Investigating Photo-Degradation as a Potential Pheromone Production Pathway in Spotted Lanternfly, Lycorma delicatula
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection and Maintenance
2.2. Whole Body Extract
2.3. Photo-Degradation Process
2.4. Lure Preparation
2.5. Gas Chromatography—Mass Spectrometry (GC-MS)
2.6. Gas Chromatography—Electroantennographic Detection (GC-EAD)
2.7. Behavioral Bioassays
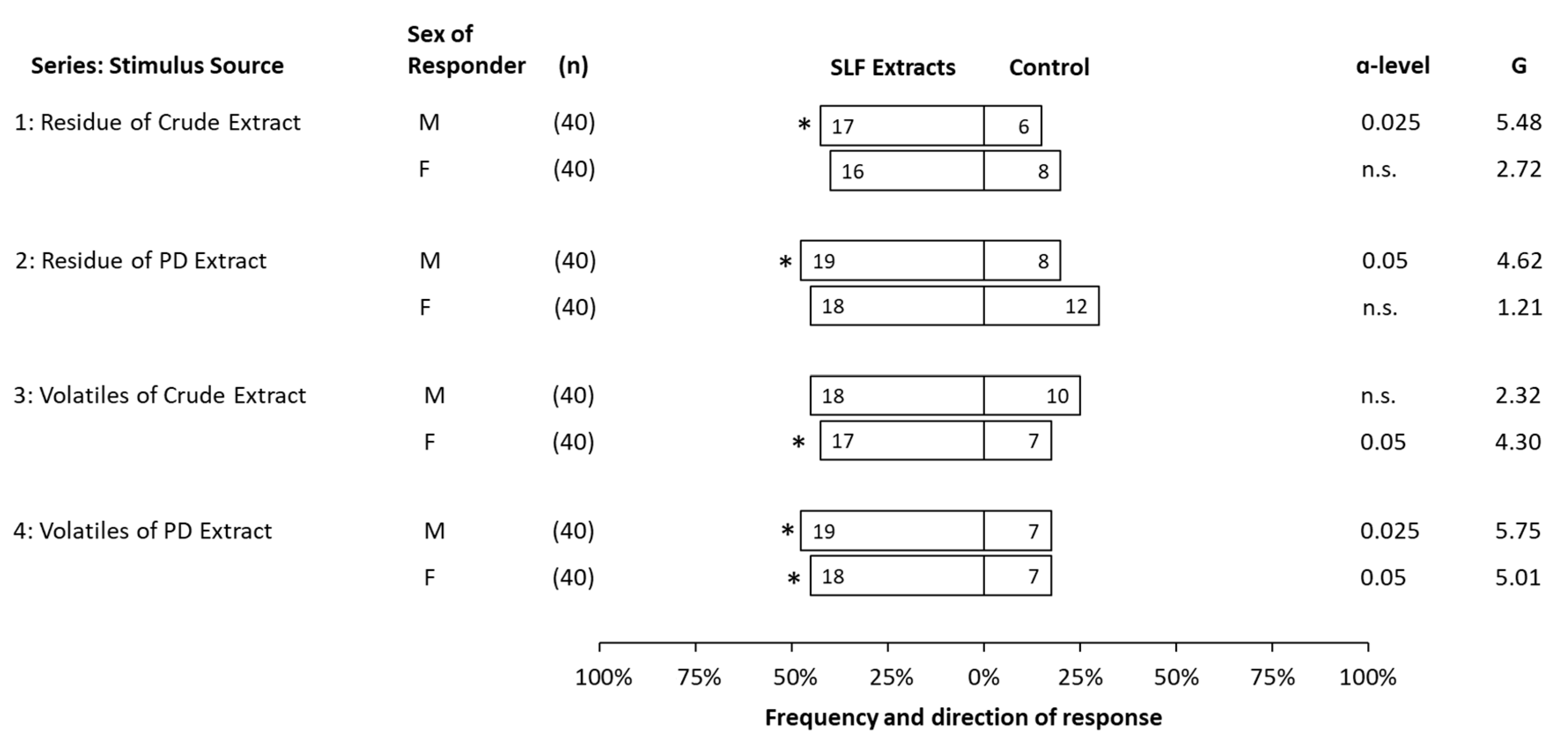
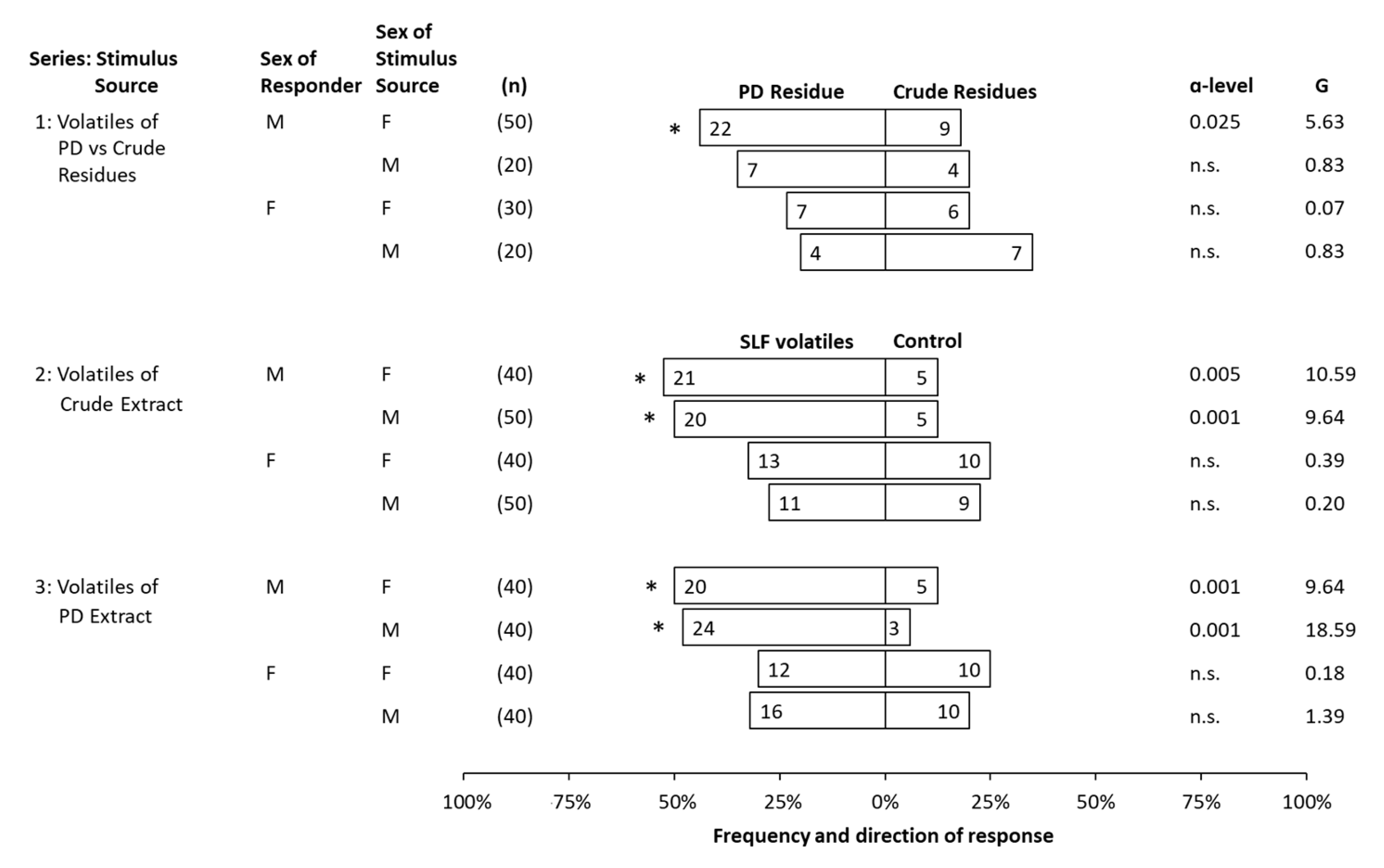
3. Results
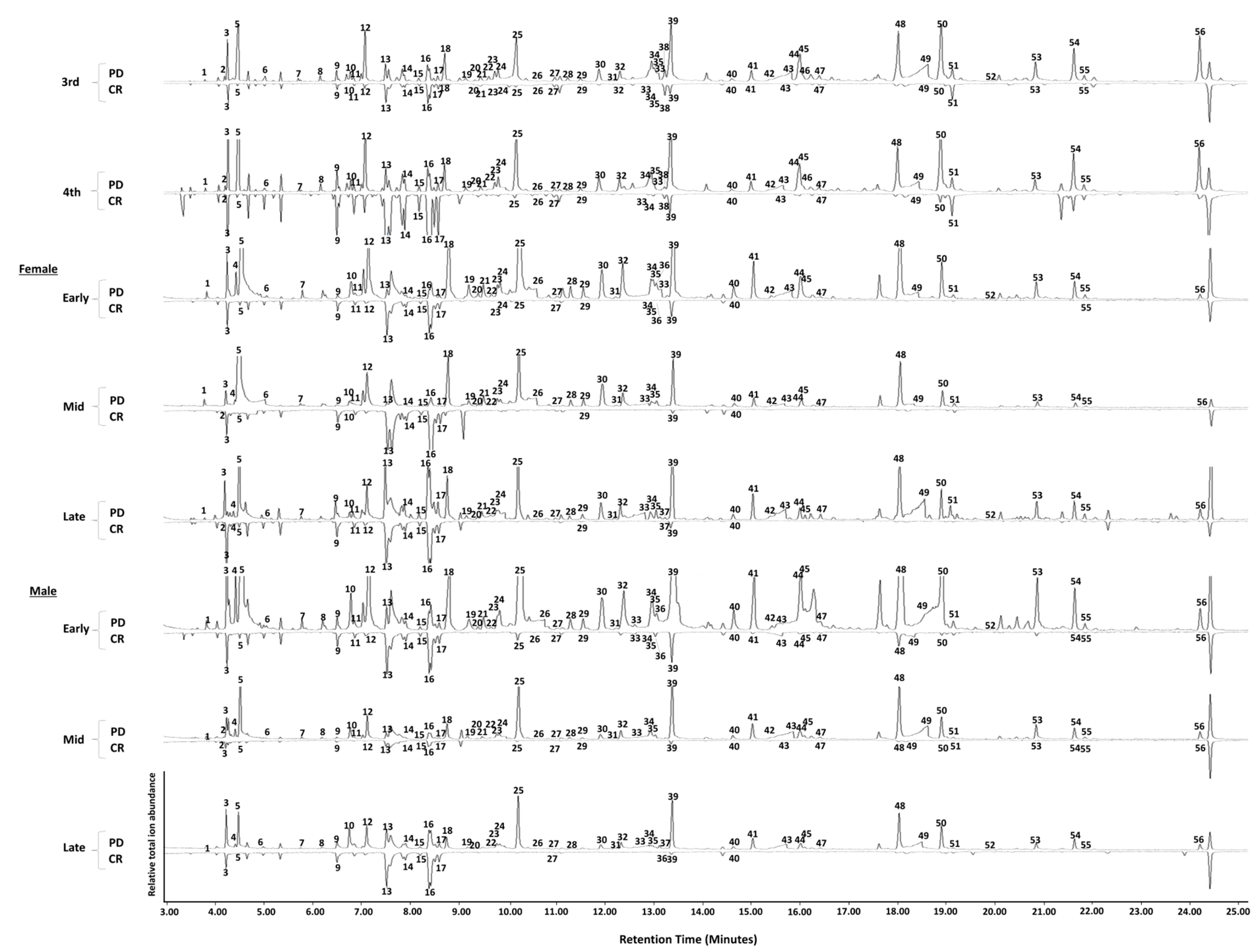
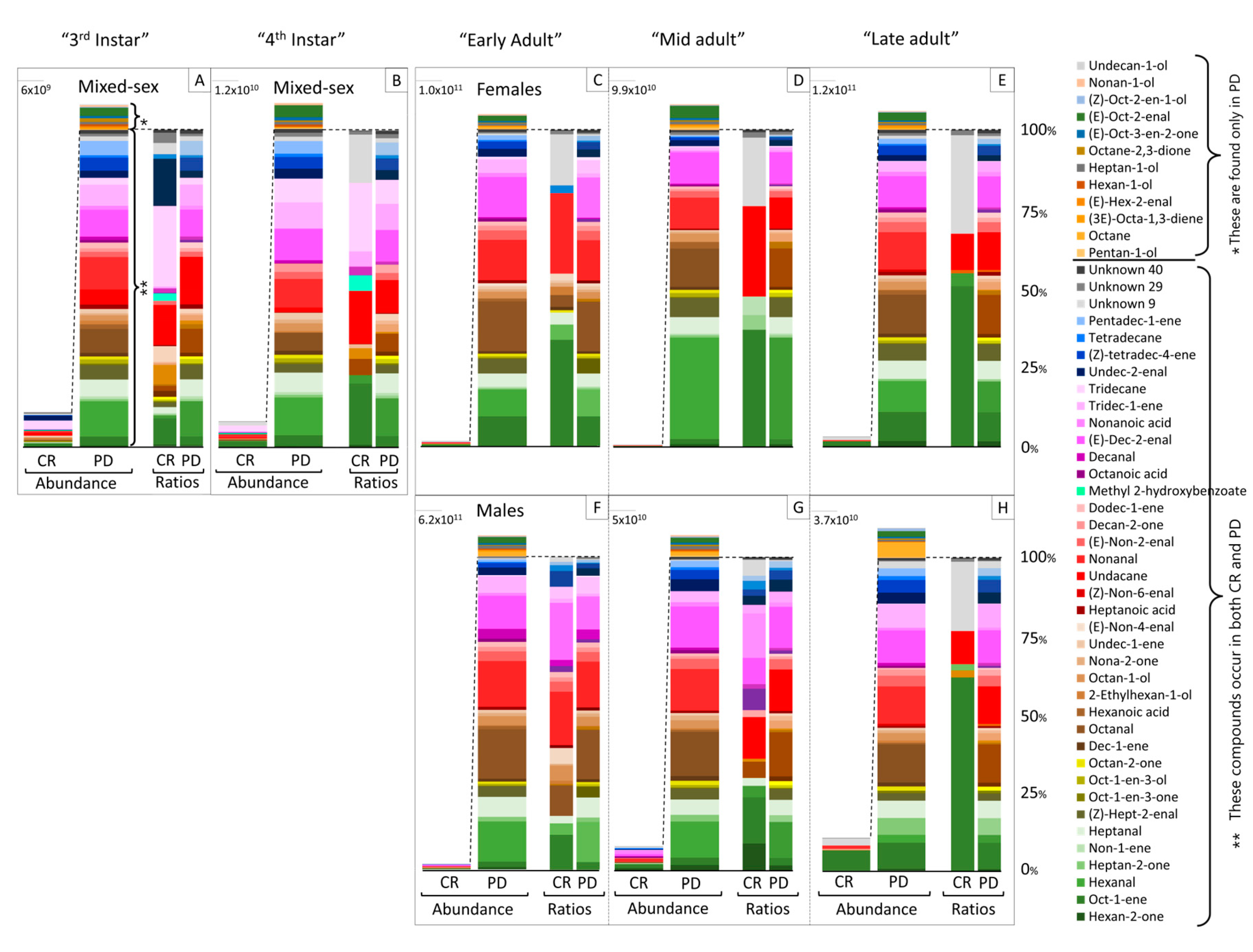
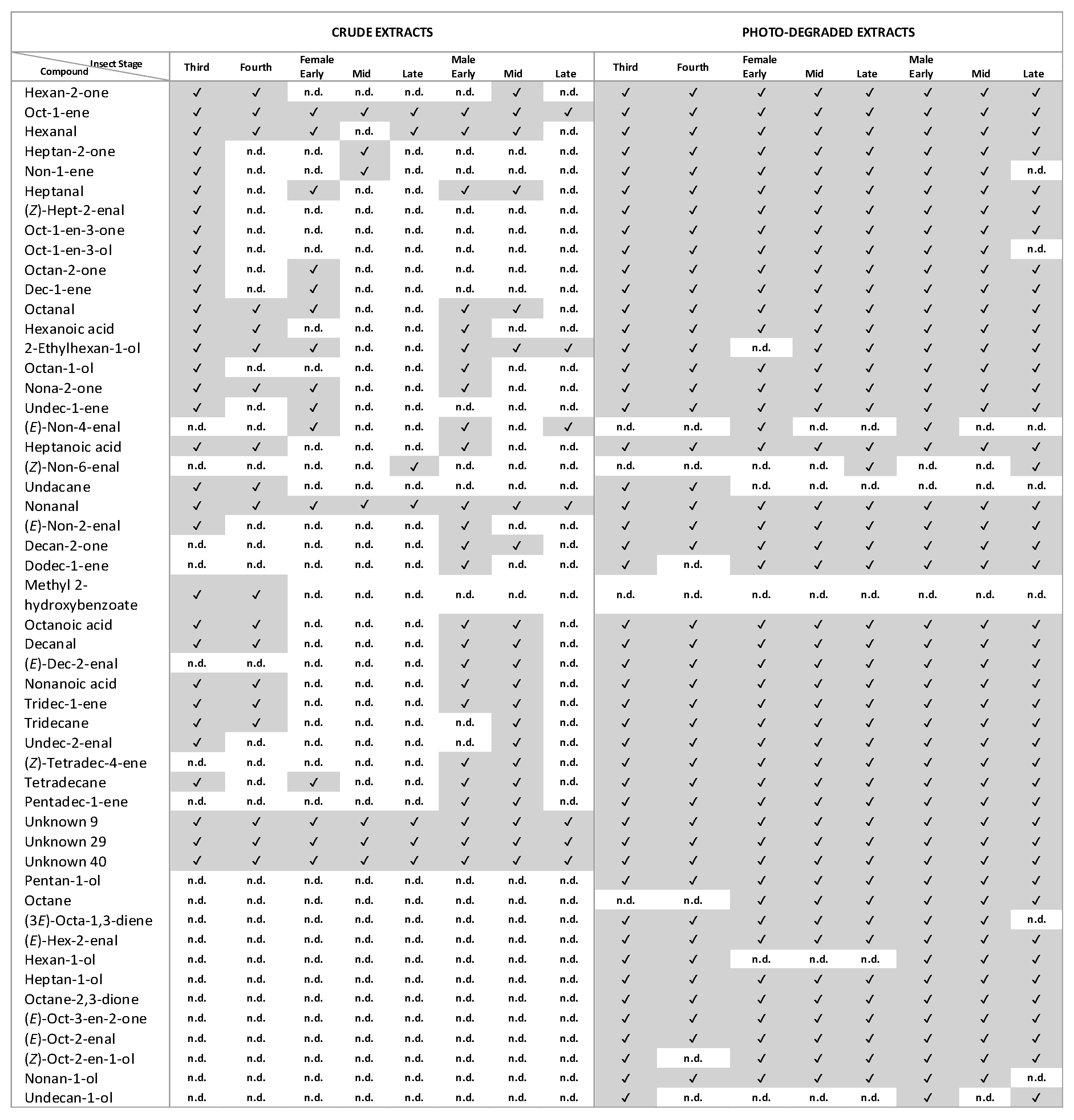
3.1. GC-MS Analysis
3.2. GC-EAD Analysis
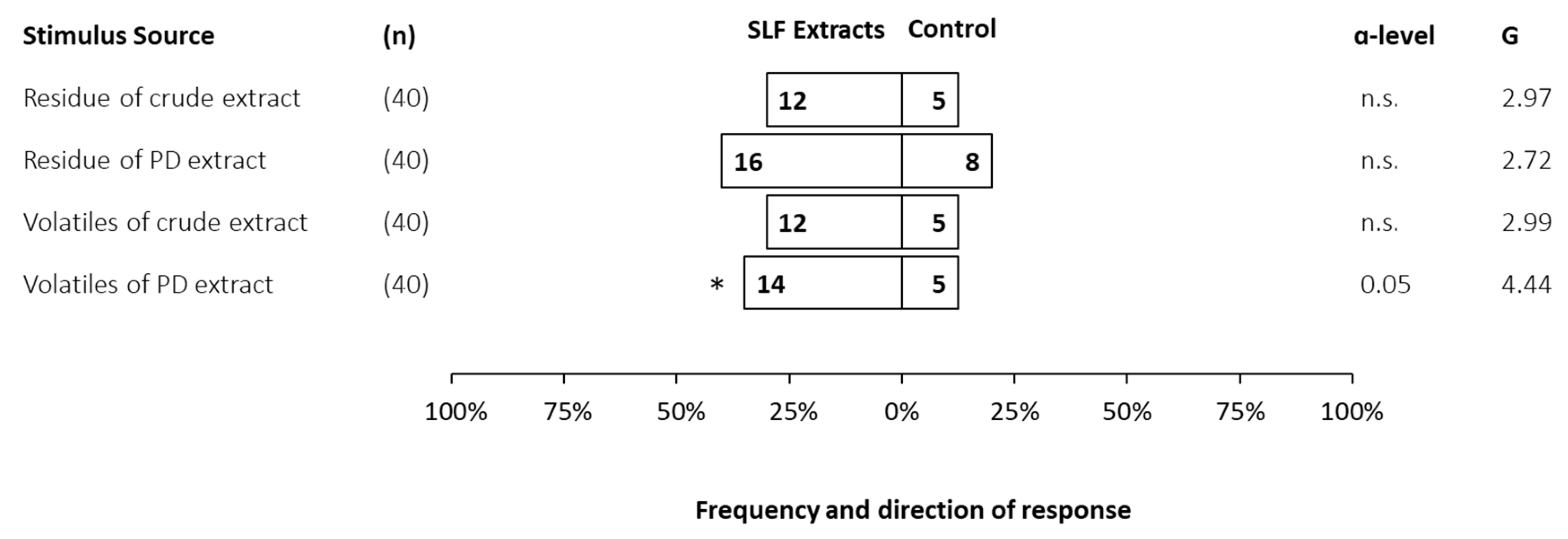
3.3. Behavioral Bioassays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barringer, L.E.; Donovall, L.R.; Spichiger, S.-E.; Lynch, D.; Henry, D. The first new world record of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae). Entomol. News 2015, 125, 20–23. [Google Scholar] [CrossRef]
- Han, J.M.; Kim, H.; Lim, E.J.; Lee, S.; Kwon, Y.J.; Cho, S. Lycorma delicatula (Hemiptera: Auchenorrhyncha: Fulgoridae: Aphaeninae) finally, but suddenly arrived in Korea. Entomol. Res. 2008, 38, 281–286. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Kwon, D.H.; Park, S.; Lee, Y.; Huang, J.; Kai, S.; Lee, H.-S.; Hong, K.-J.; Jang, Y. Molecular comparison of Lycorma delicatula (Hemiptera: Fulgoridae) isolates in Korea, China, and Japan. J. Asia-Pac. Entomol. 2013, 16, 503–506. [Google Scholar] [CrossRef]
- NYSIPM. Spotted Lanternfly. Available online: https://nysipm.cornell.edu/environment/invasive-species-exotic-pests/spotted-lanternfly/ (accessed on 18 November 2022).
- Barringer, L.; Ciafré, C.M. Worldwide feeding host plants of spotted lanternfly, with significant additions from North America. Environ. Entomol. 2020, 49, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.M.; Calvin, D.; Hills-Stevenson, J. Early response (2018–2020) to the threat of spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae) in Pennsylvania. Ann. Entomol. Soc. Am. 2021, 114, 709–718. [Google Scholar] [CrossRef]
- Urban, J.M. Perspective: Shedding light on spotted lanternfly impacts in the USA. Pest Manag. Sci. 2020, 76, 10–17. [Google Scholar] [CrossRef]
- Lee, D.-H.; Park, Y.-L.; Leskey, T.C. A review of biology and management of Lycorma delicatula (Hemiptera: Fulgoridae), an emerging global invasive species. J. Asia-Pac. Entomol. 2019, 22, 589–596. [Google Scholar] [CrossRef]
- Wolfin, M.S.; Binyameen, M.; Wang, Y.; Urban, J.M.; Roberts, D.C.; Baker, T.C. Flight dispersal capabilities of female spotted lanternflies (Lycorma delicatula) related to size and mating status. J. Insect Behav. 2019, 32, 188–200. [Google Scholar] [CrossRef]
- Keller, J.A.; Johnson, A.E.; Uyi, O.; Wurzbacher, S.; Long, D.; Hoover, K. Dispersal of Lycorma delicatula (Hemiptera: Fulgoridae) nymphs through contiguous, deciduous forest. Environ. Entomol. 2020, 49, 1012–1018. [Google Scholar] [CrossRef]
- Mauchline, N.; McKenna, C. BS1847: Spotted Lanternfly, Lycorma delicatula (White 1845) Review: Biology, Ecology and Pest Management with Reference to Kiwifruit; The New Zealand Institute for Plant and Food Research Limited: Auckland, New Zealand, 2019; pp. 1–49. [Google Scholar]
- Leach, H.; Leach, A. Seasonal phenology and activity of spotted lanternfly (Lycorma delicatula) in eastern US vineyards. J. Pest Sci. 2020, 93, 1215–1224. [Google Scholar] [CrossRef]
- Dechaine, A.C.; Sutphin, M.; Leskey, T.C.; Salom, S.M.; Kuhar, T.P.; Pfeiffer, D.G. Phenology of Lycorma delicatula (Hemiptera: Fulgoridae) in Virginia, USA. Environ. Entomol. 2021, 50, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Keena, M.A.; Nielsen, A.L. Comparison of the hatch of newly laid Lycorma delicatula (Hemiptera: Fulgoridae) eggs from the United States after exposure to different temperatures and durations of low temperature. Environ. Entomol. 2021, 50, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, D.; Keena, M.A.; Nielsen, A.L.; Hamilton, G. Effects of temperature on development and survival of nymphal Lycorma delicatula (Hemiptera: Fulgoridae). Environ. Entomol. 2021, 50, 183–191. [Google Scholar] [CrossRef]
- Nixon, L.J.; Jones, S.K.; Tang, L.; Urban, J.; Felton, K.; Leskey, T.C. Survivorship and development of the invasive Lycorma delicatula (Hemiptera: Fulgoridae) on wild and cultivated temperate host plants. Environ. Entomol. 2022, 51, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Rohde, B.B.; Cooperband, M.F.; Canlas, I.; Mankin, R.W.; Howard, D.R. Evidence of Receptivity to Vibroacoustic Stimuli in the Spotted Lanternfly. J. Econ. Entomol. 2022, 115, 2116–2120. [Google Scholar] [CrossRef] [PubMed]
- Faal, H.; Cooperband, M.F.; Canlas, I.; Carrillo, D. Evidence of Pheromone Use in a Fulgorid, Spotted Lanternfly. Forests 2022, 13, 1639. [Google Scholar] [CrossRef]
- Faal, H.; Meier, L.R.; Canlas, I.J.; Murman, K.; Wallace, M.; Carrillo, D.; Cooperband, M.F. Volatiles from male honeydew excretions attract conspecific male spotted lanternflies, Lycorma delicatula (Hemiptera: Fulgoridae). Front. Insect Sci. 2022, 2, 982965. [Google Scholar] [CrossRef]
- Cooperband, M.F.; Murman, K. Responses of adult spotted lanternflies to artificial aggregations composed of all males or females. Front. Insect Sci. 2022, 2, 981832. [Google Scholar] [CrossRef]
- Baker, T.C.; Smyers, E.C.; Urban, J.M.; Meng, Z.; Damadaram, K.J.P.; Myrick, A.J.; Cooperband, M.F.; Domingue, M.J. Progression of seasonal activities of adults of the spotted lanternfly, Lycorma delicatula, during the 2017 season of mass flight dispersal behavior in eastern Pennsylvania. J. Asia-Pac. Entomol. 2019, 22, 705–713. [Google Scholar] [CrossRef]
- Cooperband, M.F.; Wickham, J.; Cleary, K.; Spichiger, S.-E.; Zhang, L.; Baker, J.; Canlas, I.; Derstine, N.; Carrillo, D. Discovery of three kairomones in relation to trap and lure development for spotted lanternfly (Hemiptera: Fulgoridae). J. Econ. Entomol. 2019, 112, 671–682. [Google Scholar] [CrossRef]
- Derstine, N.T.; Meier, L.; Canlas, I.; Murman, K.; Cannon, S.; Carrillo, D.; Wallace, M.; Cooperband, M.F. Plant volatiles help mediate host plant selection and attraction of the spotted lanternfly (Hemiptera: Fulgoridae): A generalist with a preferred host. Environ. Entomol. 2020, 49, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Murman, K.; Setliff, G.P.; Pugh, C.V.; Toolan, M.J.; Canlas, I.; Cannon, S.; Abreu, L.; Fetchen, M.; Zhang, L.; Warden, M.L. Distribution, Survival, and Development of Spotted Lanternfly on Host Plants Found in North America. Environ. Entomol. 2020, 49, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Cooperband, M.F.; Siderhurst, M.S.; Murman, K.; Cannon, S.; Harris, S.M.W. Radiotelemetry and Harmonic Radar for Tracking Spotted Lanternfly in the Field; Animal and Plant Health Inspection Service, U.S. Department of Agriculture: Buzzards Bay, MA, USA, 2021; pp. 10–13. [Google Scholar]
- Jahdi, R.; Arabi, M. Monitoring summer solar ultraviolet (UV) radiation on the ground level over Ardabil-Sarein, NW Iran. ISPRS Ann. Photogramm. Remote Sens. Spat. Inf. Sci. 2023, 10, 327–333. [Google Scholar] [CrossRef]
- Jang, Y.; An, H.G.; Kim, H.; Kim, K.H. Spectral preferences of Lycorma delicatula (Hemiptera: Fulgoridae). Entomol. Res. 2013, 43, 115–122. [Google Scholar] [CrossRef]
- Hatano, E.; Wada-Katsumata, A.; Schal, C. Environmental decomposition of olefinic cuticular hydrocarbons of Periplaneta americana generates a volatile pheromone that guides social behaviour. Proc. R. Soc. B 2020, 287, 20192466. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Nelson, D.R.; De Renobales, M. Chemistry, biochemistry, and physiology of insect cuticular lipids. Arch. Insect Biochem. Physiol. 1987, 6, 227–265. [Google Scholar] [CrossRef]
- Millar, J.G. Chemical Synthesis of Insect Cuticular Hydrocarbons; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Cho, S.-R.; Lee, J.-E.; Jeong, J.-W.; Yang, J.-O.; Yoon, C.-M.; Kim, G.-H. Comparison of cuticular hydrocarbons of different developmental stages of the spot clothing wax cicada, Lycorma delicatula (Hemiptera: Fulgoridae). Korean J. Appl. Entomol. 2011, 50, 185–194. [Google Scholar] [CrossRef]
- Bartelt, R.J.; Cossé, A.A.; Petroski, R.J.; Weaver, D.K. Cuticular hydrocarbons and novel alkenediol diacetates from wheat stem sawfly (Cephus cinctus): Natural oxidation to pheromone components. J. Chem. Ecol. 2002, 28, 385–405. [Google Scholar] [CrossRef]
- Wickham, J.D.; Xu, Z.; Teale, S.A. Evidence for a female-produced, long range pheromone of Anoplophora glabripennis (Coleoptera: Cerambycidae). Insect Sci. 2012, 19, 355–371. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Ginzel, M.D. Chemical ecology, biochemistry, and molecular biology of insect hydrocarbons. Annu. Rev. Entomol. 2021, 66, 45–60. [Google Scholar] [CrossRef]
- Faal, H.; Silk, P.J.; LeClair, G.; Teale, S.A. Biologically active cuticular compounds of female Sirex noctilio. Entomol. Exp. Appl. 2022, 170, 327–338. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene. SpringerPlus 2013, 2, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Lipid Oxidation, 2nd ed.; Woodhead Publishing Limited: Philadelphia, PA, USA, 2012; p. 463. [Google Scholar]
- Baeckstrom, P.; Bjorkling, F.; Hogberg, H.-E.; Norin, T. Cross-coupling of vinyl cuprates and allylic halides and synthesis of the Comstock mealybug pheromone via photooxidation of 2,6-dimethyl-2, 5-heptadiene. Acta Chem. Scand. B 1984, 38, 779–782. [Google Scholar] [CrossRef]
- Staples, J.K.; Bartelt, R.J.; Cossé, A.A.; Whitman, D.W. Sex pheromone of the pine false webworm Acantholyda erythrocephala. J. Chem. Ecol. 2009, 35, 1448–1460. [Google Scholar] [CrossRef]
- Lebreton, S.; Borrero-Echeverry, F.; Gonzalez, F.; Solum, M.; Wallin, E.A.; Hedenström, E.; Hansson, B.S.; Gustavsson, A.-L.; Bengtsson, M.; Birgersson, G. A Drosophila female pheromone elicits species-specific long-range attraction via an olfactory channel with dual specificity for sex and food. BMC Biol. 2017, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stewart-Jones, A.; Poppy, G.M. Comparison of glass vessels and plastic bags for enclosing living plant parts for headspace analysis. J. Chem. Ecol. 2006, 32, 845–864. [Google Scholar] [CrossRef]
- Pfeifer, Y.V.; Kroh, L.W. Investigation of reactive α-dicarbonyl compounds generated from the Maillard reactions of l-methionine with reducing sugars via their stable quinoxaline derivatives. J. Agric. Food Chem. 2010, 58, 8293–8299. [Google Scholar] [CrossRef]
- Zunic-Kosi, A.; Chinta, S.; Headrick, D.; Cokl, A.; Millar, J. Do chemical signals mediate reproductive behavior of T rupanea vicina, an emerging pest of ornamental marigold production in C alifornia? Entomol. Exp. Et Appl. 2013, 149, 44–56. [Google Scholar] [CrossRef]
- Cooperband, M.F.; Cossé, A.A.; Jones, T.H.; Carrillo, D.; Cleary, K.; Canlas, I.; Stouthamer, R. Pheromones of three ambrosia beetles in the Euwallacea fornicatus species complex: Ratios and preferences. PeerJ 2017, 5, e3957. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Sokal, R.R. Statistical Tables, 3rd ed.; W. H. Freeman and Company: New York, NY, USA, 1995. [Google Scholar]
- Howse, P.; Stevens, J.M.; Jones, O.T. Insect Pheromones and Their Use in Pest Management; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Elkinton, J.S.; Cardé, R.T. Odor dispersion. In Chem. Ecol. Insects; Bell, W.J., Cardé, R.T., Eds.; Chapman and Hall: CRC Press, London, UK, 1984; pp. 73–91. [Google Scholar]
- Cooperband, M.F.; Wickham, J.D.; Warden, M.L. Factors guiding the orientation of nymphal spotted lanternfly. Lycorma Delicatula. Insects 2023, 14, 279. [Google Scholar] [CrossRef]
- Mason, R.T.; Fales, H.M.; Jones, T.H.; O’Brien, L.B.; Taylor, T.W.; Hogue, C.L.; Blum, M.S. Characterization of fulgorid waxes (homoptera: Fulgoridae: Insecta). Insect Biochem. 1989, 19, 737–740. [Google Scholar] [CrossRef]
- O’Brien, L.B.; Wilson, S.W. Planthopper systematics and external morphology. In Leafhoppers Planthoppers; Nault, L.R., Rodriguez, J.G., Eds.; John Wiley & Sons: New York, NY, USA, 1985; pp. 61–102. [Google Scholar]
- Fang, M.; Ivanisevic, J.; Benton, H.P.; Johnson, C.H.; Patti, G.J.; Hoang, L.T.; Uritboonthai, W.; Kurczy, M.E.; Siuzdak, G. Thermal degradation of small molecules: A global metabolomic investigation. Anal. Chem. 2015, 87, 10935–10941. [Google Scholar] [CrossRef] [PubMed]
- Arn, H.; Städler, E.; Rauscher, S. The electroantennographic detector—A selective and sensitive tool in the gas chromatographic analysis of insect pheromones. Z. Für Nat. C 1975, 30, 722–725. [Google Scholar] [CrossRef]
- Cork, A.; Beevor, P.S.; Gough, A.J.E.; Hall, D.R. Gas chromatography linked to electroantennography: A versatile technique for identifying insect semiochemicals. In Chromatogr. Isol. Insect Horm. Pheromones; McCaffery, A.R., Wilson, I.D., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 271–274. [Google Scholar]
- Bjostad, L.B. Electrophysiological methods. Methods Chem. Ecol. Chem. Methods 1998, 1, 339–375. [Google Scholar]
- Chinta, S.; Dickens, J.C.; Aldrich, J.R. Olfactory reception of potential pheromones and plant odors by tarnished plant bug, Lygus lineolaris (Hemiptera: Miridae). J. Chem. Ecol. 1994, 20, 3251–3267. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S.; Ueda, Y.; Ono, M. Attractant pheromone for male rice bug, Leptocorisa chinensis: Semiochemicals produced by both male and female. J. Chem. Ecol. 1996, 22, 1429–1437. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faal, H.; Canlas, I.J.; Cossé, A.; Jones, T.H.; Carrillo, D.; Cooperband, M.F. Investigating Photo-Degradation as a Potential Pheromone Production Pathway in Spotted Lanternfly, Lycorma delicatula. Insects 2023, 14, 551. https://doi.org/10.3390/insects14060551
Faal H, Canlas IJ, Cossé A, Jones TH, Carrillo D, Cooperband MF. Investigating Photo-Degradation as a Potential Pheromone Production Pathway in Spotted Lanternfly, Lycorma delicatula. Insects. 2023; 14(6):551. https://doi.org/10.3390/insects14060551
Chicago/Turabian StyleFaal, Hajar, Isaiah J. Canlas, Allard Cossé, Tappey H. Jones, Daniel Carrillo, and Miriam F. Cooperband. 2023. "Investigating Photo-Degradation as a Potential Pheromone Production Pathway in Spotted Lanternfly, Lycorma delicatula" Insects 14, no. 6: 551. https://doi.org/10.3390/insects14060551
APA StyleFaal, H., Canlas, I. J., Cossé, A., Jones, T. H., Carrillo, D., & Cooperband, M. F. (2023). Investigating Photo-Degradation as a Potential Pheromone Production Pathway in Spotted Lanternfly, Lycorma delicatula. Insects, 14(6), 551. https://doi.org/10.3390/insects14060551






