Host Specificity in Canopy Nesting Forms of Ochrogaster lunifer: The Larger Children Do Not Care
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Egg Mass and Larval Nest Collection
2.2. Realised Fecundity
2.3. Egg Mass Transfers
2.4. Larval Nest Transfers
2.5. Data Analysis
3. Results
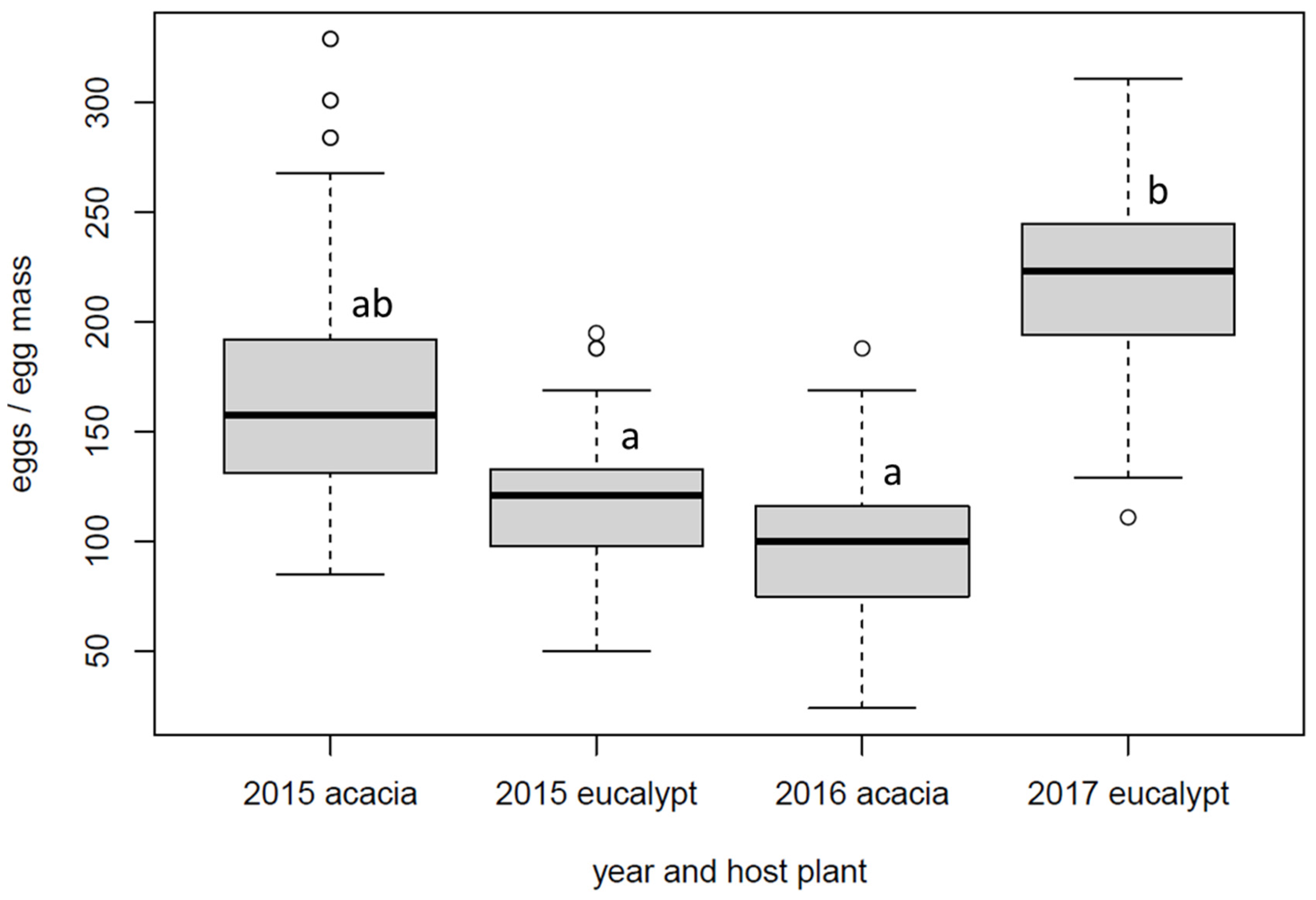
3.1. Realised Fecundity
3.2. Egg Mass and Mature Nest Transfers
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Courtney, S.P.; Kibota, T.T. Mother doesn’t know best: Selection of hosts by ovipositing insects. In Insect-Plant Interactions; Bernays, E.A., Ed.; CRC Press: Boca Raton, FL, USA, 1990; Volume II, pp. 161–188. [Google Scholar]
- Mayhew, P.J. Adaptive patterns of host–plant selection by phytophagous insects. Oikos 1997, 79, 417–428. [Google Scholar] [CrossRef]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference–performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.C.; Rafter, M.A.; Walter, G.H. Insects allocate eggs adaptively across their native host plants. Arthropod. Plant Interact. 2019, 13, 181–191. [Google Scholar] [CrossRef]
- Common, I.F.B. Moths of Australia; Melbourne University Press: Melbourne, Australia, 1990. [Google Scholar]
- Floater, G.J. Life history comparisons of ground- and canopy nesting populations of Ochrogaster lunifer Herrich-Schäffer [Lepidoptera: Thaumetopoeidae]: Evidence for two species? Austral. J. Entomol. 1996, 35, 223–230. [Google Scholar] [CrossRef]
- Steinbauer, M.J. Wing pattern polyphenism in two behavioural forms of Ochrogaster lunifer [Lepidoptera: Notodontidae]. Austral Entomol. 2019, 58, 432–442. [Google Scholar] [CrossRef]
- van Schagen, J.; Hobbs, R.J.; Majer, J.D. Defoliation of trees in roadside corridors and remnant vegetation in the Western Australian wheatbelt. J. R. Soc. West. Aust. 1992, 75, 75–81. [Google Scholar]
- van Schagen, J.; Majer, J.D.; Hobbs, R.J. Biology of Ochrogaster lunifer Herrich-Schaeffer [Lepidoptera: Thaumetopoeidae], a defoliator of Acacia acuminata Bentham, in the Western Australian wheatbelt. Austr. Entomol. Mag. 1992, 19, 19–24. [Google Scholar]
- Cawdell-Smith, A.J.; Todhunter, K.H.; Anderson, S.T.; Perkins, N.R.; Bryden, W.L. Equine amnionitis and fetal loss: Mare abortion following experimental exposure to processionary caterpillars [Ochrogaster lunifer]. Equine Vet. J. 2012, 44, 282–288. [Google Scholar] [CrossRef]
- Perkins, L.E.; Zalucki, M.P.; Perkins, N.R.; Cawdell-Smith, A.J.; Todhunter, K.H.; Bryden, W.L.; Cribb, B.W. The urticating setae of Ochrogaster lunifer, an Australian processionary caterpillar of veterinary importance. Med. Vet. Entomol. 2016, 30, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Perkins, L.E.; Cribb, B.W.; Pagendam, D.; Zalucki, M.P. Variation in morphology and air-borne dispersal of the urticating apparatus of Ochrogaster lunifer, an Australian processionary caterpillar, and implications for livestock and humans. J. Insect Sci. 2019, 19, 6. [Google Scholar] [CrossRef]
- Floater, G.J. The Brooks-Dyar rule and morphometrics of the processionary caterpillar, Ochrogaster lunifer Herrich-Schäffer (Lepidoptera: Thaumetopoeidae). Austral. J. Entomol. 1996, 35, 271–278. [Google Scholar] [CrossRef]
- Floater, G.J. Estimating movement of the processionary caterpillar, Ochrogaster lunifer Herrich-Schäffer (Lepidoptera: Thaumetopoeidae) between discrete resource patches. Austral. J. Entomol. 1996, 35, 279–283. [Google Scholar] [CrossRef]
- Floater, G.J. Rainfall, nitrogen and host plant condition: Consequences for the processionary caterpillar, Ochrogaster lunifer (Thaumetopoeidae). Ecol. Entomol. 1997, 22, 247–255. [Google Scholar] [CrossRef]
- Floater, G.J. Tuft scales and egg protection in Ochrogaster lunifer Herrich-Schäffer (Lepidoptera: Thaumetopoeidae). Austral. J. Entomol. 1998, 37, 34–39. [Google Scholar] [CrossRef]
- Floater, G.J.; Zalucki, M.P. Life tables of the processionary caterpillar Ochrogaster lunifer Herrich-Schaffer at local and regional scales. Austral. J. Entomol. 1999, 38, 330–339. [Google Scholar] [CrossRef]
- Floater, G.J.; Zalucki, M.P. Habitat structure and egg distributions in the processionary caterpillar Ochrogaster lunifer: Lessons for conservation and pest management. J. Appl. Ecol. 2000, 37, 87–99. [Google Scholar] [CrossRef]
- Perkins, L.E.; Uemura, M.; Zalucki, M. A trunk-nesting form of the processionary caterpillar Ochrogaster lunifer (Lepidoptera: Notodontidae) restricted to a single host species Corymbia tessellaris (Myrtaceae), with some comparisons to the ground-nesting form. Austral Entomol. 2023. [Google Scholar] [CrossRef]
- Mather, A.; Zalucki, M.P.; Farrell, J.; Perkins, L.E.; Cook, L.G. The Australian processionary caterpillar, Ochrogaster lunifer Herrich-Schäffer (Lepidoptera: Notodontidae), comprises cryptic species. Austral Entomol. 2019, 58, 816–825. [Google Scholar] [CrossRef]
- Hnatiuk, R.J.; Maslin, B.R. Phytogeography of Acacia in Australia in relation to climate and species-richness. Austral. J. Bot. 1988, 36, 361–383. [Google Scholar] [CrossRef]
- Maslin, B.R.; Miller, J.T.; Seigler, D.S. Overview of the generic status of Acacia (Leguminosae: Mimosoideae). Austral. Syst. Bot. 2003, 16, 1–18. [Google Scholar] [CrossRef]
- Uemura, M.; Perkins, L.; Battisti, A.; Zalucki, M.P. Egg mass structure of the processionary caterpillar Ochrogaster lunifer (Lepidoptera: Notodontidae): Is the outer egg layer sacrificed for attack by the egg parasitoid Anastatus fuligispina (Hymenoptera: Chalcidoidea: Eupelmidae)? Austral Entomol. 2019, 58, 810–815. [Google Scholar] [CrossRef]
- Jones, L.C. Insects allocate eggs adaptively according to plant age, stress, disease or damage. Proc. R. Soc. B 2022, 289, 20220831. [Google Scholar] [CrossRef] [PubMed]
- Staples, T.L.; Mayfield, M.M.; England, J.R.; Dwyer, J.M. Drivers of Acacia and Eucalyptus growth rate differ in strength and direction in restoration plantings across Australia. Ecol. Appl. 2022, 32, e2636. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Walter, G.H.; Moore, C.J. Host plant adaptations in myrtaceous-feeding Pergid sawflies: Essential oils and the morphology and behaviour of Pergagrapta larvae (Hymenoptera, Symphyta, Pergidae). Biol. J. Linn. Soc. 2000, 70, 15–26. [Google Scholar] [CrossRef]
- Stigter, H.; Geraedts, W.H.J.M.; Spijkers, H.C.P. Thaumetopoea processionea in The Netherlands: Present status and management perspectives (Lepidoptera: Notodontidae). Proc. Exper. Appl. Entomol. 1997, 8, 3–16. [Google Scholar]
- Janz, N.; Söderlind, L.; Nylin, S. No effect of larval experience on adult host preferences in Polygonia c-album (Lepidoptera: Nymphalidae): On the persistence of Hopkins’ host selection principle. Ecol. Entomol. 2009, 34, 50–57. [Google Scholar] [CrossRef]
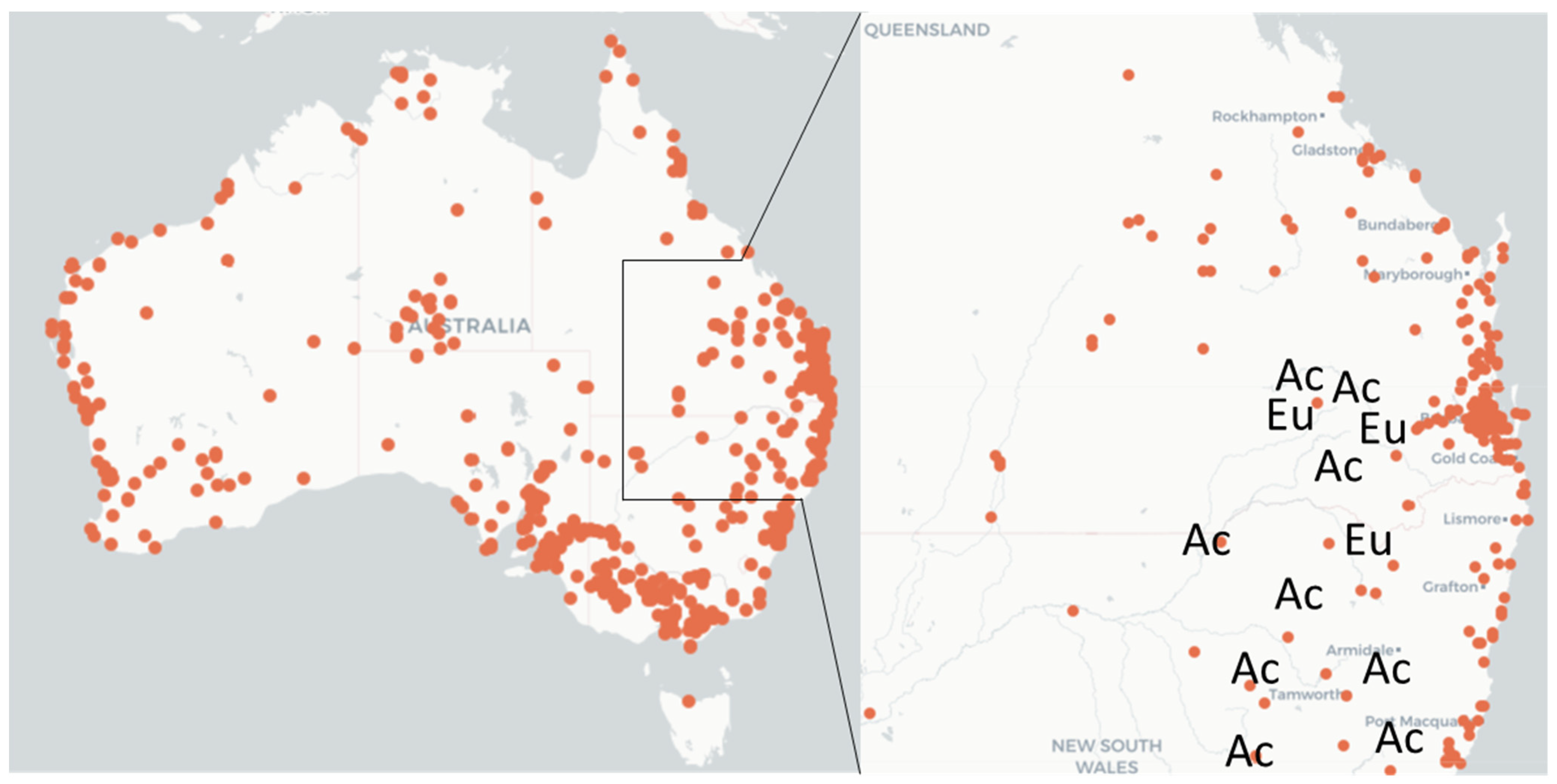


| Location | Co-Ordinates | Host Species | Egg Masses (n) | Larval Nests (n) |
|---|---|---|---|---|
| Allora, Qld | 27°59′00″ S 151°57′33″ E | E. orgadophila | 92 | 13 |
| Ashford, NSW | 29°10′12″ S 151°09′49″ E | E. melanophloia | 8 | 0 |
| Bylong Valley Way HV, NSW | 32°20′39″ S 150°34′40″ E | A. pendula | 20 | 0 |
| Edderton Rd HV, NSW | 32°19′40″ S 150°49′19″ E | A. hakeoides | 28 | 0 |
| Foxes Lane Moree, NSW | 29°00′15″ S 150°01′26″ E | A. pendula | 80 | 10 |
| Gulargambone, NSW | 31°18′50″ S 148°16′05″ E | A. pendula | 10 | 0 |
| Jerrys Plains HV, NSW | 32°25′18″ S 150°41′48″ E | A. pendula | 25 | 11 |
| Jondaryan, Qld | 27°22′19″ S 151°35′25″ E | A. pendula, A. stenophylla | 5 | 0 |
| Malu, Qld | 27°20′22″ S 151°32′45″ E | A. stenophylla | 0 | 5 |
| Oakey, Qld | 27°26′02″ S 151°43′16″ E | A. salicina | 0 | 3 |
| Pittsworth, Qld | 27°42′30″ S 151°38′46″ E | E. orgadophila | 105 | 18 |
| Scone HV, NSW | 32°06′53″ S 150°58′47″ E | A. pendula | 0 | 3 |
| Natal Species | Recipient Species | Egg Masses | Larval Nests |
|---|---|---|---|
| Acacia (pendula, salicina, sp.) | Acacia (concurrens, fimbriata, iteaphylla, pendula, salicina, stenophylla) | 56 | 10 |
| Acacia (pendula, salicina, sp.) | Eucalyptus (orgadophila, sp.) | 17 | 7 |
| Eucalyptus (orgadophila, sp.) | Eucalyptus (orgadophila, melanophloia) | 29 | 24 |
| Eucalyptus (orgadophila, sp.) | Acacia (concurrens, fimbriata, pendula, salicina, stenophylla) | 121 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrell, J.; Zalucki, M.P.; Battisti, A. Host Specificity in Canopy Nesting Forms of Ochrogaster lunifer: The Larger Children Do Not Care. Insects 2023, 14, 420. https://doi.org/10.3390/insects14050420
Farrell J, Zalucki MP, Battisti A. Host Specificity in Canopy Nesting Forms of Ochrogaster lunifer: The Larger Children Do Not Care. Insects. 2023; 14(5):420. https://doi.org/10.3390/insects14050420
Chicago/Turabian StyleFarrell, Julianne, Myron P. Zalucki, and Andrea Battisti. 2023. "Host Specificity in Canopy Nesting Forms of Ochrogaster lunifer: The Larger Children Do Not Care" Insects 14, no. 5: 420. https://doi.org/10.3390/insects14050420
APA StyleFarrell, J., Zalucki, M. P., & Battisti, A. (2023). Host Specificity in Canopy Nesting Forms of Ochrogaster lunifer: The Larger Children Do Not Care. Insects, 14(5), 420. https://doi.org/10.3390/insects14050420







