronnet, S.; Harrington, R. Aphids in the face of global changes. Comptes Rendus—Biol. 2010, 333, 497–503. [Google Scholar] [CrossRef]
- McPherson, R.M.; Brann, D.E. Seasonal abundance and species composition of aphids (Homoptera: Aphididae) in Virginia small grains. J. Econ. Entomol. 1983, 76, 272–274. [Google Scholar] [CrossRef]
- Klein, M.L.; Rondon, S.I.; Walenta, D.L.; Zeb, Q.; Murphy, A.F. Spatial and temporal dynamics of aphids (Hemiptera: Aphididae) in the Columbia Basin and Northeastern Oregon. J. Econ. Entomol. 2017, 110, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Leslie, T.W.; Van Der Werf, W.; Bianchi, F.J.J.A.; Honěk, A. Population dynamics of cereal aphids: Influence of a shared predator and weather. Agric. For. Entomol. 2009, 11, 73–82. [Google Scholar] [CrossRef]
- Andrade, T.O.; Outreman, Y.; Krespi, L.; Plantegenest, M.; Vialatte, A.; Gauffre, B.; Van Baaren, J. Spatiotemporal variations in aphid-parasitoid relative abundance patterns and food webs in agricultural ecosystems. Ecosphere 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Clemente-Orta, G.; Albajes, R.; Batuecas, I.; Achon, M.A. Planting period is the main factor for controlling maize rough dwarf disease. Sci. Rep. 2021, 11, 977. [Google Scholar] [CrossRef]
- Yang, L.; Liu, B.; Zhang, Q.; Zeng, Y.; Pan, Y.; Li, M.; Lu, Y. Landscape structure alters the abundance and species composition of early-season aphid populations in wheat fields. Agric. Ecosyst. Environ. 2019, 269, 167–173. [Google Scholar] [CrossRef]
- Alignier, A.; Raymond, L.; Deconchat, M.; Menozzi, P.; Monteil, C.; Sarthou, J.P.; Vialatte, A.; Ouin, A. The effect of semi-natural habitats on aphids and their natural enemies across spatial and temporal scales. Biol. Control 2014, 77, 76–82. [Google Scholar] [CrossRef]
- Caballero-López, B.; Bommarco, R.; Blanco-Moreno, J.M.; Sans, F.X.; Pujade-Villar, J.; Rundlöf, M.; Smith, H.G. Aphids and their natural enemies are differently affected by habitat features at local and landscape scales. Biol. Control 2012, 63, 222–229. [Google Scholar] [CrossRef]
- Bahlai, C.A.; Sikkema, S.; Hallett, R.H.; Newman, J.; Schaafsma, A.W. Modeling distribution and abundance of soybean aphid in soybean fields using measurements from the surrounding landscape. Environ. Entomol. 2010, 39, 50–56. [Google Scholar] [CrossRef]
- Baillod, A.; Tscharntke, T.; Clough, Y.; Batáry, P. Landscape-scale interactions of spatial and temporal cropland heterogeneity drive biological control of cereal aphids. J. Appl. Ecol. 2017, 54, 1804–1813. [Google Scholar] [CrossRef]
- Martin, E.A.; Reineking, B.; Seo, B.; Steffan-Dewenter, I. Pest control of aphids depends on landscape complexity and natural enemy interactions. PeerJ 2015, 3, e1095. [Google Scholar] [CrossRef]
| City, State | Location a | Date Range (2020) b | Date Range (2021) b |
|---|---|---|---|
| Brewton, Alabama | South AL | 9 May–31 October | 9 May–31 October |
| Tifton, Georgia | GA | 2 May–19 September | 18 April–3 October |
| Blackville, South Carolina | SC | 4 April–31 October | 4 April–1 October |
| College Station, Texas | TX | 2 May–25 July | 6 June–25 July |
| Belle Mina, Alabama | North AL | 4 April–31 October | 1 June 31 October |
| Shorter, Alabama | Central AL | 4 April–31 October | 23 May–31 October |
| Apex, North Carolina | NC | 9 May–31 October | 4 April–24 October |
| Suffolk, Virginia | VA | 4 April–29 August | 4 April–19 September |
| Jackson, Tennessee | TN | 30 May–22 August | 6 June–22 August |
| Stoneville, Mississippi | MS | 2 May–4 July | 4 July–31 August |
| Jay, Florida | FL | 8 August–22 August | 27 June–22 August |
| Dependent Variable | Independent Variable | Sum of Squares | df | F | p |
|---|---|---|---|---|---|
| Aphis gossypii | Week | 74.12 | 6 | 42.00 | <0.001 |
| Location | 119.64 | 8 | 50.86 | <0.001 | |
| Year | 7.22 | 1 | 24.55 | <0.001 | |
| Week/Location | 97.05 | 48 | 6.88 | <0.001 | |
| Week/Year | 11.69 | 6 | 6.63 | <0.001 | |
| Location/Year | 70.91 | 8 | 30.14 | <0.001 | |
| Week/Location/Year | 97.31 | 48 | 6.89 | <0.001 | |
| Protaphis middletonii | Week | 18.28 | 6 | 7.94 | <0.001 |
| Location | 470.30 | 8 | 153.26 | <0.001 | |
| Year | 0.71 | 1 | 1.85 | 0.170 | |
| Week/Location | 98.60 | 48 | 5.36 | <0.001 | |
| Week/Year | 6.16 | 6 | 2.68 | 0.015 | |
| Location/Year | 43.03 | 8 | 14.02 | <0.001 | |
| Week/Location/Year | 58.55 | 48 | 3.18 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahas, J.W.; Mahas, J.B.; Ray, C.; Kesheimer, A.; Steury, T.D.; Conzemius, S.R.; Crow, W.; Gore, J.; Greene, J.K.; Kennedy, G.G.; et al. The Spatiotemporal Distribution, Abundance, and Seasonal Dynamics of Cotton-Infesting Aphids in the Southern U.S. Insects 2023, 14, 639. https://doi.org/10.3390/insects14070639
Mahas JW, Mahas JB, Ray C, Kesheimer A, Steury TD, Conzemius SR, Crow W, Gore J, Greene JK, Kennedy GG, et al. The Spatiotemporal Distribution, Abundance, and Seasonal Dynamics of Cotton-Infesting Aphids in the Southern U.S. Insects. 2023; 14(7):639. https://doi.org/10.3390/insects14070639
Chicago/Turabian StyleMahas, John W., Jessica B. Mahas, Charles Ray, Adam Kesheimer, Todd D. Steury, Sophia R. Conzemius, Whitney Crow, Jeffrey Gore, Jeremy K. Greene, George G. Kennedy, and et al. 2023. "The Spatiotemporal Distribution, Abundance, and Seasonal Dynamics of Cotton-Infesting Aphids in the Southern U.S." Insects 14, no. 7: 639. https://doi.org/10.3390/insects14070639
APA StyleMahas, J. W., Mahas, J. B., Ray, C., Kesheimer, A., Steury, T. D., Conzemius, S. R., Crow, W., Gore, J., Greene, J. K., Kennedy, G. G., Kerns, D., Malone, S., Paula-Moraes, S., Roberts, P., Stewart, S. D., Taylor, S., Toews, M., & Jacobson, A. L. (2023). The Spatiotemporal Distribution, Abundance, and Seasonal Dynamics of Cotton-Infesting Aphids in the Southern U.S. Insects, 14(7), 639. https://doi.org/10.3390/insects14070639







