Simple Summary
Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) is a natural predatory enemy insect widely distributed in north China and has been widely used in agricultural practice. Owing to a low daily average temperature from December to February of the following year in greenhouses and the facultative diapause of adult H. axyridis, the application of this ladybird in greenhouses in north China is limited. It is of great importance to regulate its predatory ability to improve its biological control efficiency in greenhouses. The 5-hydroxytryptamine (5-HT) was found to play a regulatory role in the predation of H. axyridis in our previous study, but the 5-HT receptor of H. axyridis has not been clearly identified so far, and its expression characteristics are poorly understood. In this study, the 5-HT receptor gene of H. axyridis was cloned and identified by phylogenetic tree construction and multiple sequence alignments. The expression patterns of each receptor in different developmental stages and tissues were analyzed by quantitative real-time PCR (qRT-PCR). The results showed that H. axyridis expressed four 5-HT receptor subtypes, named 5-HT1AHar, 5-HT1BHar, 5-HT2Har, and 5-HT7Har. All 4 receptors were expressed at high levels in the adult stage, especially in 2-day-old adults, with expression levels of 18.72-fold (male) and 14.21-fold (female) of that in eggs for 5-HT1AHar, 32.27-fold (male) and 83.58-fold (female) of that in eggs for 5-HT1BHar, 36.82-fold (male) and 119.35-fold (female) of that in eggs for 5-HT2Har, and 165.47-fold (male) and 115.59-fold (female) of that in eggs for 5-HT7Har. The level of expression for each receptor gene decreased with the advance of day-age in adults. The levels of expression of 5-HT1BHar, 5-HT2Har, and 5-HT7Har were low at the egg, larval, and pupal stages, and 5-HT1AHar was not expressed in the larval stage. The four receptors were expressed in the nervous system, digestive tract, pectoral muscles, and male and female gonads of adults. The 5-HT1AHar was expressed at a high level in the pectoral muscle (6.75-fold of that in the nervous system), 5-HT1BHar in male gonads (1.02-fold of that in the nervous system) and the nervous system, 5-HT2Har in male gonads (5.74-fold of that in the nervous system), and 5-HT7Har in the digestive tract (1.81-fold of that in the nervous system). The results of this study will lay a foundation for research on the function of the 5-HT receptor by RNA interference in the regulation of predation by H. axyridis.
Abstract
It has been found that 5-hydroxytryptamine (5-HT) modulates the feeding of some insects, and this phenomenon was found in Harmonia axyridis (Pallas) by our previous study. An understanding of the 5-HT system in this beetle is helpful for utilizing 5-HT to modulate its predation to improve biological control efficiency, especially in greenhouses in winter in north China. This is because 5-HT influences diapause in insects by modulating the synthesis and release of prothoracic hormone (PTTH) and, therefore, influences feeding. To elucidate the molecular basis of the H. axyridis 5-HT system, reverse-transcription polymerase chain reaction (RT-PCR), multiple sequence alignment, and phylogenetic tree construction were used to identify the 5-HT receptor in H. axyridis, and quantitative real-time PCR (qRT-PCR) was used to analyze the expression pattern of these receptor genes in different developmental stages and in the nervous system (brain + ventral nerve cord), digestive tract, pectoral muscles, and gonads of the adult ladybird. The results showed that four 5-HT receptors were identified in H. axyridis, named 5-HT1AHar, 5-HT1BHar, 5-HT2Har, and 5-HT7Har. The four receptors were expressed at high levels in the adult stage, especially in 2-day-old adults, with expression levels of 18.72-fold (male) and 14.21-fold (female) of that in eggs for 5-HT1A, 32.27-fold (male) and 83.58-fold (female) of that in eggs for 5-HT1B, 36.82-fold (male) and 119.35-fold (female) of that in eggs for 5-HT2, and 165.47-fold (male) and 115.59-fold (female) of that in eggs for 5-HT7. The level of expression decreased with the advance of day-age in adults. The levels of expression of 5-HT1BHar, 5-HT2Har, and 5-HT7Har were low at the egg, larval, and pupal stages, and 5-HT1AHar was not expressed in the larval stage. The four receptors were expressed in the nervous system, digestive tract, pectoral muscles, and male and female gonads. The 5-HT1AHar was expressed at a high level in the pectoral muscle (6.75-fold of that in the nervous system), 5-HT1BHar in male gonads (1.02-fold of that in the nervous system) and the nervous system, 5-HT2Har in male gonads (5.74-fold of that in the nervous system), and 5-HT7Har in the digestive tract (1.81-fold of that in the nervous system). The results of this study will lay a foundation for research on the function of the 5-HT receptor by RNA interference in the regulation of predation by H. axyridis.
1. Introduction
The 5-hydroxytryptamine (5-HT), also known as serotonin, is an ancient and conserved small biological molecule widely distributed in nature. As a neurotransmitter, which is a chemical that transmits information between neurons or between neurons and effector cells such as muscle cells, gland cells, etc., 5-HT controls and regulates various important physiological activities of organisms including humans, nematodes, and insects [1]. The 5-HT in insects can regulate growth and development, reproduction [2,3,4,5], diapause [6], feeding [7,8], learning and memory [9], behavior [10,11,12], olfaction [13], circadian rhythm [14,15,16], immune response [17], movement [18], cardiac rhythm regulation [19], and gregarization [20], etc. Studying the 5-HT of insects is necessary to reveal the behavior and physiological mechanisms of insects, which are commonly the basis of beneficial insect utilization and pest control.
The 5-HT works primarily by acting on specific receptors. The 5-HT receptor is a phylogenetically ancient receptor that evolved 750 million years ago and is present in invertebrates and higher mammals [21]. At present, 5-HT receptors have been cloned in more than 10 species of insects; most of these receptors belong to the G-protein-coupled receptor superfamily [22]. G-protein-coupled receptors (GPCRs) are a receptor protein superfamily that contains seven α-helical transmembrane (TM) structures. GPCRs activate the second messenger pathway to transfer ligand signals into cells to regulate specific physiological processes via conjugated heterotrimer G proteins (α, β, γ subunit) [23,24]. GPCRs are involved in chemo- and photoreception, hormonal physiology, synaptic function, and a variety of other processes [25]. Three types of 5-HT receptor have been cloned in insects, namely 5-HT1, 5-HT2, and 5-HT7 [22]. In most insects, there are two subtypes of 5-HT1, namely 5-HT1A and 5-HT1B; there are also two subtypes of 5-HT2, namely 5-HT2A and 5-HT2B [22,26,27]. Qi et al. [28] identified a novel 5-HT8 receptor without homology with vertebrate 5-HTs in Pieris rapae. Li et al. [29] found the 5-HT1D subtype of 5-HT1 and the 5-HT7A and 5-HT7B subtypes of 5HT7 in Aedes aegypti.
Harmonia axyridis (Pallas) is a highly adaptable, natural predatory enemy insect widely distributed in north China. It has a strong ability to prey on aphids, Tetranychus mites, scale insects, and the eggs of some Lepidopteran pests. As an important natural enemy for biological control, it has been widely used in agricultural production in China [30]. Owing to a low daily average temperature from December to February of the following year in greenhouses and the facultative diapause of adult H. axyridis, the application of this ladybird in greenhouses in north China is limited, for the food intake by ladybirds in diapause is low. It is of great importance to regulate its predatory ability to improve its biological control efficiency in greenhouses. Wang et al. [6] found that 5HTRB locked the gate of PTTH release and synthesis in the Chinese silk moth, Antheraea Pernyi, and led to a delay of emergence of adults. We suspect that the 5-HT pathway may be involved in control of diapause in H. axyridis, and, therefore, influences its predation. A previous injection experiment showed that 5-HT plays an obvious regulatory role in the predation of H. axyridis (unpublished data), so it is important to study the 5-HT receptors of H. axyridis; but the 5-HT receptor of H. axyridis has not been clearly identified so far, and its expression characteristics are poorly understood. In this study, the 5-HT receptor gene of H. axyridis was cloned and identified by phylogenetic tree construction and multiple sequence alignments. The expression patterns of each receptor in different developmental stages and tissues were analyzed by quantitative real-time PCR (qRT-PCR). The purpose of this study was to lay a foundation for the functional study of the 5-HT receptor in H. axyridis.
2. Materials and Methods
2.1. Insects
The H. axyridis eggs were collected from peach trees on the campus at Qingdao Agricultural University in April 2019, and fed on Myzus persicae after hatching to establish a laboratory colony. The laboratory colony was established at 20 ± 1 °C and 65 ± 10% relative humidity under a photoperiod of 12 h light:12 h dark in a manual climatic box (RGN-300, Ningbo Southeast Instrument Co., Ltd., Ningbo City, China). M. persicae were cultivated on Chinese cabbage seedlings under the same conditions of temperature, light, and humidity in another manual climatic box. The H. axyridis used for experiments were collected from the laboratory colony.
Virgin H. axyridis adults at 14 days old were collected for 5-HT receptor cloning. Twenty eggs at one-day-old; fifteen first instar larvae at two-day-old; ten second instar larvae at two-day-old; five third instar larvae at two-day-old; two fourth instar larvae at two-day-old; two pupae at two-day-old; two virgin females at two-day-old, seven-day-old and fourteen-day-old; and two virgin males at two-day-old, seven-day-old, and fourteen-day-old were collected for analysis of the expression pattern of 5-HT receptor genes in different developmental stages. Five to ten virgin adults at sixteen-day-old were collected and anesthetized on ice to isolate the nervous system (brain + ventral nerve cord), digestive tract, pectoral muscles, and gonads for analysis of the expression pattern of the 5-HT receptor gene in different tissues; each tissue was removed to an Rnase-free Eppendorf centrifuge tube charged with ice-cold 0.9% normal saline. All the samples were frozen in liquid nitrogen and quickly transferred into a freezer at −80 °C for later use.
2.2. The mRNA Isolation and cDNA Synthesis
The samples were fully ground in liquid nitrogen, and total RNA was isolated with TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). The RNA isolated from each sample was dissolved in DEPC-treated water (Sangon Biotech, Shanghai, China), and the quality of the RNA was assessed on a 1% agarose gel, and quantities were determined on a spectrophotometer (Nano Photometer TM-Class, Implen GmbH, Munich, Germany). The isolated RNA with A260/A280 values ranging from 1.8 to 2.0 was used for cDNA synthesis. Single-strand cDNA was synthesized from 3μL RNA of 200 μg/μL using a ReverTra Ace-a-kit (TaKaRa, Dalian, China).
2.3. Cloning of the Serotonin Receptor Gene from H. axyridis
According to the predicted 5-HT receptor gene open reading frame (ORF) of H. axyridis in the InsectBase (www.insect-genome.com accessed on 9 November 2021), primers were designed using Primer 5.0 (the base sequences of the primers are listed in Table 1). PCR reactions were carried out with a 2× Spark FastHiFi PCR Master Mix kit (Qingdao Haosail Science Co., Ltd., Qingdao, China) according to the manufacturer’s instructions and consisted of one cycle at 94 °C for 3 min, 40 cycles at 94 °C for 30 s, 50 °C (for Haxy004670.1) or 52 °C (for Haxy019702.1 and Haxy005697.1) or 55 °C (for Haxy019754) for 30 s, and 72 °C for 30 s, with an out-of-cycle extension step of 72 °C for 10 min. The annealing temperatures for each gene are listed in Table 1. The PCR product was separated by electrophoresis on a 1.0% agarose gel, and the purified product was sent to Sangon Biotech Co., LTD. (Shanghai, China) for sequencing using a 3730 XL DNA analyzer (Applied Biosystems, Carlsbad, CA, USA).

Table 1.
Primers used for gene cloning.
2.4. Multiple Sequence Alignment and Phylogenetic Analysis
The derived amino acid sequences were used for phylogenetic analysis. The phylogenetic tree and molecular evolutionary analyses were performed using MEGA 7.0 software with the neighbor-joining method [31]. The accession numbers of the 5-HT receptor genes of other insect species are listed in Table 2. Multiple sequence alignments were identified by BLAST programs from the NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi accessed on 11 October 2022). Multiple sequence alignments of the complete amino acid sequences were perform ed with ClustalX (http://www.genome.jp/tools-bin/clustalw accessed on 11 October 2022). The ENDscript/ESPript website (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi accessed on 12 October 2022) was used to map the sequence alignment results. The transmembrane segments were predicted with Deep TMHMM 2.0 (https://dtu.biolib.com/DeepTMHMM accessed on 7 March 2023).

Table 2.
Accession numbers of 5-HT receptors in different insect species.
2.5. Quantitative Real-Time PCR (qRT-PCR)
According to the target genes sequence obtained in Section 2.3, primers for qRT-PCR were designed using Primer 5.0, and the 18sRNA (F: ACGGACTTCGGTAGGACG; R: CGCAGACAATCCCGAAA) gene was used as the reference gene for qRT-PCR. The base sequences of the primers are listed in Table 3. The qRT-PCR reactions were carried out with a Spark Taq PCR Master Mix kit (Qingdao Haosail Science Co., Ltd., Qingdao, China) according to the manufacturer’s instructions, and consisted of 1 cycle at 94 °C for 180 s, 40 cycles of 94 °C for 15 s, 55 °C (for Haxy019702.1, Haxy004670.1 and Haxy005697.1) or 60 °C (for Haxy019754) for 15 s, and 72 °C for 20 s. The annealing temperatures for each gene are listed in Table 3. There were three biological replicates for each treatment and three technical replicates for each biological replicate.

Table 3.
Primers for fluorescent quantitative PCR reaction.
2.6. Statistical Analysis
Relative quantitative data were calculated according to the 2−ΔΔCt method [32]. The expression level of the 5-HT receptor genes at the egg stage was set to be 1, and those in different developmental stages were compared with those in the egg stage. The expression level of 5-HT receptor genes in the nervous system was set to be 1, and those in different tissues were compared with those in the nervous system. Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. Statistical significance was associated with values of p < 0.05.
3. Results
3.1. Cloning of the 5-HT Receptor Gene
The electrophoretogram of cloned products was shown in Figure 1. A Haxy004670.1 fragment of 772 bp, Haxy005697.1 fragment of 834 bp, Haxy019702.1 fragment of 1061 bp, and Haxy019754 fragment of 1053 bp were cloned, respectively.
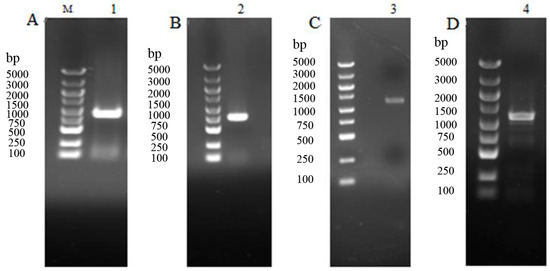
Figure 1.
Electrophoretogram of PCR products of 5-HT receptor genes in Harmonia axyridis. M: BM 5000+ marker, lanes 1–4: PCR amplification products of Haxy004670.1, Haxy005697.1, Haxy019702.1, and Haxy019754. (A) PCR products of Haxy004670.1. (B) PCR products of Haxy005697.1. (C) PCR products of Haxy019702.1. (D) PCR products of Haxy019754.
3.2. Multiple Sequence Analysis of 5-HT Receptor Genes
The amino acid sequences deduced from the Harmonia 5-HT receptor genes were compared with those of other insects, and the result indicated that the Harmonia 5-HT receptor was structurally similar to the 5-HT receptors of other known insects, all with similarity exceeding 50%. Haxy004670.1 was 68.06% identical to the 5-HT1A receptors of Tribolium castaneum. Haxy005697.1 was 78.06% and 66.00% identical to the 5-HT1B receptors of T. castaneum and A. pernyi, respectively. Haxy019702.1 was 65.48% and 59.57% identical to the 5-HT2A receptors of Drosophila melanogaster and Apis mellifera, respectively. Haxy019754 was 78.70% and 55.70% identical to the 5-HT7 receptors of Aedes aegypti and A. mellifera, respectively.
The cDNA fragment from Haxy004670.1 obtained through gene cloning encoded three transmembrane (TM) segments corresponding to the fifth to seventh TM segments of GPCRs (TM5, TM6, and TM7 in Figure 2A). The molecular phylogenetics of 5-HT1A receptors were analyzed using the deduced amino acid sequence of the partial cDNA fragment of Haxy004670.1 and those of the corresponding part of various 5-HT receptors in other species; the results indicated that Haxy004670.1 was closely related to the insect 5-HT1A proteins (Figure 3A).
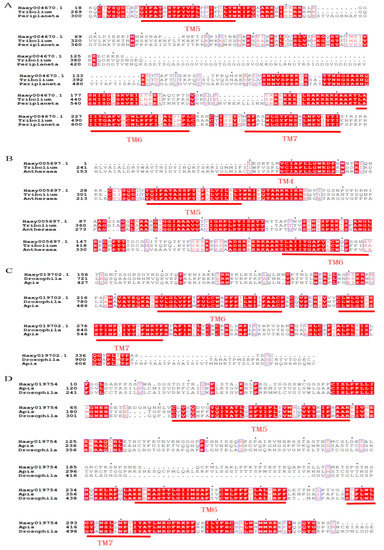
Figure 2.
Amino acid sequence alignment of 5-HT receptors from Harmonia axyridis and other insect species. (A) Alignment against sequences of Haxy004670.1 with 5-HT1A receptors from T. Castaneum and P. americana. (B) Alignment against sequences of Haxy005697.1 with 5-HT1B receptors from T. Castaneum and A. pernyi. (C) Alignment against sequences of Haxy019702.1 with 5-HT2A receptors from D. melanogaster and A. mellifera. (D) Alignment against sequences of Haxy019754 with 5-HT7 receptors from D. melanogaster and A. mellifera. The alignment was generated using ClustalX alignment software. Putative transmembrane domains are indicated by red bars (TM1-7). Identical residues between the receptors are shown as white characters against a red background. Conservatively substituted residues are framed in blue. The accession numbers of 5-HT in relevant species in GenBank are listed in Table 2.
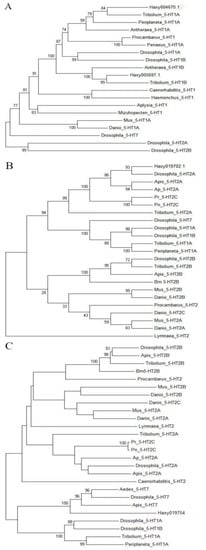
Figure 3.
Phylogenetic tree based on amino acid sequences of 5-HT receptors of Harmonia axyridis and other species (1000 replicates). The GenBank accession numbers of the proteins used for phylogenetic analysis are listed in Table 2. (A) Phylogenetic tree for Haxy004670.1. and Haxy005697.1. (B) Phylogenetic tree for Haxy019702.1. (C) Phylogenetic tree for Haxy019754.
The cDNA fragment from Haxy019754 obtained through gene cloning encoded three transmembrane (TM) segments, corresponding to the fourth to sixth TM segments of GPCRs (TM4, TM5, and TM6 in Figure 2B). The molecular phylogenetics of 5-HT1B receptors were analyzed using the deduced amino acid sequence of the partial cDNA fragment of Haxy005697.1 and those of the corresponding parts of various 5-HT receptors in other species; the results indicated that Haxy005697.1 was closely related to the insect 5-HT1B proteins (Figure 3A).
The cDNA fragment of Haxy019702.1 obtained through gene cloning encoded two transmembrane (TM) segments corresponding to the fourth to sixth TM segments of GPCRs (TM6 and TM7 in Figure 2C). The molecular phylogenetics of 5-HT2 receptors were analyzed using the deduced amino acid sequence of the partial cDNA fragment of Haxy019702.1 and those of the corresponding parts of various 5-HT receptors in other species; the results indicated that Haxy019702.1 was closely related to the insect 5-HT2 proteins (Figure 3B).
The cDNA fragment of Haxy019754 obtained through gene cloning encoded two transmembrane (TM) segments corresponding to the fifth to seventh TM segments of GPCRs (TM5, TM6, and TM7 in Figure 2D). The molecular phylogenetics of 5-HT7 receptors were analyzed using the deduced amino acid sequence of the partial cDNA fragment of Haxy019754 and those of the corresponding parts of various 5-HT receptors in other species; the results indicated that Haxy019754 was closely related to the insect 5-HT7 proteins (Figure 3C).
By multiple sequence alignment and construction of a phylogenetic tree, Haxy004670.1 was identified as 5-HT1A, Haxy005697.1 was identified as 5-HT1B, Haxy019702.1 was identified as 5-HT2, and Haxy019754 was identified as 5-HT7. According to the sequence similarity and the current nomenclature rules of 5-HT receptors, each newly identified gene was named after the receptor type plus genus name [33], so they were named 5-HT1AHar (GenBank accession number: OQ724534), 5-HT1BHar (GenBank accession number: OQ724535), 5-HT2Har (GenBank accession number: OQ724536), and 5-HT7Har (GenBank accession number: OQ724536).
3.3. Expression Pattern of 5-HT Receptor Genes in Different Developmental Stages in H. axyridis
The expression patterns of the 5-HT receptor genes in different developmental stages were investigated using RT-qPCR analysis. The following twelve developmental stages, egg, first instar larva, second instar larva, third instar larva, fourth instar larva, pupa, two-day-old adult, seven-day-old adult, and fourteen-day-old adult were subjected to RT-qPCR analysis. As shown in Figure 4, the expression profiles of 5-HT receptor genes in different developmental stages of H. axyridis are generally consistent with only slight differences. All receptor genes were expressed at high levels in the adult stage; the levels of expression in the 2-day-old and 7-day-old adults were significantly higher than that in the preadult stage. In the adult stage, the level of expression decreased with age, and a sharp drop was found in the 14-day-old female adults. The 5-HT1AHar was not expressed in the larval stage and had its highest level of expression in 2-day-old adult males (18.72-fold of that in eggs), differing from that in 2-day-old females (14.21-fold of that in eggs). The highest levels of expression of 5-HT1BHar and 5-HT2Har were both recorded in 2-day-old female adults (83.58-fold of that in eggs for 5-HT1BHar and 119.35-fold of that in eggs for 5-HT2Har), and both differed from their expression in 2-day-old male adults (32.27-fold of that in eggs for 5-HT1BHar and 36.82-fold of that in eggs for 5-HT2Har). The highest expression level of 5-HT7Har was recorded in 2-day-old male adults (165.47-fold of that in eggs), which differed from that in female adults (115.59-fold of that in eggs). The level of expression of 5-HT7Har in the larval stage increased with the increase in instar, and the level of expression in the fourth instar larvae was highest (24.70-fold of that in eggs), differing from that in other instars (2.86~10.14-fold of that in eggs). The levels of expression of all the 5-HT receptors genes were low in the pupal stage.
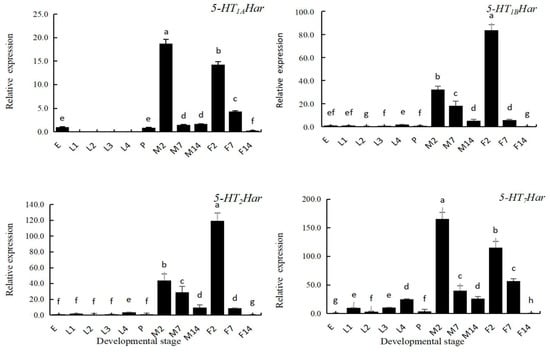
Figure 4.
The expression pattern of four 5-HT receptor genes in different developmental stages in Harmonia axyridis by qPCR. Abbreviations: egg (E), first instar larvae (L1), second instar larvae (L2), third instar larvae (L3), fourth instar larvae (L4), pupa (P), 2-day-old-male (M2), 7-day-old male (M7), 14-day-old male (M14), 2-day-old female (F2), 7-day-old female (F7), 14-day-old female (F14). The expression level of 5-HT receptor genes at egg stage was set to be 1, and those in different developmental stages were compared with that in egg stage. Different lowercase letters on the columns indicate significant differences in gene expression between different stages. (p < 0.05, Tukey’s test).
3.4. The Expression Pattern of 5-HT Receptor Genes in Different Tissues in H. axyridis
As shown in Section 3.3, the level of expression of 5-HT receptor genes in H. axyridis is highest at the adult stage. Therefore, adult H. axyridis were collected for analysis of the expression pattern of 5-HT receptor genes in different tissues. Five tissues of the ladybird including the nervous system, digestive tract, pectoral muscles, female gonads and male gonads were subjected to RT-qPCR analysis. As shown in Figure 5, the highest level of expression of 5-HT1AHar was recorded in the pectoral muscles (6.75-fold of that in the nervous system), followed by the male gonads (2.66-fold of that in the nervous system), nervous system, digestive tract (0.60-fold of that in the nervous system) and female gonads (0.04-fold of that in the nervous system), and the level of expression in different tissues differed. The highest level of expression of 5-HT1BHar was recorded in the male gonads (1.02-fold of that in the nervous system) and the nervous system (the levels of expression did not differ from each other), followed by the digestive tract (0.08-fold of that in the nervous system), female gonads (0.12-fold of that in the nervous system), and pectoral muscles (0.16-fold of that in the nervous system), and the level of expression in the digestive tract did not differ from that in the female gonads. The highest level of expression of 5-HT2Har was recorded in the male gonads (5.74-fold of that in the nervous system), followed by the female gonads (1.71-fold of that in the nervous system), nervous system, pectoral muscles (0.27-fold of that in the nervous system), and digestive tract (0.23-fold of that in the nervous system), and the levels of expression in the last two tissues did not differ from each other. The highest level of expression of 5-HT7Har was recorded in the digestive tract (1.81-fold of that in the nervous system), followed by the male gonads (1.06-fold of that in the nervous system) and the nervous system, the pectoral muscle (0.63-fold of that in the nervous system) and the female gonads (0.05-fold of that in the nervous system), and the level of expression in the nervous system did not differ from that in the male gonads.
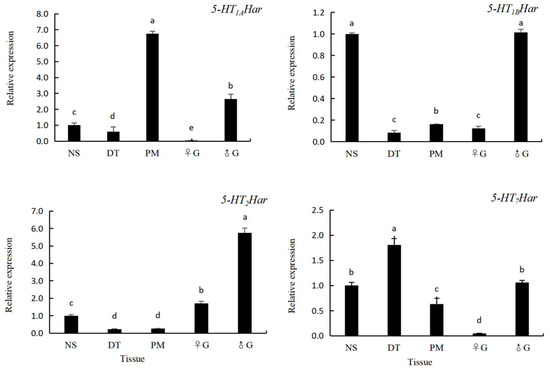
Figure 5.
The expression pattern of four 5-HT receptor genes in different tissues in 14-day-old adult Harmonia axyridis by qPCR. Abbreviations: nervous system (NS), digestive tract (DT), pectoral muscles (PM), female gonads (♀G), and male gonad (♂G). The expression level of 5-HT receptor genes in the nervous system was set to be 1, and those in different tissues were compared with that in the nervous system. Different lowercases letters on the columns indicate significant differences between expression levels in different tissues. (p < 0.05, Tukey’s test).
4. Discussion
In this study, four 5-HT receptor genes, 5-HT1AHar, 5-HT1BHar, 5-HT2Har, and 5-HT7Har, were identified in H. axyridis. Sequence alignment revealed that the amino acid residues of the 5-HT receptor were conserved among different insect species. Phylogenetic analysis showed that the same 5-HT receptor family subtypes in different organisms clustered preferentially in one clade, followed by 5-TH receptors in different species clustered in different clades based on genetic distance.. The same family of receptors first clustered into one clade, suggesting that 5-HT1, 5-HT2, and 5-HT7 receptors were formed before the divergence of vertebrates from invertebrates. Each receptor type was differentiated into different subtypes; for example, insect 5-HT1 diverged into 5-HT1A and 5-HT1B, and 5-HT2 diverged into 5-HT2A and 5-HT2B. These isoforms of the 5-HT receptor each clustered into a small clade that may have developed independently in vertebrates and invertebrates.
It was shown that 5-HT receptor genes were expressed differently in different developmental stages of H. axyridis. Furthermore, 5-HT1BHar, 5-HT2Har, and 5-HT7Har were expressed in all the developmental stages of H. axyridis, while 5-HT7Har was expressed at all stages except larva. The levels of expression of the four receptors in the adult stage were significantly higher than those in the preadult stage; this discovery coincides with that of Wang et al. [33] in their study of expression patterns of four 5-HT receptors (5-HT1A, 5-HT1B, 5-HT2A, and 5-HT2B) in different developmental stages in Helicoverpa armigera (Hübner). Four 5-HT receptor genes in H. axyridis were expressed at high levels in the adult stage of H. axyridis, indicating that these receptors may be involved in physiological functions in adults. The 5-HT1AHar was not expressed in the larval stage but only in the adult stage and the developmental stage adjacent to the adult, indicating that this gene may be related to physiological phenomena specific to the adult. The expression of 5-HT7Har in the larval stage of H. axyridis showed an upward trend with the increase in instar and reached the highest level at the fourth instar. The studies of Zhao [34] and Yu [35] all showed that daily predation by H. axyridis larvae was greatest at the fourth instar, so we suspected that the expression of 5-HT7Har was related to predation. The 5-HT7 receptor affects feeding by mediating gastrointestinal smooth muscle relaxation in mammals [36], and this phenomenon was also found in the honeybee, Apis mellifera mellifera [8]. However, the relationship between the expression of 5-HT7Har and predation by H. axyridis needs further study. Moreover, 5-HT innervation and 5-HT receptors were found in the alimentary tract of A. mellifera mellifera [8], Leucophaea maderae [37], Camponotus mus (Roger) [38], and Locusta migratoria (Fabricius) [39]. A study of the distribution of 5-HT innervation and 5-HT receptors may be helpful for understanding the mechanism by which 5-HT regulates predation by H. axyridis. It was also found in our study that the expression of four 5-HT receptor genes in H. axyridis decreased with the advance of adult day-age, indicating that these 5-HT receptors were related to some age-dependent physiological functions of adults. The levels of expression of 5-HT1AHar and 5-HT7Har were significantly higher in males than in females, while the levels of expression of 5-HT1BHar and 5-HT2Har were higher in females; these phenomena suggested that the expression of these genes may be associated with sex-related physiological functions.
The expression patterns of 5-HT receptors in different tissues can help generate hypotheses related to their biological significance in insects. Tissue localization of 5-HT receptors has been reported in a variety of insects and arthropods. H. armigera (Hübner) 5-HT1 (including 5-HT1A and 5-HT1B) [33], Gryllus bimaculatus 5-HT1B [40], and Bombyx mori 5-HT1 [41] were all reported to be expressed at high levels in the nervous system but expressed at low levels or not expressed in the intestinal tract of H. armigera. In this study, we found that 5-HT1A Har was expressed at a high level in the pectoral muscle and 5-HT1B Har in the nervous system, but both showed low expression in the digestive tract; this result was generally consistent with that found in the above three insects. In addition, 5-HT2 receptors have been found to be expressed at high levels in the salivary glands of Calliphora vicina and G. bimaculatus, and the level of expression in the brain of C. vicina is relatively high [26,40]. In this study, the level of expression of the 5-HT2 Har receptor in the nervous system was lower than that in the gonads, which was inconsistent with the expression pattern in G. bimaculatus and C. vicina. Furthermore, 5-HT7 Har is reported to be expressed at a high level in the midgut of G. bimaculatus [41] and Eriocheir sinensis [42], which is consistent with our findings in H. axyridis. In this study, the Harmonia 5-HT receptor was found to be expressed in various tissues at differential expression levels, suggesting that the 5-HT system is widely distributed in this ladybird.
The results for the expression profile of the H. axyridis 5-HT receptors in this article will provide a basis for the functional study of them, and further studies on expression sites, timing of expression, pharmacological reaction and demographical effect, etc., are needed to elucidate the exact function of these receptors.
Author Contributions
Conceptualization, L.S. and C.Z.; methodology, Q.Z. and L.S.; investigation, Q.Z. and Y.C.; writing—the original draft preparation, Q.Z. and Y.C.; writing—the review and editing, Q.Z. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Pilot Project of National Key Research and Development Program (2017YFD0201000) and Shandong Modern Agricultural Technology and Industry System (SDAIT-05).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors are very grateful to Yinjun Fan for much advice on the experiment.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.B. A neurohormonal role for serotonin in the control of locust oviducts. Arch. Insect Biochem. Physiol. 2004, 56, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Neckameyer, W.S. A trophic role for serotonin in the development of a simple feeding circuit. Dev. Neurosci. 2010, 32, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Li, A.Y.; Witt, C.M.; Pérez de León, A.A. Effects of reserpine on reproduction and serotonin immunoreactivity in the stable fly Stomoxys calcitrans (L.). J. Insect Physiol. 2013, 59, 974–982. [Google Scholar] [CrossRef]
- Ling, L.; Raikhel, A.S. Serotonin signaling regulates insulin-like peptides for growth, reproduction, and metabolism in the disease vector Aedes aegypti. Proc. Natl. Acad. Sci. USA 2018, 115, E9822–E9831. [Google Scholar] [CrossRef]
- Wang, Q.; Mohamed, A.A.M.; Takeda, M. Serotonin receptor B may lock the gate of PTTH release/synthesis in the Chinese silk moth, Antheraea pernyi; A diapause initiation/maintenance mechanism? PLoS ONE 2013, 8, e79381. [Google Scholar] [CrossRef]
- Liscia, A.; Solari, P.; Gibbons, S.T.; Gelperin, A.; Stoffolano, J.G., Jr. Effect of serotonin and calcium on the supercontractile muscles of the adult blowfly crop. J. Insect Physiol. 2012, 58, 356–366. [Google Scholar] [CrossRef]
- French, A.S.; Simcock, K.L.; Rolke, D.; Gartside, S.E.; Blenau, W.; Wright, G.A. The role of serotonin in feeding and gut contractions in the honeybee. J. Insect Physiol. 2014, 61, 8–15. [Google Scholar] [CrossRef]
- Wright, G.A. The role of dopamine and serotonin in conditioned food aversion learning in the honeybee. Commun. Integr. Biol. 2011, 4, 318–320. [Google Scholar] [CrossRef]
- Dierick, H.A.; Greenspan, R.J. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 2007, 39, 678–682. [Google Scholar] [CrossRef]
- Moncalvo, V.; Campos, A.R. Role of serotonergic neurons in the Drosophila larval response to light. BMC Neurosci. 2009, 10, 66. [Google Scholar]
- Thamm, M.; Balfanz, S.; Scheiner, R.; Baumann, A.; Blenau, W. Characterization of the 5-HT1A receptor of the honeybee (Apis mellifera) and involvement of serotonin in phototactic behavior. Cell. Mol. Life Sci. 2010, 67, 2467–2479. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, P.; Mercer, A.R. Serotonin modulation of moth central olfactory neurons. Annu. Rev. Entomol. 2008, 53, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, K.; Ikeda, M.; Nagao, T.; Tamotsu, S. Involvement of serotonin in the circadian rhythm of an insect visual system. Naturwissenschaften 1993, 80, 137–139. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, F.; Zheng, X.; Sehgal, A. Serotonin modulates circadian entrainment in Drosophila. Neuron 2005, 47, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Joiner, W.J.; Sehgal, A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr. Biol. 2006, 16, 1051–1062. [Google Scholar] [CrossRef]
- Kim, G.S.; Kim, Y. Up-regulation of circulating hemocyte population in response to bacterial challenge is mediated by octopamine and 5-hydroxytryptamine via rac1 signal in Sodoptera exigua. J. Insect Physiol. 2010, 56, 559–566. [Google Scholar] [CrossRef]
- Silva, B.; Goles, N.I.; Varas, R.; Campusano, J.M. Serotonin receptors expressed in Drosophila mushroom bodies differentially modulate larval locomotion. PLoS ONE 2014, 9, e89641. [Google Scholar] [CrossRef]
- Majeed, Z.R.; Stacy, A.; Cooper, R.L. Pharmacological and genetic identification of serotonin receptor subtypes on Drosophila larval heart and aorta. J. Comp. Physiol. 2014, 184, 205–219. [Google Scholar] [CrossRef]
- Anstey, M.L.; Rogers, S.M.; Ott, S.R.; Burrows, M.; Simpson, S.J. Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science 2009, 323, 62730. [Google Scholar] [CrossRef]
- Peroutka, S.J.; Howell, T.A. The molecular evolution of G protein-coupled receptors: Focus on 5-hydroxytryptamine receptors. Neuropharmacology 1994, 33, 319–324. [Google Scholar] [CrossRef]
- Vleugels, R.; Velinden, H.; Broeckj, V. Serotonin, serotonin receptors and their actions in insects. Neurotransimitter 2015, 2, e314. [Google Scholar]
- Ji, T.H.; Grossmann, M.; Ji, I. G protein-coupled receptors. I. Diversity of receptor-ligand interactions. J. Biol. Chem. 1998, 273, 17299–17302. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.A.; Fox, A.N.; Pitts, R.J.; Kent, L.B.; Tan, P.L.; Chrystal, M.A.; Cravchik, A.; Collins, F.H.; Robertson, H.M.; Zwiebel, L.J. G protein-coupled receptors in Anopheles gambiae. Science 2002, 298, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Brody, T.; Cravchik, A. Drosophila melanogaster G protein–coupled receptors. J. Cell Biol. 2000, 150, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Röser, C.; Jordan, N.; Balfanz, S.; Baumann, A.; Walz, B.; Baumann, O.; Blenau, W. Molecular and pharmacological characterization of serotonin 5-HT2α and 5-HT7 receptors in the salivary glands of the blowfly Calliphora vicina. PLoS ONE 2012, 7, e49459. [Google Scholar] [CrossRef]
- Qi, Y.X.; Jin, M.; Ni, X.Y.; Ye, G.Y.; Lee, Y.; Huang, J. Characterization of three serotonin receptors from the small white butterfly, Pieris rapae. Insect Biochem. Mol. Bology 2017, 87, 107–116. [Google Scholar] [CrossRef]
- Qi, Y.X.; Xia, R.Y.; Wu, Y.S.; Stanley, D.; Huang, J.; Ye, G.Y. Larvae of the small white butterfly, Pieris rapae, express a novel serotonin receptor. J. Neurochem. 2014, 131, 767–777. [Google Scholar] [CrossRef]
- Li, M.Z.; Li, Y.X.; Chen, J.; Zhang, L.; Liao, C.H.; Han, Q. Bioinformatics analysis of the Aedes aegypti 5-HT receptor family and construction of spatial and temporal expression profiles. J. South China Univ. Trop. Agric. 2021, 12, 347–355+403. [Google Scholar]
- Wang, S.; Zhang, R.Z.; Zhang, F. Research progress on biology and ecology of Hamonia axyridis Pallas (Coleoptera: Coccinellidae). Chin. J. Appl. Ecol. 2007, 18, 2117–2126. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, H.X.; Chen, S.T.; Wang, K.; Gao, X.Y.; Xie, G.Y.; Chen, W.P.; Zhao, X.C. Cloning and expression analysis of the 5-HT1 and 5-HT2 genes in Helicoverpa armigera. Plant Prot. 2023, 49, 147–156+163. [Google Scholar]
- Zhao, T.X.; Yuan, M.L. Biological and ecological characteristics of Harmonia axyridis in China. Pratacultural Sci. 2017, 34, 614–629. [Google Scholar]
- Yu, H.X. Effects of Photoperiod on Biological and Physiological Characteristics and Predation of Harmonia axyridis (Pallas) under Low Temperature. Master’s Thesis, Qingdao Agricultural University, Qingdao, China, 2022. [Google Scholar]
- Vanhoenacker, P.; Haegeman, G.; Leysen, J.E. 5-HT7 receptors: Current knowledge and future prospects. Trends Pharmacol. Sci. 2000, 21, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Osborne, R.H.; Banner, S.E.; Wood, S.J. The Pharmacology of the gut of the desert locust Schhistocerca gregaria and other Insects. Comp. Biochem. Physiol. 1990, 96, 1–9. [Google Scholar]
- Falibene, A.; Rössler, W.; Josens, R. Serotonin depresses feeding behaviour in ants. J. Insect Physiol. 2012, 58, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Molaei, G.; Lange, A.B. The association of serotonin with the alimentary canal of the African migratory locust, Locusta migratoria: Distribution, physiology and pharmacological profile. J. Insect Physiol. 2003, 49, 1073–1082. [Google Scholar] [CrossRef]
- Watanabe, T.; Sadamoto, H.; Aonuma, H. Identification and expression analysis of the genes involved in serotonin biosynthesis and transduction in the field cricket Gryllus bimaculatus. Insect Mol. Biol. 2011, 20, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Li, Y.; Chen, X.; Chen, P. Gene cloning and expression analysis of 5-HT receptors in silkworm (Bombyx mori). Sci. Agric. Sin. 2015, 48, 987–1001. [Google Scholar]
- Huang, G.Y. Cloning and Expression Analysis of 5-HT7 and DA2 in Several Physiological Processes of Eriocheir sinensis. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).