Characterization of Glutamate-Gated Chloride Channel in Tribolium castaneum
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Total RNA Isolation and Reverse Transcription
2.3. Cloning and Sequence Analysis of TcGluCl
2.4. Homology Modelling of TcGluCl
2.5. Reverse Transcription Quantitative PCR (RT-qPCR)
2.6. Statistical Analysis
3. Results
3.1. Cloning and Structural Characteristics of TcGluCl
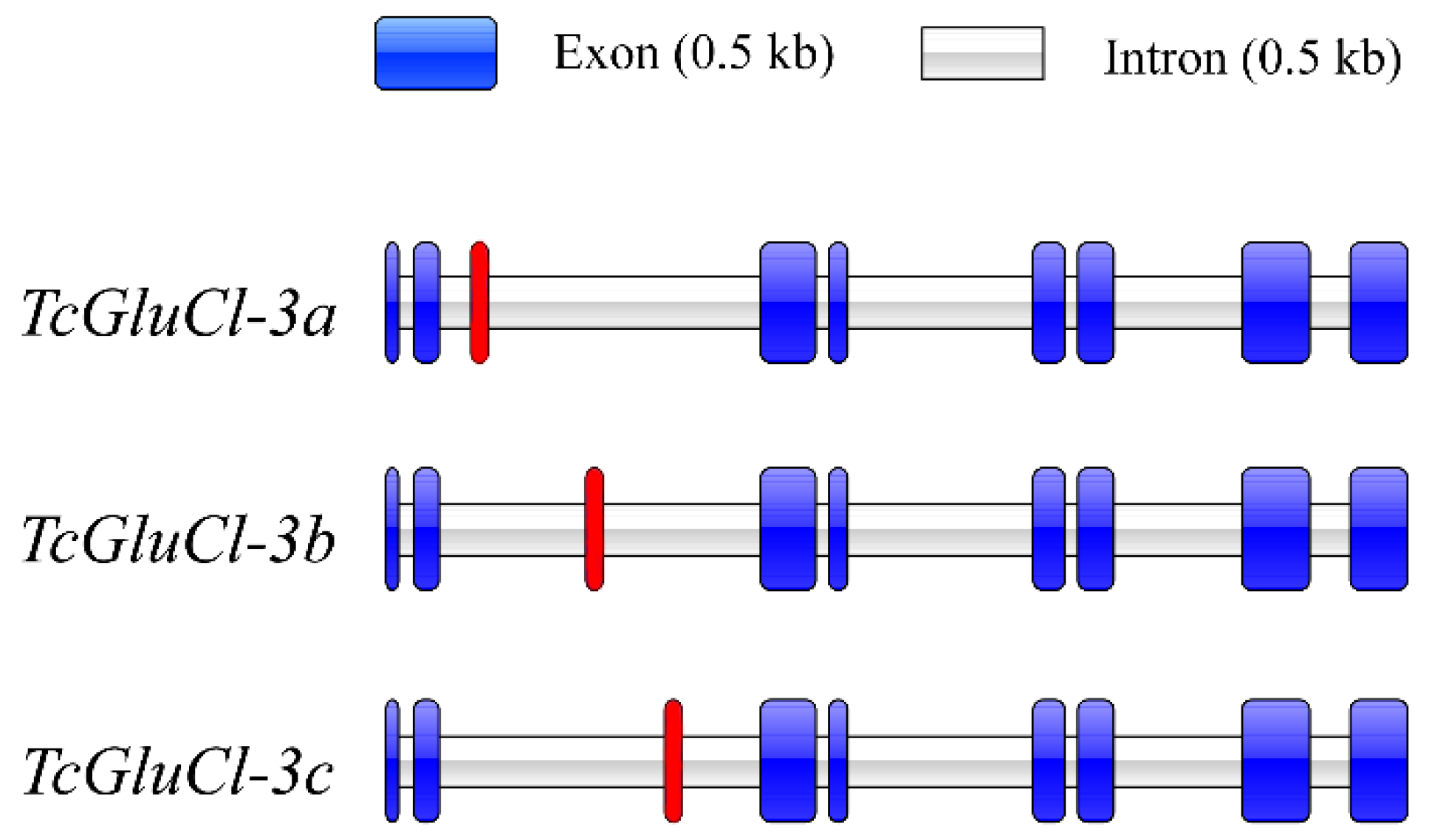
3.2. Genomic Structures of Three TcGluCl Variants
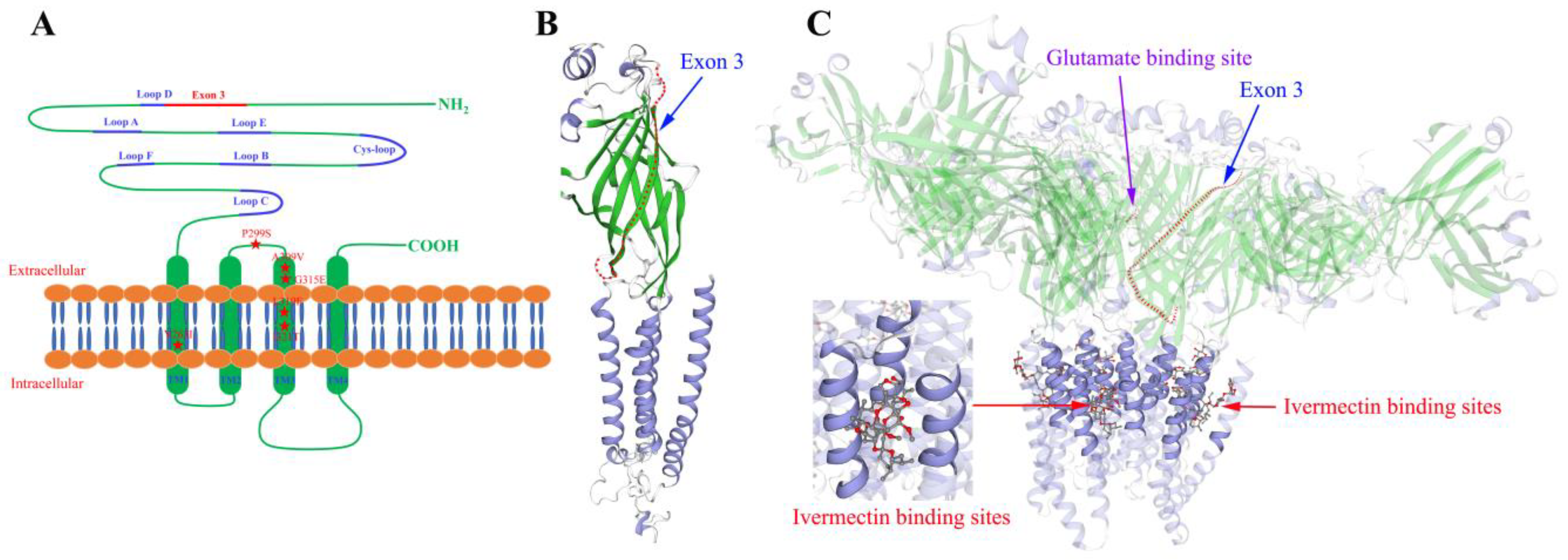
3.3. Alternative Splicing of Exon 3 of TcGluCl
3.4. Molecular Modeling of TcGluCl
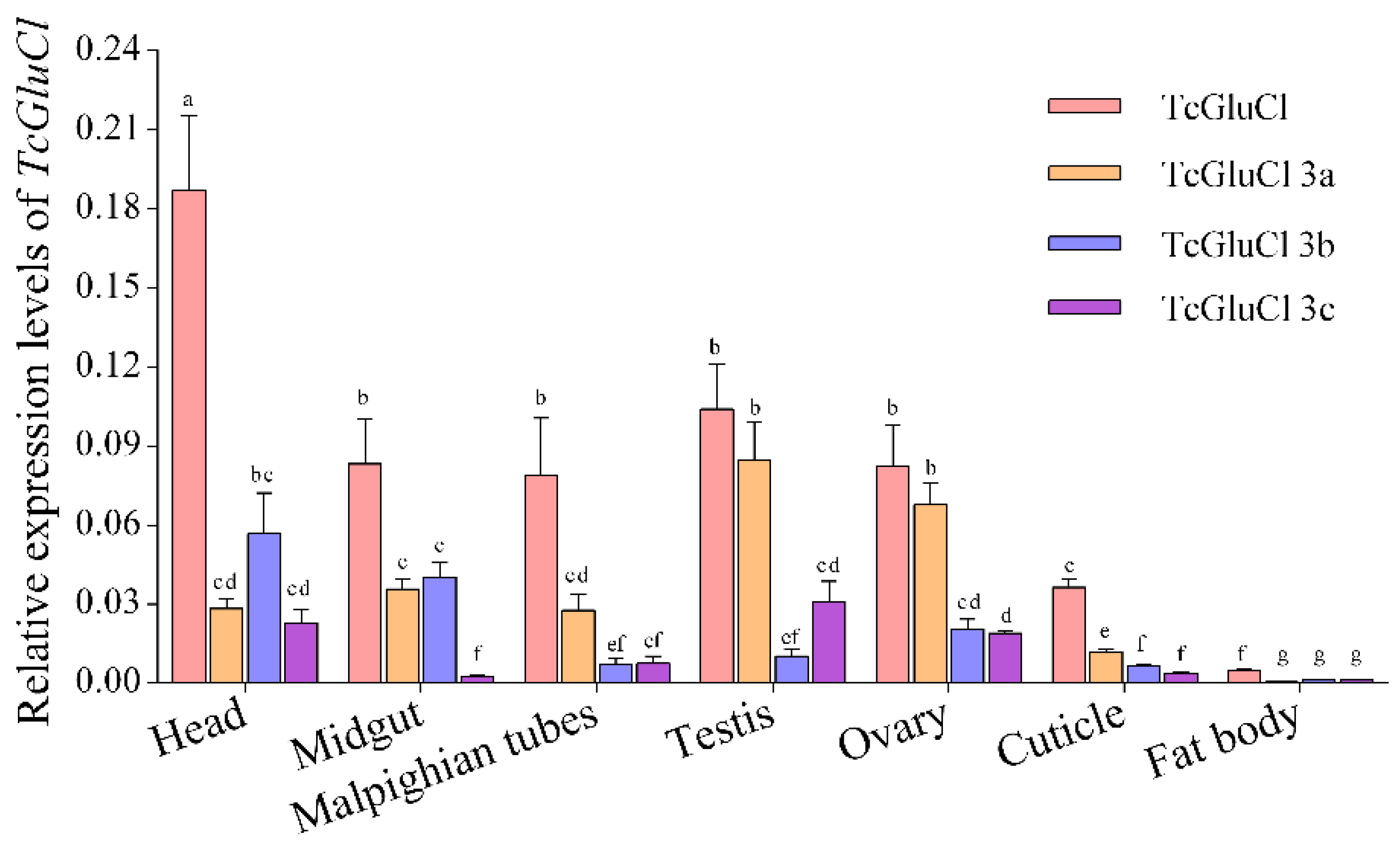
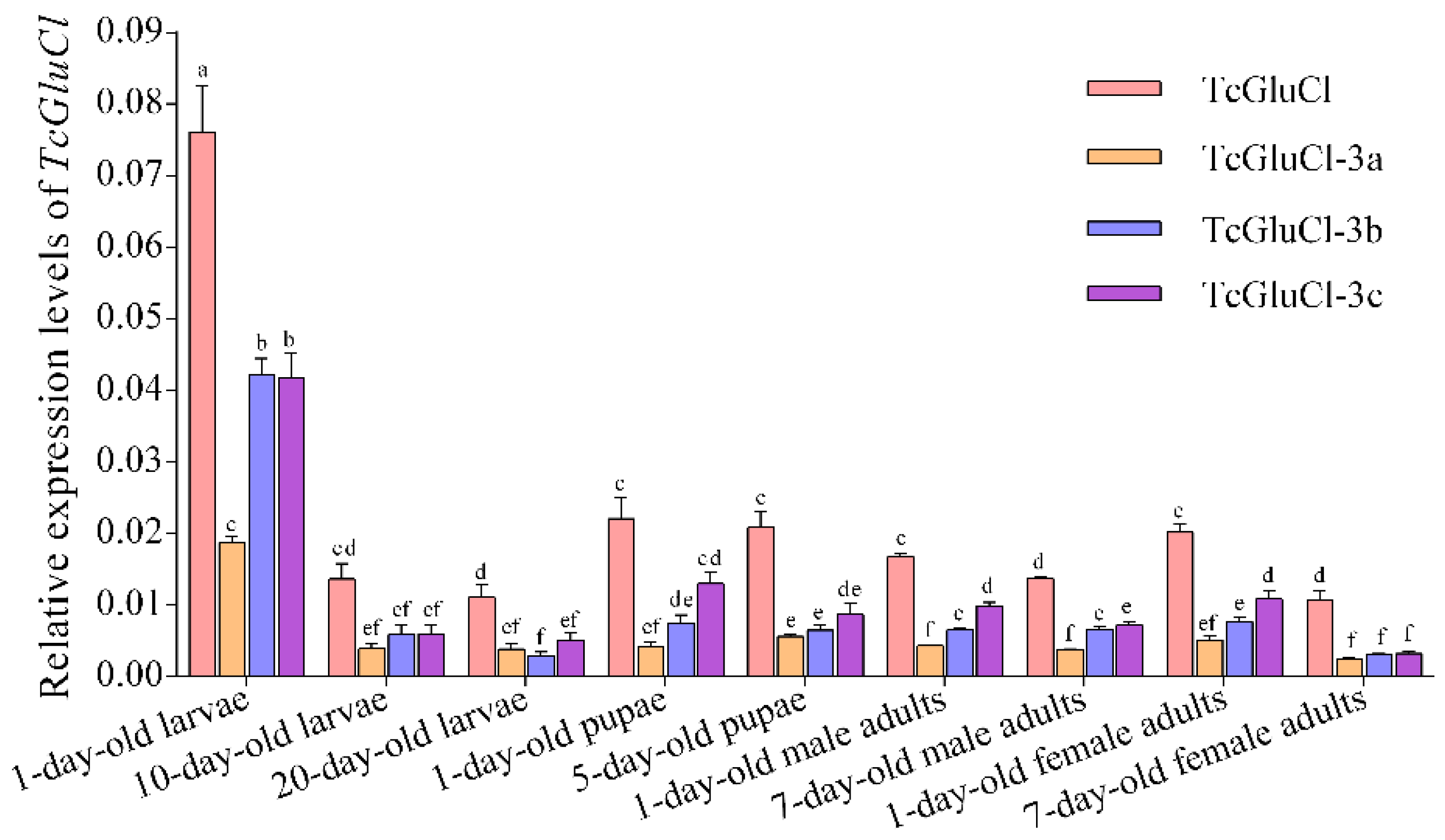
3.5. Spatial and Temporal Expressions of TcGluCls in T. castaneum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wolstenholme, A.J. Glutamate-gated Chloride Channels. J. Biol. Chem. 2012, 287, 40232–40238. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.K.; Miao, L.J.; Ge, H.C.; Yang, X.M.; Dong, F.; Xu, X.; Wu, Z.L.; Qian, K.; Wang, J.J. Molecular characterization of glutamate-gated chloride channel and its possible roles in development and abamectin susceptibility in the rice stem borer, Chilo suppressalis. Pestic. Biochem. Physiol. 2019, 155, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Narahashi, T.; Zhao, X.; Ikeda, T.; Salgado, V.L.; Yeh, J.Z. Glutamate-activated chloride channels: Unique fipronil targets present in insects but not in mammals. Pestic. Biochem. Physiol. 2010, 97, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, A.J.; Rogers, A.T. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology 2005, 131, S85–S95. [Google Scholar] [CrossRef] [PubMed]
- Ozoe, Y. γ-Aminobutyrate- and glutamate-gated chloride channels as targets of insecticides. Adv. Insect Physiol. 2013, 44, 211–286. [Google Scholar]
- Jones, A.K.; Sattelle, D.B. The cys-loop ligand-gated ion channel gene superfamily of the nematode, Caenorhabditis elegans. Invertebr. Neurosci. 2008, 8, 41–47. [Google Scholar] [CrossRef]
- Cully, D.F.; Wilkinson, H.; Vassilatis, D.K.; Etter, A.; Arena, J.P. Molecular biology and electrophysiology of glutamate-gated chloride channels of invertebrates. Parasitology 1996, 113, S191–S200. [Google Scholar] [CrossRef]
- Dermauw, W.; Ilias, A.; Riga, M.; Tsagkarakou, A.; Grbić, M.; Tirry, L.; Van Leeuwen, T.; Vontas, J. The cys-loop ligand-gated ion channel gene family of Tetranychus urticae: Implications for acaricide toxicology and a novel mutation associated with abamectin resistance. Insect Biochem. Mol. Biol. 2012, 42, 455–465. [Google Scholar] [CrossRef]
- Cully, D.F.; Paress, P.S.; Liu, K.K.; Schaeffer, J.M.; Arena, J.P. Identification of a Drosophila melanogaster glutamate-gated chloride channel sensitive to the antiparasitic agent avermectin. J. Biol. Chem. 1996, 271, 20187–20191. [Google Scholar] [CrossRef]
- Furutani, S.; Ihara, M.; Nishino, Y.; Akamatsu, M.; Jones, A.K.; Sattelle, D.B.; Matsuda, K. Exon 3 splicing and mutagenesis identify residues influencing cell surface density of heterologously expressed silkworm (Bombyx mori) glutamate-gated chloride channels. Mol. Pharmacol. 2014, 86, 686–695. [Google Scholar] [CrossRef]
- Janssen, D.; Derst, C.; Buckinx, R.; Van den Eynden, J.; Rigo, J.M.; Van Kerkhove, E. Dorsal unpaired median neurons of Locusta migratoria express ivermectin- and fipronil-sensitive glutamate-gated chloride channels. J. Neurophysiol. 2007, 97, 2642–2650. [Google Scholar] [CrossRef]
- El Hassani, A.K.; Schuster, S.; Dyck, Y.; Demares, F.; Leboulle, G.; Armengaud, C. Identification, localization and function of glutamate-gated chloride channel receptors in the honeybee brain. Eur. J. Neurosci. 2012, 36, 2409–2420. [Google Scholar] [CrossRef]
- Raymond, V.; Sattelle, D.B.; Lapied, B. Co-existence in DUM neurones of two GluCl channels that differ in their picrotoxin sensitivity. Neuroreport 2000, 11, 2695–2701. [Google Scholar] [CrossRef]
- Barbara, G.S.; Zube, C.; Rybak, J.; Gauthier, M.; Grunewald, B. Acetylcholine, GABA and glutamate induce ionic currents in cultured antennal lobe neurons of the honeybee, Apis mellifera. J. Comp. Physiol. A 2005, 191, 823–836. [Google Scholar] [CrossRef]
- Ast, G. How did alternative splicing evolve? Nat. Rev. Genet. 2004, 5, 773–782. [Google Scholar] [CrossRef]
- Kita, T.; Ozoe, F.; Ozoe, Y. Expression pattern and function of alternative splice variants of glutamate-gated chloride channel in the housefly Musca domestica. Insect Biochem. Mol. Biol. 2014, 45, 1–10. [Google Scholar] [CrossRef]
- Wu, S.F.; Mu, X.C.; Dong, Y.X.; Wang, L.X.; Wei, Q.; Gao, C.F. Expression pattern and pharmacological characterisation of two novel alternative splice variants of the glutamate-gated chloride channel in the small brown planthopper Laodelphax striatellus. Pest Manag. Sci. 2017, 73, 590–597. [Google Scholar] [CrossRef]
- Jones, A.K.; Sattelle, D.B. The cys-loop ligand-gated ion channel gene superfamily of the red flour beetle, Tribolium castaneum. BMC Genom. 2007, 8, 327. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Y.; Ma, J.; Luo, X.; Nie, P.; Zuo, Z.; Lahrmann, U.; Zhao, Q.; Zheng, Y.; Zhao, Y.; et al. IBS: An illustrator for the presentation and visualization of biological sequences. Bioinformatics 2015, 31, 3359–3361. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Hibbs, R.E.; Gouaux, E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 2011, 474, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Wang, R.; Yang, Y.H.; Wu, S.W.; O’Reilly, A.O.; Wu, Y.D. A point mutation in the glutamate-gated chloride channel of Plutella xylostella is associated with resistance to abamectin. Insect Mol. Biol. 2016, 25, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.I.; Gray, M.; Kuklinski, W.; Johnson, L.B.; Snow, C.D.; Black, W.C.; Partin, K.M.; Foy, B.D. Characterization of the target of ivermectin, the glutamate-gated chloride channel, from Anopheles gambiae. J. Exp. Biol. 2015, 218, 1478–1486. [Google Scholar] [CrossRef]
- Semenov, E.P.; Pak, W.L. Diversification of Drosophila chloride channel gene by multiple posttranscriptional mRNA modifications. J. Neurochem. 1999, 72, 66–72. [Google Scholar] [CrossRef]
- Wang, X.L.; O’Reilly, A.O.; Williamson, M.S.; Puinean, A.M.; Yang, Y.H.; Wu, S.W.; Wu, Y.D. Function and pharmacology of glutamate-gated chloride channel exon 9 splice variants from the diamondback moth Plutella xylostella. Insect Biochem. Mol. Biol. 2019, 104, 58–64. [Google Scholar] [CrossRef]
- Démares, F.; Raymond, V.; Armengaud, C. Expression and localization of glutamate-gated chloride channel variants in honeybee brain (Apis mellifera). Insect Biochem. Mol. Biol. 2013, 43, 115–124. [Google Scholar] [CrossRef]
- Liu, H.P.; Lin, S.C.; Lin, C.Y.; Yeh, S.R.; Chiang, A.S. Glutamate-gated chloride channels inhibit juvenile hormone biosynthesis in the cockroach, Diploptera punctata. Insect Biochem. Mol. Biol. 2005, 35, 1260–1268. [Google Scholar] [CrossRef]
- Chiang, A.S.; Pszczolkowski, M.A.; Liu, H.P.; Lin, S.C. Ionotropic glutamate receptors mediate juvenile hormone synthesis in the cockroach, Diploptera punctata. Insect Biochem. Mol. Biol. 2002, 32, 669–678. [Google Scholar] [CrossRef]
- Boumghar, K.; Couret-Fauvel, T.; Garcia, M.; Armengaud, C. Evidence for a role of GABA- and glutamate-gated chloride channels in olfactory memory. Pharmacol. Biochem. Behav. 2012, 103, 69–75. [Google Scholar] [CrossRef]
- Demares, F.; Drouard, F.; Massou, I.; Crattelet, C.; Loeuillet, A.; Bettiol, C.; Raymond, V.; Armengaud, C. Differential involvement of glutamate-gated chloride channel splice variants in the olfactory memory processes of the honeybee APIS Mellifera. Pharmacol. Biochem. Behav. 2014, 124, 137–144. [Google Scholar] [CrossRef]
- Collins, B.; Kane, E.A.; Reeves, D.C.; Akabas, M.H.; Blau, J. Balance of activity between LNvs and glutamatergic dorsal clock neurons promotes robust circadian rhythms in Drosophila. Neuron 2012, 74, 706–718. [Google Scholar] [CrossRef]
- Liu, W.W.; Wilson, R.I. Glutamate is an inhibitory neurotransmitter in the Drosophila olfactory system. Proc. Natl. Acad. Sci. USA 2013, 110, 10294–10299. [Google Scholar] [CrossRef]
- Li, B.W.; Rush, A.C.; Weil, G.J. High level expression of a glutamate-gated chloride channel gene in reproductive tissues of Brugia malayi may explain the sterilizing effect of ivermectin on filarial worms. Int. J. Parasitol-Drug 2014, 4, 71–76. [Google Scholar] [CrossRef]
- Wang, J.; Gu, L.; Knipple, D.C. Evaluation of some potential target genes and methods for RNAi-mediated pest control of the corn earworm Helicoverpa zea. Pestic. Biochem. Physiol. 2018, 149, 67–72. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, K.; Jiang, C.; Guan, D.; Zhuang, A.; Meng, X.; Wang, J. Characterization of Glutamate-Gated Chloride Channel in Tribolium castaneum. Insects 2023, 14, 580. https://doi.org/10.3390/insects14070580
Qian K, Jiang C, Guan D, Zhuang A, Meng X, Wang J. Characterization of Glutamate-Gated Chloride Channel in Tribolium castaneum. Insects. 2023; 14(7):580. https://doi.org/10.3390/insects14070580
Chicago/Turabian StyleQian, Kun, Chengyun Jiang, Daojie Guan, Anxiang Zhuang, Xiangkun Meng, and Jianjun Wang. 2023. "Characterization of Glutamate-Gated Chloride Channel in Tribolium castaneum" Insects 14, no. 7: 580. https://doi.org/10.3390/insects14070580
APA StyleQian, K., Jiang, C., Guan, D., Zhuang, A., Meng, X., & Wang, J. (2023). Characterization of Glutamate-Gated Chloride Channel in Tribolium castaneum. Insects, 14(7), 580. https://doi.org/10.3390/insects14070580





