Natural Trypanosoma cruzi Infection and Climatic Season Influence the Developmental Capacity in Field-Caught Mepraia spinolai Nymphs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
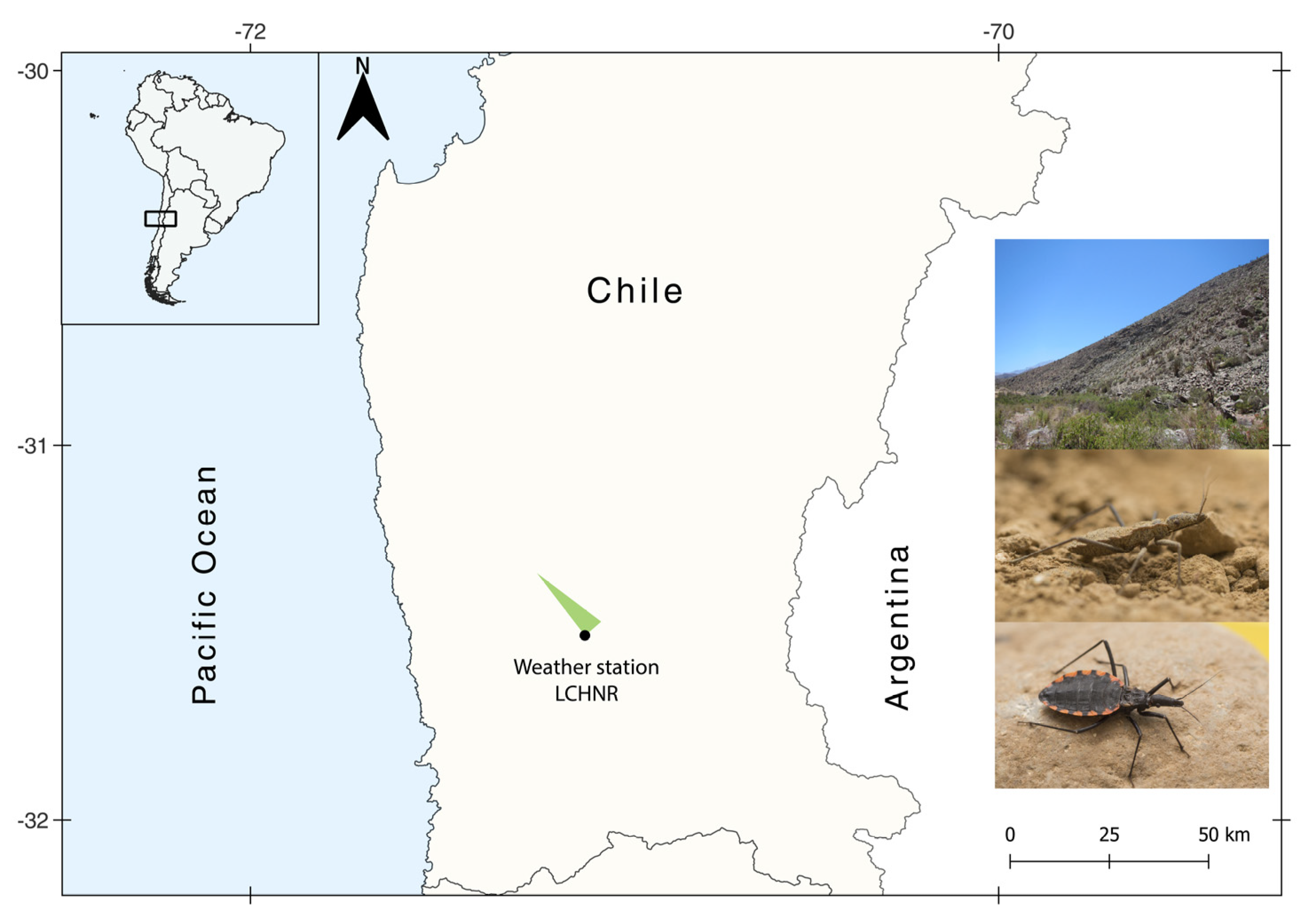
2.1. Study Area and Triatomine Collection
2.2. Triatomine Fecal Sample Collection
2.3. DNA Extraction and T. cruzi Infection Detection
2.4. Statistical Analysis
3. Results
3.1. M. spinolai Populations and Climatic Conditions
3.2. Molting Events: Climatic Period and T. cruzi Infection
3.3. Nymphs Molting Twice: Developmental Stage, T. cruzi Infection, and Climatic Period
3.4. Non-Molting Nymphs: Infection and Climatic Period
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Telleria, J.; Tibayrenc, M. American Trypanosomiasis Chagas Disease: One Hundred Years of Research, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2017; p. 844. [Google Scholar]
- Rabinovich, J.E. Vital statistics of Triatominae (Hemiptera: Reduviidae) under laboratory conditions. I. Triatoma infestans Klug. J. Med. Entomol. 1972, 9, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Gorla, D.E.; Schofield, C.J. Population dynamics of Triatoma infestans under natural climatic conditions in the Argentine Chaco. Med. Vet. Entomol. 1989, 3, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Figueiras, A.N.L.; Lazzari, C.R. Aggregation in the haematophagous bug Triatoma infestans: A novel assembling factor. Physiol. Entomol. 1998, 23, 33–37. [Google Scholar] [CrossRef]
- Lorenzo, M.G.; Guarneri, A.A.; Pires, H.H.; Diotaiuti, L.; Lazzari, C.R. Microclimatic properties of the Triatoma brasiliensis habitat. Cad. Saude Publica 2000, 16, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, E.; Gourbiere, S.; Barrera-Perez, M.; Rodriguez-Felix, E.; Ruiz-Pina, H.; Banos-Lopez, O.; Ramirez-Sierra, M.J.; Menu, F.; Rabinovich, J.E. Geographic distribution of Triatoma dimidiata and transmission dynamics of Trypanosoma cruzi in the Yucatan peninsula of Mexico. Am. J. Trop. Med. Hyg. 2002, 67, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Menu, F.; Ginoux, M.; Rajon, E.; Lazzari, C.R.; Rabinovich, J.E. Adaptive developmental delay in Chagas disease vectors: An evolutionary ecology approach. PLoS Negl. Trop. Dis. 2010, 4, e691. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, C.R.; Pereira, M.H.; Lorenzo, M.G. Behavioural biology of Chagas disease vectors. Mem. Inst. Oswaldo Cruz 2013, 108, 34–47. [Google Scholar] [CrossRef]
- Pelosse, P.; Kribs-Zaleta, C.M.; Ginoux, M.; Rabinovich, J.E.; Gourbière, S.; Menu, F. Influence of vectors’ risk-spreading strategies and environmental stochasticity on the epidemiology and evolution of vector-borne diseases: The example of Chagas’ disease. PLoS ONE 2013, 8, e70830. [Google Scholar] [CrossRef]
- Elliot, S.L.; Adler, F.R.; Sabelis, M.W. How virulent should a parasite be to its vector? Ecology 2003, 84, 2568–2574. [Google Scholar] [CrossRef]
- Elliot, S.L.; Rodrigues, J.d.O.; Lorenzo, M.G.; Martins-Filho, O.A.; Guarneri, A.A. Trypanosoma cruzi, etiological agent of Chagas disease, is virulent to its Triatomine vector Rhodnius prolixus in a temperature-dependent manner. PLoS Negl. Trop. Dis. 2015, 9, e0003646. [Google Scholar] [CrossRef] [PubMed]
- Schofield, C.J. The role of blood intake in density regulation of populations of Triatoma infestans (Klug) (Hemiptera:Reduviidae). Bull. Ent. Res. 1982, 72, 617–629. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. Factors controlling moulting and ‘metamorphosis’ in an insect. Nature 1934, 133, 725–726. [Google Scholar] [CrossRef]
- Steel, C.G.; Vafopoulou, X. Circadian orchestration of developmental hormones in the insect, Rhodnius prolixus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Reis dos Santos, J.; Lacombe, D. Estudos relativos à duraçao da ecdise e ovopisiçao de Triatoma infestans infectado pela Trypanosoma cruzi. An. Acad. Brasil. Ciênc. 1985, 57, 127. [Google Scholar]
- Schaub, G.A. Developmental time and mortality of larvae of Triatoma infestans infected with Trypanosoma cruzi. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 94–96. [Google Scholar] [CrossRef]
- Lima, M.M.; Pereira, J.B.; Santos, J.A.A.; Pinto, Z.T.; Braga, M.V. Development and reproduction of Panstrongylus megistus (Hemiptera: Reduviidae) infected with Trypanosoma cruzi, under laboratory conditions. Ann. Entomol. Soc. Am. 1992, 85, 458–461. [Google Scholar] [CrossRef]
- Oliveira, T.G.; Carvalho-Costa, F.A.; Gomes, T.F.; Sarquis, O.; Sposina, R.; Lima, M.M. Developmental and reproductive patterns of Triatoma brasiliensis infected with Trypanosoma cruzi under laboratory conditions. Mem. Inst. Oswaldo Cruz 2010, 105, 1057–1060. [Google Scholar] [CrossRef]
- Botto-Mahan, C. Trypanosoma cruzi induces life-history trait changes in the wild kissing bug Mepraia spinolai: Implications for parasite transmission. Vector-Borne Zoonotic Dis. 2009, 9, 505–510. [Google Scholar] [CrossRef]
- Guarneri, A.A.; Lazzari, C.; Xavier, A.A.P.; Diotaiuti, L.; Lorenzo, M.G. The effect of temperature on the behaviour and development of Triatoma brasiliensis. Physiol. Entomol. 2003, 28, 185–191. [Google Scholar] [CrossRef]
- Joerg, M.E. Influencia de temperaturas fijas en períodos anuales sobre metamorfosis y fertilidad de Triatoma infestans. Bol. Chil. Parasitol. 1962, 17, 17–19. [Google Scholar] [PubMed]
- Catalá, S. The biting rate of Triatoma infestans in Argentina. Med. Vet. Entomol. 1991, 5, 325–333. [Google Scholar] [CrossRef]
- Lande, R. Genetics and demography in biological conservation. Science 1988, 241, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Numata, H.; Miyazaki, Y.; Ikeno, T. Common features in diverse insect clocks. Zool. Lett. 2015, 1, 10. [Google Scholar] [CrossRef]
- Saunders, D.S.; Bertossa, R.C. Deciphering time measurement: The role of circadian ‘clock’ genes and formal experimentation in insect photoperiodism. J. Insect. Physiol. 2011, 57, 557–566. [Google Scholar] [CrossRef]
- Goto, S.G. Roles of circadian clock genes in insect photoperiodism. Entomol. Sci. 2013, 16, 1–16. [Google Scholar] [CrossRef]
- Garrido, R.; Bacigalupo, A.; Peña-Gómez, F.; Bustamante, R.O.; Cattan, P.E.; Gorla, D.; Botto-Mahan, C. Potential impact of climate change on geographical distribution of two wild vectors of Chagas disease in Chile: Mepraia spinolai and Mepraia gajardoi. Parasit. Vectors 2019, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- di Castri, F.; Hajek, E.R. Bioclimatología de Chile; Editorial Universidad Católica de Chile: Santiago, Chile, 1976. [Google Scholar]
- Ihle-Soto, C.; Costoya, E.; Correa, J.P.; Bacigalupo, A.; Cornejo-Villar, B.; Estadella, V.; Solari, A.; Ortiz, S.; Hernández, H.J.; Botto-Mahan, C.; et al. Spatio-temporal characterization of Trypanosoma cruzi infection and discrete typing units infecting hosts and vectors from non-domestic foci of Central Chile. PLoS Negl. Trop. Dis. 2019, 13, e7170. [Google Scholar] [CrossRef]
- Botto-Mahan, C.; Bacigalupo, A.; Correa, J.P.; Fontúrbel, F.E.; Cattan, P.E.; Solari, A. Prevalence, infected density or individual probability of infection? Assessing vector infection risk in the wild transmission of Chagas disease. Proc. R. Soc. B 2020, 287, 20193018. [Google Scholar] [CrossRef]
- San Juan, E.; Araya-Donoso, R.; Sandoval-Rodríguez, A.; Yáñez-Meza, A.; Quiroga, N.; Botto-Mahan, C. Lizards and rabbits may increase Chagas infection risk in the Mediterranean-type ecosystem of South America. Sci. Rep. 2020, 10, 1853. [Google Scholar] [CrossRef] [PubMed]
- Canals, M.; Cruzat, L.; Molina, M.C.; Ferreira, A.; Cattan, P. Blood host source of Mepraia spinolai (Heteroptera, Reduviidae) wild vector of Chagas disease in Chile. J. Med. Entomol. 2001, 38, 303–307. [Google Scholar] [CrossRef]
- Chacon, F.; Bacigalupo, A.; Quiroga, M.; Ferreira, A.; Cattan, P.; Ramirez-Toloza, G. Feeding profile of Mepraia spinolai, a sylvatic vector of Chagas disease in Chile. Acta Trop. 2016, 162, 171–173. [Google Scholar] [CrossRef]
- De Bona, S.; Correa, J.P.; San Juan, E.; Estay-Olea, D.; Quiroga, N.; Bacigalupo, A.; Araya-Donoso, R.; Botto-Mahan, C. Opportunistic or selective? stage-dependent feeding behavior in a wild vector of Chagas disease. Int. J. Parasitol. 2023, 53, 55–64. [Google Scholar] [CrossRef]
- Rozas, M.; Botto-Mahan, C.; Coronado, X.; Ortiz, S.; Cattan, P.E.; Solari, A. Co-existence of Trypanosoma cruzi genotypes in wild and peridomestic mammals in Chile. Am. J. Trop. Med. Hyg. 2007, 77, 647–653. [Google Scholar] [CrossRef]
- Correa, J.P.; Bacigalupo, A.; Fontúrbel, F.; Oda, E.; Cattan, P.E.; Solari, A.; Botto-Mahan, C. Spatial distribution of an infectious disease in a native small mammal community. Sci. Nat. 2015, 102, 51. [Google Scholar] [CrossRef]
- Correa, J.P.; Bacigalupo, A.; Yefi-Quinteros, E.; Rojo, G.; Solari, A.; Cattan, P.E.; Botto-Mahan, C. Trypanosomatid infections among vertebrates of Chile: A systematic review. Pathogens 2020, 9, 661. [Google Scholar] [CrossRef]
- Quiroga, N.; Campos-Soto, R.; Yañez-Meza, A.; Rodríguez-San Pedro, A.; Allendes, J.L.; Bacigalupo, A.; Botto-Mahan, C.; Correa, J.P. Trypanosoma cruzi DNA in Desmodus rotundus (common vampire bat) and Histiotus montanus (small big-eared brown bat) from Chile. Acta Trop. 2022, 225, 106206. [Google Scholar] [CrossRef]
- Botto-Mahan, C.; Correa, J.P.; Araya-Donoso, R.; Farías, F.; Quiroga, N.; Reyes-Olivares, C.; San Juan, E.; Campos-Soto, R.; González-Acuña, D. Lizards as silent hosts of Trypanosoma cruzi. Emerg. Infect. Dis. 2022, 28, 1250–1253. [Google Scholar] [CrossRef]
- Garrido, R.; Campos-Soto, R.; Quiroga, N.; Botto-Mahan, C. Bloodmeal-stealing in wild-caught Mepraia spinolai (Hemiptera: Reduviidae), a sylvatic vector of Trypanosoma cruzi. Ecol. Entomol. 2021, 46, 681–683. [Google Scholar] [CrossRef]
- Canals, M.; Ehrenfeld, M.; Solís, R.; Cruzat, L.; Pinochet, A.; Tapia, C.; Cattan, P.E. Comparative biology of Mepraia spinolai in laboratory and field conditions: Five years study. Parasitol. Dia. 1998, 22, 72–78. [Google Scholar] [CrossRef]
- Frías-Lasserre, D.; González, C.R.; Valenzuela, C.R.; de Carvalho, D.B.; Oliveira, J.; Canals, M.; da Rosa, J.A. Wing polymorphism and Trypanosoma cruzi infection in wild, peridomestic, and domestic collections of Mepraia spinolai (Hemiptera: Reduviidae) from Chile. J. Med. Entomol. 2017, 54, 1061–1066. [Google Scholar] [CrossRef]
- Botto-Mahan, C.; Cattan, P.E.; Canals, M.; Acuña, M. Seasonal variation in the home range and host availability of the blood-sucking insect Mepraia spinolai in wild environment. Acta Trop. 2005, 95, 160–163. [Google Scholar] [CrossRef]
- Lent, H.; Jurberg, J. Algumas informações sôbre Triatoma spinolai Porter, 1934 com um estudo sôbre as genitálias externas (Hemiptera, Reduviidae). Rev. Brasil Biol. 1967, 28, 499–520. [Google Scholar]
- Ehrenfeld, M.J.; Canals, M.; Cattan, P.E. Population parameters of Triatoma spinolai (Heteroptera: Reduviidae) under different environmental conditions and densities. J. Med. Entomol. 1998, 35, 740–744. [Google Scholar] [CrossRef]
- Canals, M.; Solis, R.; Valderas, J.; Ehrenfeld, M.; Cattan, P.E. Preliminary studies on temperature selection and activity cycles of Triatoma infestans and T. spinolai (Heteroptera: Reduviidae), Chilean vectors of Chagas’ disease. J. Med. Entomol. 1997, 34, 11–17. [Google Scholar] [CrossRef]
- Wincker, P.; Britto, C.; Pereira, J.B.; Cardoso, M.A.; Oelemann, O.; Morel, C.M. Use of a simplified polymerase chain reaction procedure to detect Trypanosoma cruzi in blood samples from chronic chagasic patients in a rural endemic area. Am. J. Trop. Med. Hyg. 1994, 51, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Cortés, V.; Cruz, A.; Onetti, S.; Kinzel, D.; Garcia, J.; Ortiz, S.; López, A.; Cattan, P.E.; Botto-Mahan, C.; Solari, A. Trypanosoma cruzi infection follow-up in a sylvatic vector of Chagas disease: Comparing early and late stage nymphs. PLoS Negl. Trop. Dis. 2021, 15, e0009729. [Google Scholar] [CrossRef]
- Guhl, F.; Jaramillo, C.; Carranza, J.C.; Vallejo, G.A. Molecular characterization and diagnosis of Trypanosoma cruzi and T. rangeli. Arch. Med. Res. 2002, 33, 362–370. [Google Scholar] [CrossRef]
- Egaña, C.; Pinto, R.; Vergara, F.; Ortiz, S.; Campos-Soto, R.; Solari, A. Fluctuations in Trypanosoma cruzi discrete typing unit composition in two naturally infected triatomines: Mepraia gajardoi and M. spinolai after laboratory feeding. Acta Trop. 2016, 160, 9–14. [Google Scholar] [CrossRef]
- Garcia, V.; Graterol, J.; López, A.; Ortiz, S.; Solari, A. Influence of Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) infection on mortality of the sylvatic Triatomine vector, Mepraia spinolai (Heteroptera: Reuviidae), under fasting. J. Med. Entomol. 2019, 56, 1384–1388. [Google Scholar] [CrossRef]
- McCabe, A.; Yañez, F.; Pinto, R.; López, A.; Ortiz, S.; Muñoz-San Martin, C.; Botto-Mahan, C.; Solari, A. Survivorship of wild caught Mepraia spinolai nymphs: The effect of seasonality and Trypanosoma cruzi infection after feeding and fasting in the laboratory. Infect. Genet. Evol. 2019, 71, 197–204. [Google Scholar] [CrossRef]
- Noireau, F.; Dujardin, J.P. Flight and nutritional status of sylvatic Triatoma sordida and Triatoma guasayana. Mem. Inst. Oswaldo Cruz 2001, 96, 385–389. [Google Scholar] [CrossRef]
- Sarquis, O.; Carvalho-Costa, F.A.; Oliveira, L.S.; Duarte, R.; Andrea, P.S.D.; de Oliveira, T.G.; Lima, M.M. Ecology of Triatoma brasiliensis in northeastern Brazil: Seasonal distribution, feeding resources, and Trypanosoma cruzi infection in a sylvatic population. J. Vector Ecol. 2010, 35, 385–394. [Google Scholar] [CrossRef]
- Anwyl, R. The structure and properties of an abdominal stretch receptor in Rhodnius prolixus. J. Insect Physiol. 1972, 18, 2143–2147, 2149–2153. [Google Scholar] [CrossRef]
- Chiang, R.G.; Davey, K.G. A novel receptor capable of monitoring applied pressure in the abdomen of an insect. Science 1988, 241, 1665–1667. [Google Scholar] [CrossRef]
- Botto-Mahan, C.; Cattan, P.E.; Medel, R. Chagas disease parasite induces behavioural changes in the kissing bug Mepraia spinolai. Acta Trop. 2006, 98, 219–223. [Google Scholar] [CrossRef]
- Estay-Olea, D.; de Bona, S.; Bacigalupo, A.; Quiroga, N.; San Juan, E.; Correa, J.P.; Solari, A.; Botto-Mahan, C. Trypanosoma cruzi could affect wild triatomine approaching behaviour to humans by altering vector nutritional status: A field test. Acta Trop. 2020, 210, 105574. [Google Scholar] [CrossRef]
- Córdoba-Aguilar, A. Chagas bugs and Trypanosoma cruzi: Puppets and puppeteer? Acta Trop. 2020, 211, 105600. [Google Scholar] [CrossRef]
- Díaz, S.; Villavicencio, B.; Correia, N.; Costa, J.; Haag, K.L. Triatomine bugs, their microbiota and Trypanosoma cruzi: Asymmetric responses of bacteria to an infected blood meal. Parasites Vectors 2016, 9, 636. [Google Scholar] [CrossRef]
- Oliveira, J.L.; Cury, J.C.; Gurgel-Gonçalves, R.; Bahia, A.C.; Monteiro, F.A. Field-collected Triatoma sordida from Central Brazil display high microbiota diversity that varies with regard to developmental stage and intestinal segmentation. PLoS Negl. Trop. Dis. 2018, 12, e0006709. [Google Scholar] [CrossRef]
- Guarneri, A.A.; Schaub, G.A. Interaction of Triatomines with their bacterial microbiota and Trypanosomes. In Triatominae. The Biology of Chagas Disease Vectors; Guarneri, A., Lorenzo, M., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 345–386. [Google Scholar] [CrossRef]
- Vázquez, D.P.; Canale, D.; Gürtler, R.E. Effects of non-susceptible hosts on the infection with Trypanosoma cruzi of the vector Triatoma infestans: An experimental model. Mem. Inst. Oswaldo Cruz 1999, 94, 413–419. [Google Scholar] [CrossRef]
- de Fuentes-Vicente, J.A.; Gutiérrez-Cabrera, A.E.; Flores-Villegas, A.L.; Lowenberger, C.; Benelli, G.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A. What makes an effective Chagas disease vector? Factors underlying Trypanosoma cruzi-triatomine interactions. Acta Trop. 2018, 183, 23–31. [Google Scholar] [CrossRef]
- Asin, S.; Catala, S. Development of Trypanosoma cruzi in Triatoma infestans: Influence of temperature and blood consumption. J. Parasitol. 1995, 81, 1–7. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Teixeira, C.F.; de Sousa, V.F.A.; Guarneri, A.A. Effect of temperature and vector nutrition on the development and multiplication of Trypanosoma rangeli in Rhodnius prolixus. Parasitol. Res. 2018, 117, 1737–1744. [Google Scholar] [CrossRef]
- Tamayo, L.D.; Guhl, F.; Vallejo, G.A.; Ramírez, J.D. The effect of temperature increase on the development of Rhodnius prolixus and the course of Trypanosoma cruzi metacyclogenesis. PLoS Negl. Trop. Dis. 2018, 12, e0006735. [Google Scholar] [CrossRef]
- Castañera, M.B.; Aparicio, J.P.; Gürtler, R.E. A stage-structured stochastic model of the population dynamics of Triatoma infestans, the main vector of Chagas disease. Ecol. Model. 2003, 162, 33–53. [Google Scholar] [CrossRef]
- Pittendrigh, C.S. The circadian oscillation in Drosophila pseudosobscura pupae: A model for the photoperiodic clock. Z. Pflanzenphysiol. 1966, 54, 275–307. [Google Scholar]

| Population | Period | Month/Year | Nº of Captures | L:D | Min T° | Max T° |
|---|---|---|---|---|---|---|
| C1 | Cooling | May 2016 | 79 | 10.5:13.5 | 8.37 ± 0.53 | 21.51 ± 0.99 |
| C2 | Cooling | July 2017 | 60 | 10.1:13.9 | 4.34 ± 0.15 | 17.71 ± 0.79 |
| C3 | Cooling | June 2018 | 100 | 10.1:13.9 | 4.29 ± 0.22 | 24.00 ± 1.07 |
| C4 | Cooling | August 2018 | 104 | 10.5:13.5 | 2.91 ± 0.29 | 20.20 ± 0.50 |
| W1 | Warming | October 2016 | 100 | 12.8:11.2 | 5.09 ± 0.27 | 24.57 ± 0.91 |
| W2 | Warming | September 2017 | 47 | 11.5:12.5 | 3.73 ± 0.63 | 20.34 ± 0.71 |
| W3 | Warming | November 2017 | 40 | 13.7:10.3 | 7.91 ± 0.28 | 24.91 ± 0.76 |
| W4 | Warming | October 2018 | 179 | 13.0:11.0 | 4.49 ± 0.20 | 23.09 ± 1.02 |
| Nº Molting Events | |||||
|---|---|---|---|---|---|
| Population | Nymphal Stage | Total Number | One | Two | None |
| C1 | I-II-III-IV | 16-28-13-22 | 14-24-12-14 | 2-4-1-8 | 0-0-0-0 |
| C2 | I-II-III-IV | 24-16-10-10 | 18-13-7-4 | 6-2-2-6 | 0-1-1-0 |
| C3 | I-II-III-IV | 21-31-38-10 | 10-13-29-4 | 8-13-1-1 | 3-5-8-5 |
| C4 | I-II-III-IV | 15-43-33-13 | 15-17-15-10 | 0-22-16-2 | 0-4-2-1 |
| W1 | I-II-III-IV | 28-46-20-6 | 11-29-12-5 | 16-14-2-1 | 1-3-6-0 |
| W2 | I-II-III-IV | 15-16-5-11 | 2-2-4-3 | 13-14-1-8 | 0-0-0-0 |
| W3 | I-II-III-IV | 18-12-5-5 | 5-7-3-3 | 13-5-2-2 | 0-0-0-0 |
| W4 | I-II-III-IV | 27-33-35-84 | 19-21-27-70 | 8-11-5-0 | 0-1-3-14 |
| Molting Events | ||||||
|---|---|---|---|---|---|---|
| Cooling Period | Warming Period | |||||
| Nymphal Stage | One | Two | None | One | Two | None |
| I | 57 (14) | 16 (2) | 3 (0) | 37 (19) | 50 (24) | 1 (1) |
| II | 67 (9) | 41 (9) | 10 (3) | 59 (31) | 44 (33) | 4 (2) |
| III | 63 (15) | 20 (8) | 11 (1) | 46 (26) | 10 (8) | 9 (3) |
| IV | 32 (7) | 17 (6) | 6 (1) | 81 (43) | 11 (10) | 14 (8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botzotz, J.; Méndez-Valdés, G.; Ortiz, S.; López, A.; Botto-Mahan, C.; Solari, A. Natural Trypanosoma cruzi Infection and Climatic Season Influence the Developmental Capacity in Field-Caught Mepraia spinolai Nymphs. Insects 2023, 14, 272. https://doi.org/10.3390/insects14030272
Botzotz J, Méndez-Valdés G, Ortiz S, López A, Botto-Mahan C, Solari A. Natural Trypanosoma cruzi Infection and Climatic Season Influence the Developmental Capacity in Field-Caught Mepraia spinolai Nymphs. Insects. 2023; 14(3):272. https://doi.org/10.3390/insects14030272
Chicago/Turabian StyleBotzotz, Juan, Gabriel Méndez-Valdés, Sylvia Ortiz, Angélica López, Carezza Botto-Mahan, and Aldo Solari. 2023. "Natural Trypanosoma cruzi Infection and Climatic Season Influence the Developmental Capacity in Field-Caught Mepraia spinolai Nymphs" Insects 14, no. 3: 272. https://doi.org/10.3390/insects14030272
APA StyleBotzotz, J., Méndez-Valdés, G., Ortiz, S., López, A., Botto-Mahan, C., & Solari, A. (2023). Natural Trypanosoma cruzi Infection and Climatic Season Influence the Developmental Capacity in Field-Caught Mepraia spinolai Nymphs. Insects, 14(3), 272. https://doi.org/10.3390/insects14030272








