Granger Causality Analysis of Transient Calcium Dynamics in the Honey Bee Antennal Lobe Network
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bee Preparation
2.2. Olfactory Stimulation
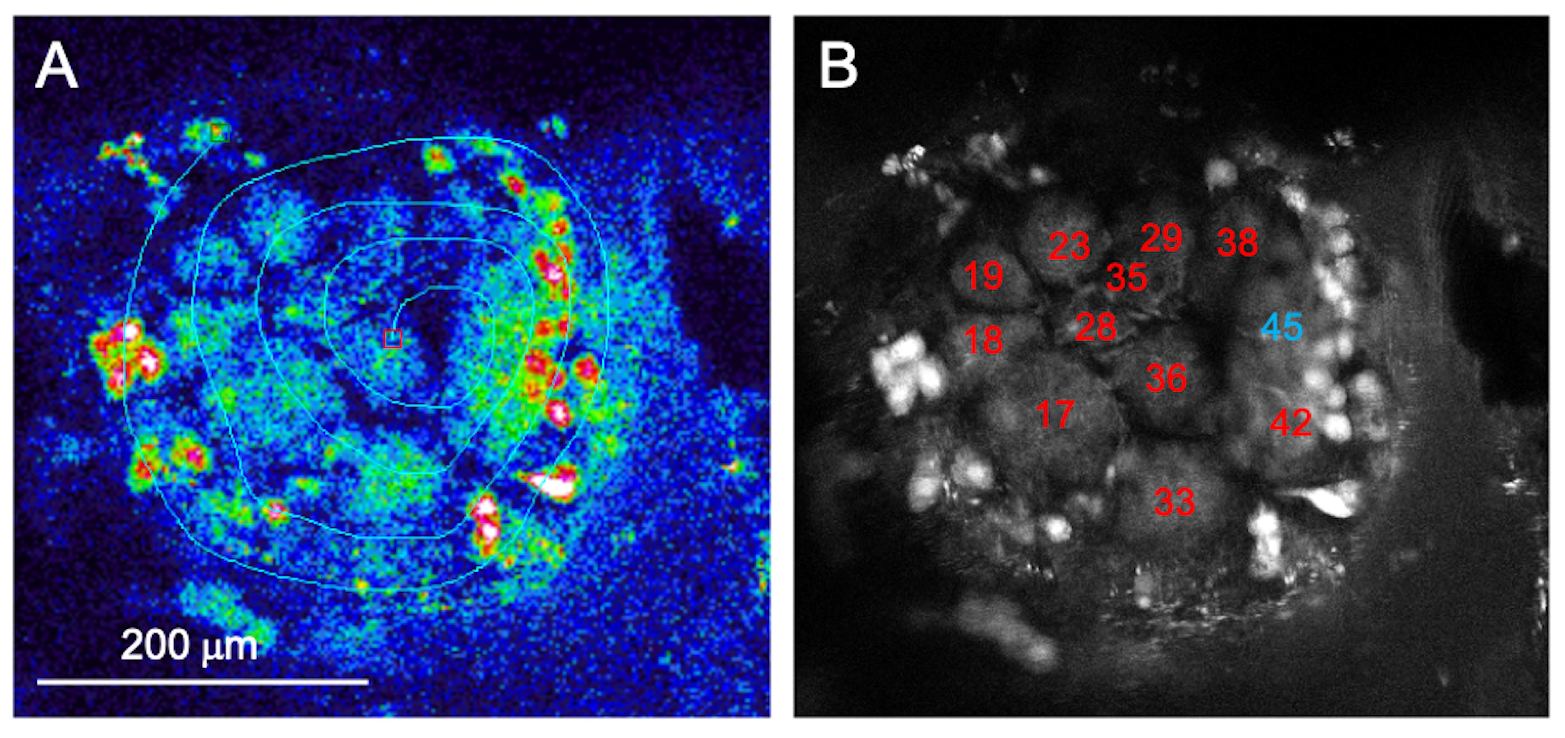
2.3. Calcium Imaging Acquisition and Data Processing
2.4. Granger Causality
2.5. Obtaining GC Maps
2.6. Network Analysis
2.7. Stereotypy Analysis
3. Results
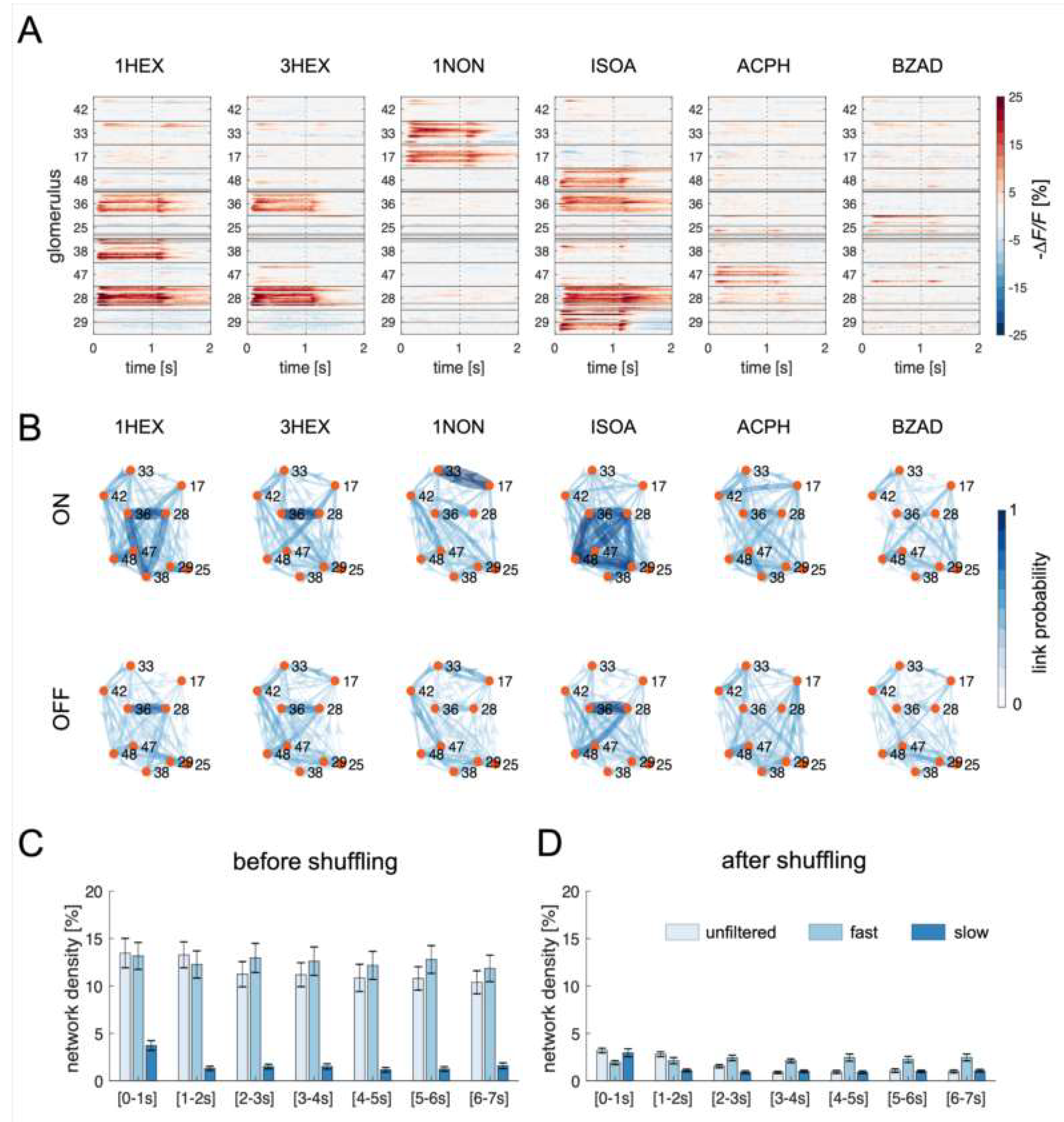
3.1. Node-Centered Odor Response Maps
3.2. Edge-Centered Odor Response Maps
3.2.1. Difference between Resting-State and Odor-Induced Connectivity
3.2.2. GC Maps Do Not Primarily Reflect Glomerular Response Similarity
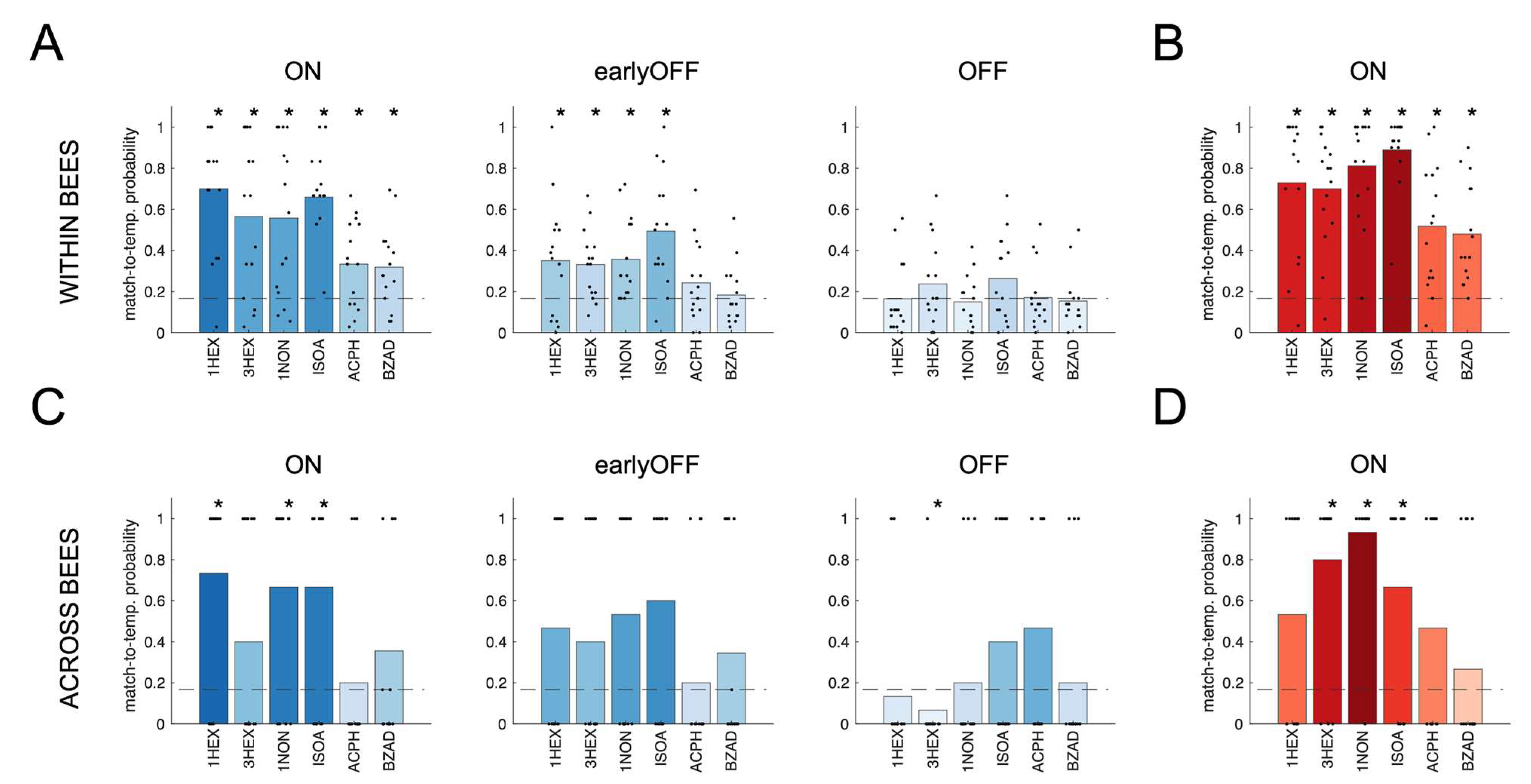
3.3. Causal Functional Connectivity Contains Odor Information
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paoli, M.; Galizia, G.C. Olfactory Coding in Honeybees. Cell. Tissue Res. 2021, 383, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Galizia, C.G.; Sachse, S.; Rappert, A.; Menzel, R. The Glomerular Code for Odor Representation Is Species Specific in the Honeybee Apis Mellifera. Nat. Neurosci. 1999, 2, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Moreaux, L.; Laurent, G. Estimating Firing Rates from Calcium Signals in Locust Projection Neurons in Vivo. Front. Neural Circuits 2007, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Sachse, S.; Rappert, A.; Galizia, C.G. The Spatial Representation of Chemical Structures in the Antennal Lobe of Honeybees: Steps towards the Olfactory Code. Eur. J. Neurosci. 1999, 11, 3970–3982. [Google Scholar] [CrossRef]
- Sachse, S.; Galizia, C.G. The Coding of Odour-Intensity in the Honeybee Antennal Lobe: Local Computation Optimizes Odour Representation. Eur. J. Neurosci. 2003, 18, 2119–2132. [Google Scholar] [CrossRef]
- Guerrieri, F.; Schubert, M.; Sandoz, J.C.; Giurfa, M. Perceptual and Neural Olfactory Similarity in Honeybees. PLoS Biol. 2005, 3, 0718–0732. [Google Scholar] [CrossRef]
- Haase, A.; Rigosi, E.; Trona, F.; Anfora, G.; Vallortigara, G.; Antolini, R.; Vinegoni, C. In-Vivo Two-Photon Imaging of the Honey Bee Antennal Lobe. Biomed. Opt. Express 2011, 2, 131. [Google Scholar] [CrossRef]
- Yaksi, E.; Wilson, R.I. Electrical Coupling between Olfactory Glomeruli. Neuron 2010, 67, 1034–1047. [Google Scholar] [CrossRef]
- Paoli, M.; Albi, A.; Zanon, M.; Zanini, D.; Antolini, R.; Haase, A. Neuronal Response Latencies Encode First Odor Identity Information across Subjects. J. Neurosci. 2018, 38, 9240–9251. [Google Scholar] [CrossRef]
- Paoli, M.; Weisz, N.; Antolini, R.; Haase, A. Spatially Resolved Time-Frequency Analysis of Odour Coding in the Insect Antennal Lobe. Eur. J. Neurosci. 2016, 44, 2387–2395. [Google Scholar] [CrossRef]
- Olsen, S.R.; Bhandawat, V.; Wilson, R.I. Excitatory Interactions between Olfactory Processing Channels in the Drosophila Antennal Lobe. Neuron 2007, 54, 89–103. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, W.; Qiao, W.; Hu, A.; Wang, Z. Functional Connectivity and Selective Odor Responses of Excitatory Local Interneurons in Drosophila Antennal Lobe. Neuron 2010, 67, 1021–1033. [Google Scholar] [CrossRef]
- Das, S.; Trona, F.; Khallaf, M.A.; Schuh, E.; Knaden, M.; Hansson, B.S.; Sachse, S. Electrical Synapses Mediate Synergism between Pheromone and Food Odors in Drosophila Melanogaster. Proc. Natl. Acad. Sci. USA 2017, 114, E9962–E9971. [Google Scholar] [CrossRef]
- Girardin, C.C.; Kreissl, S.; Galizia, C.G. Inhibitory Connections in the Honeybee Antennal Lobe Are Spatially Patchy. J. Neurophysiol. 2012, 109, 332–343. [Google Scholar] [CrossRef]
- Linster, C.; Sachse, S.; Galizia, C.G. Computational Modeling Suggests That Response Properties Rather than Spatial Position Determine Connectivity between Olfactory Glomeruli. J. Neurophysiol. 2005, 93, 3410–3417. [Google Scholar] [CrossRef]
- Geweke, J. Measurement of Linear Dependence and Feedback Between Multiple Time Series. J. Am. Stat. Assoc. 1982, 77, 304. [Google Scholar] [CrossRef]
- Chiarion, G.; Sparacino, L.; Antonacci, Y.; Faes, L.; Mesin, L. Connectivity Analysis in EEG Data: A Tutorial Review of the State of the Art and Emerging Trends. Bioengineering 2023, 10, 372. [Google Scholar] [CrossRef]
- Faes, L.; Erla, S.; Nollo, G. Measuring Connectivity in Linear Multivariate Processes: Definitions, Interpretation, and Practical Analysis. Comput. Math. Methods Med. 2012, 2012, 140513. [Google Scholar] [CrossRef]
- Sheikhattar, A.; Miran, S.; Liu, J.; Fritz, J.B.; Shamma, S.A.; Kanold, P.O.; Babadi, B. Extracting Neuronal Functional Network Dynamics via Adaptive Granger Causality Analysis. Proc. Natl. Acad. Sci. USA 2018, 115, E3869–E3878. [Google Scholar] [CrossRef]
- Chen, X.; Ginoux, F.; Carbo-Tano, M.; Mora, T.; Walczak, A.M.; Wyart, C. Granger Causality Analysis for Calcium Transients in Neuronal Networks, Challenges and Improvements. Elife 2023, 12, e81279. [Google Scholar] [CrossRef]
- Paoli, M.; Haase, A. In Vivo Two-Photon Imaging of the Olfactory System in Insects. In Olfactory Receptors; Simoes de Souza, F.A.G., Ed.; Humana Press: New York, NY, USA, 2018; pp. 179–219. ISBN 978-1-4939-8609-5. [Google Scholar]
- Sachse, S.; Galizia, C.G. Role of Inhibition for Temporal and Spatial Odor Representation in Olfactory Output Neurons: A Calcium Imaging Study. J. Neurophysiol. 2002, 87, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Hourcade, B.; Perisse, E.; Devaud, J.-M.; Sandoz, J.-C. Long-Term Memory Shapes the Primary Olfactory Center of an Insect Brain. Learn. Mem. 2009, 16, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.F.; Fernandez, P.C.; Villareal, F.; Muezzinoglu, K.; Huerta, R.; Galizia, C.G.; Smith, B.H. Nonassociative Plasticity Alters Competitive Interactions among Mixture Components in Early Olfactory Processing. Eur. J. Neurosci. 2013, 37, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Bestea, L.; Paoli, M.; Arrufat, P.; Ronsin, B.; Carcaud, J.; Sandoz, J.-C.; Velarde, R.; Giurfa, M.; de Brito Sanchez, M.G. The Short Neuropeptide F Regulates Appetitive but Not Aversive Responsiveness in a Social Insect. iScience 2022, 25, 103619. [Google Scholar] [CrossRef] [PubMed]
- Paoli, M.; Anesi, A.; Antolini, R.; Guella, G.; Vallortigara, G.; Haase, A. Differential Odour Coding of Isotopomers in the Honeybee Brain. Sci. Rep. 2016, 6, 21893. [Google Scholar] [CrossRef] [PubMed]
- Boch, R.; Shearer, D.A.; Stone, B.C. Identification of Iso-Amyl Acetate as an Active Component in the Sting Pheromone of the Honey Bee. Nature 1962, 195, 1018–1020. [Google Scholar] [CrossRef]
- Galizia, C.G.; McIlwrath, S.L.; Menzel, R. A Digital 3-Dimensional Atlas of the Honeybee Antennal Lobe Based on Optical Sections Acquired Using Confocal Micoscropy. Cell Tissue Res. 1999, 295, 383–394. [Google Scholar] [CrossRef]
- Barnett, L.; Seth, A.K. Granger Causality for State-Space Models. Phys. Rev. E 2015, 91, 040101. [Google Scholar] [CrossRef]
- Hochberg, Y.; Tamhane, A.C. Multiple Comparison Procedures; Wiley: New York, NY, USA, 1987; ISBN 9780470316672. [Google Scholar]
- Nollo, G.; Faes, L.; Pellegrini, B.; Porta, A.; Antolini, R. Synchronization Index for Quantifying Nonlinear Causal Coupling between RR Interval and Systolic Arterial Pressure after Myocardial Infarction. Comput. Cardiol. 2000, 27, 143–146. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex Network Measures of Brain Connectivity: Uses and Interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Münch, D.; Galizia, C.G. DoOR 2.0—Comprehensive Mapping of Drosophila Melanogaster Odorant Responses. Sci. Rep. 2016, 6, 21841. [Google Scholar] [CrossRef]
- Barnett, L.; Barrett, A.B.; Seth, A.K. Granger Causality and Transfer Entropy Are Equivalent for Gaussian Variables. Phys. Rev. Lett. 2009, 103, 238701. [Google Scholar] [CrossRef]
- Galán, R.F.; Weidert, M.; Menzel, R.; Herz, A.V.M.; Galizia, C.G. Sensory Memory for Odors Is Encoded in Spontaneous Correlated Activity Between Olfactory Glomeruli. Neural Comput. 2006, 18, 10–25. [Google Scholar] [CrossRef]
- Kazama, H.; Wilson, R.I. Origins of Correlated Activity in an Olfactory Circuit. Nat. Neurosci. 2009, 12, 1136–1144. [Google Scholar] [CrossRef]
- Stopfer, M.; Bhagavan, S.; Smith, B.H.; Laurent, G. Impaired Odour Discrimination on Desynchronization of Odour-Encoding Neural Assemblies. Nature 1997, 390, 70–74. [Google Scholar] [CrossRef]
- Assisi, C.; Bazhenov, M. Synaptic Inhibition Controls Transient Oscillatory Synchronization in a Model of the Insect Olfactory System. Front. Neuroeng. 2012, 5, 7. [Google Scholar] [CrossRef]
- Assisi, C.; Stopfer, M.; Bazhenov, M. Using the Structure of Inhibitory Networks to Unravel Mechanisms of Spatiotemporal Patterning. Neuron 2011, 69, 373–386. [Google Scholar] [CrossRef]
- MacLeod, K.; Bäcker, A.; Laurent, G. Who Reads Temporal Information Contained across Synchronized and Oscillatory Spike Trains? Nature 1998, 395, 693–698. [Google Scholar] [CrossRef]
- Linster, C.; Cleland, T.A. How Spike Synchronization Among Olfactory Neurons Can Contribute to Sensory Discrimination. J. Comput. Neurosci. 2001, 10, 187–193. [Google Scholar] [CrossRef]
- Andrione, M.; Timberlake, B.F.; Vallortigara, G.; Antolini, R.; Haase, A. Morphofunctional Experience-Dependent Plasticity in the Honeybee Brain. Learn. Mem. 2017, 24, 622–629. [Google Scholar] [CrossRef]
- Nouvian, M.; Mandal, S.; Jamme, C.; Claudianos, C.; D’Ettorre, P.; Reinhard, J.; Barron, A.B.; Giurfa, M. Cooperative Defence Operates by Social Modulation of Biogenic Amine Levels in the Honey Bee Brain. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172653. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. Functional and Effective Connectivity in Neuroimaging: A Synthesis. Hum. Brain Mapp. 1994, 2, 56–78. [Google Scholar] [CrossRef]
- Schäfer, S.; Bicker, G. Distribution of GABA-like Immunoreactivity in the Brain of the Honeybee. Neuroscience 1986, 246, 287–300. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paoli, M.; Antonacci, Y.; Albi, A.; Faes, L.; Haase, A. Granger Causality Analysis of Transient Calcium Dynamics in the Honey Bee Antennal Lobe Network. Insects 2023, 14, 539. https://doi.org/10.3390/insects14060539
Paoli M, Antonacci Y, Albi A, Faes L, Haase A. Granger Causality Analysis of Transient Calcium Dynamics in the Honey Bee Antennal Lobe Network. Insects. 2023; 14(6):539. https://doi.org/10.3390/insects14060539
Chicago/Turabian StylePaoli, Marco, Yuri Antonacci, Angela Albi, Luca Faes, and Albrecht Haase. 2023. "Granger Causality Analysis of Transient Calcium Dynamics in the Honey Bee Antennal Lobe Network" Insects 14, no. 6: 539. https://doi.org/10.3390/insects14060539
APA StylePaoli, M., Antonacci, Y., Albi, A., Faes, L., & Haase, A. (2023). Granger Causality Analysis of Transient Calcium Dynamics in the Honey Bee Antennal Lobe Network. Insects, 14(6), 539. https://doi.org/10.3390/insects14060539







