Management of Panonychus ulmi with Various Miticides and Insecticides and Their Toxicity to Predatory Mites Conserved for Biological Mite Control in Eastern U.S. Apple Orchards
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Orchard and Pesticide Treatments
2.2. Pesticide Treatment Application and Study Orchard Maintenance
2.3. Sampling and Data Analysis
3. Results and Discussions
3.1. Prophylactic Avermectin Treatments
3.2. Impacts on Predatory Mites
3.3. Economic Threshold Mite Treatments (Treatments #1–7, 10)
3.3.1. Panonychus ulmi Motiles
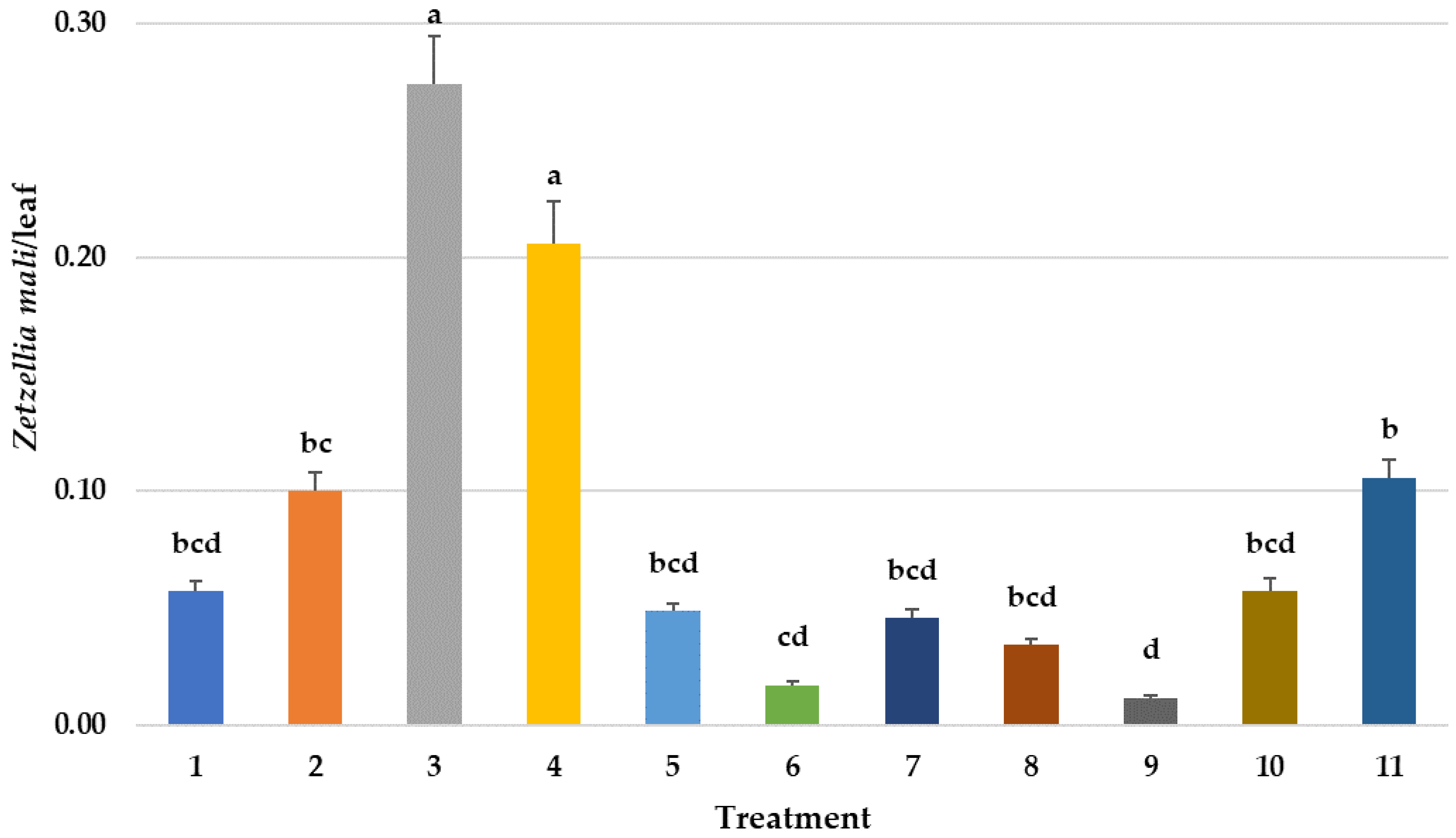
3.3.2. Effect on P. ulmi Eggs
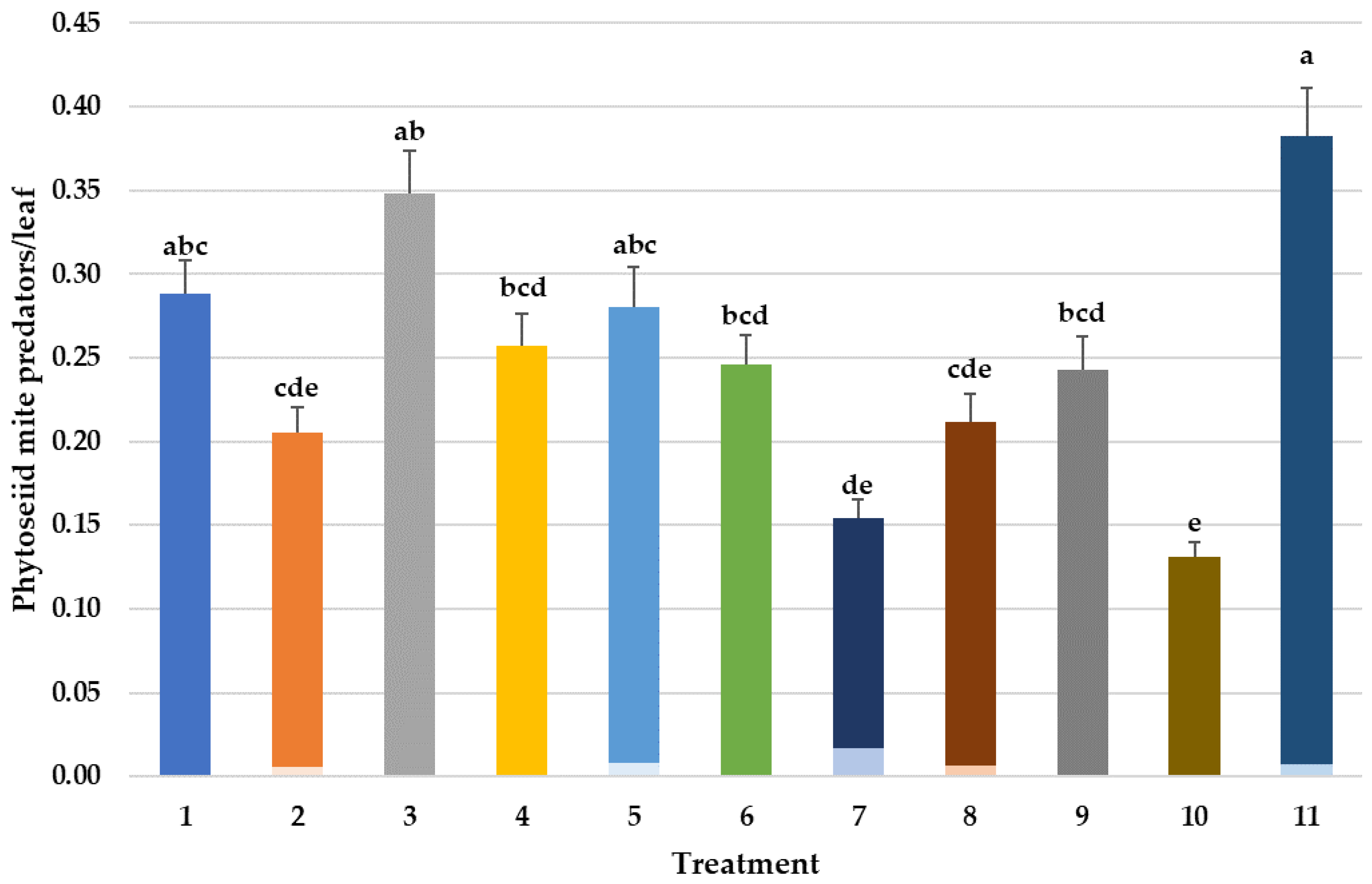
3.4. Effects on Predatory Mites Populations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boudreaux, H.B. Biological Aspects of Some Phytophagous Mites. Annu. Rev. Entomol. 1963, 8, 137–154. [Google Scholar] [CrossRef]
- Krantz, G.W.; Lindquist, E.E. Evolution of Phytophagous Mites (Acari). Annu. Rev. Entomol. 1979, 24, 121–158. [Google Scholar] [CrossRef]
- Moran, V.C. The Phytophagous Insects and Mites of Cultivated Plants in South Africa: Patterns and Pest Status. J. Appl. Ecol. 1983, 20, 450. [Google Scholar] [CrossRef]
- Helle, W.; Sabelis, M. Spider Mites: Their Biology, Natural Enemies and Control; Elsevier: Amsterdam, The Netherlands, 1985; Volume 1, ISBN 0444423729. [Google Scholar]
- Newcomer, E.; Yothers, M. Biology of the European Red Mite in the Pacific Northwest; Technical Bulletin No. 89; United States Department of Agriculture: Washington, DC, USA, 1929; p. 70. [Google Scholar]
- Collyer, E. Integrated Control of Apple Pests in New Zealand 6. Incidence of European Red Mite, Panonychus Ulmi (Koch), and Its Predators. N. Z. J. Zool. 1976, 3, 39–50. [Google Scholar] [CrossRef]
- Cutright, C.R. The European Red Mite in Ohio; Ohio Agricultural Experiment Station: Wooster, OH, USA, 1963. [Google Scholar]
- Hardman, J.M.; Herbert, H.J.; Sanford, K.H.; Hamilton, D. Effect of Populations of the European Red Mite, Panonychus Ulmi, on the Apple Variety Red Delicious in Nova Scotia. Can. Entomol. 1985, 117, 1257–1265. [Google Scholar] [CrossRef]
- Ewing, H.E. The Occurrence of the Citrus Red Spider, Tetranychus Mytilaspidis Riley, on Stone and Pomaceous Fruit Trees in Oregon. J. Econ. Entomol. 1912, 5, 414–415. [Google Scholar] [CrossRef]
- Groves, J.R.; Massee, A.M. A Synopsis of the World Literature on the Fruit Tree Red Spider Mite, Metatetranychus Ulmi Koch and Its Predators; Commonwealth Institute of Entomology: London, UK, 1951; ISBN 19510303093. [Google Scholar]
- Herbert, H.J. Biology, Life Tables, and Intrinsic Rate of Increase of the European Red Mite, Panonychus Ulmi (Acarina: Tetranychidae). Can. Entomol. 1981, 113, 65–71. [Google Scholar] [CrossRef]
- Crassweller, R.M.; Baugher, T.A.; Ford, T.G.; Marini, R.P.; Schupp, J.R.; Weber, D.; Krawczyk, G.; Biddinger, D.J.; López-Uribe, M.M.; Hopwood, J.; et al. Penn State Tree Fruit Production Guide 2020–2021; Penn State Extension: University Park, PA, USA, 2020. [Google Scholar]
- Hull, L.A.; Beersi, E.H. Validation of Injury Thresholds for European Red MMite (Acari: Tetranychidae) on ‘Yorking’ and ‘Delicious’ Apple. J. Econ. Entomol. 1990, 83, 2026–2031. [Google Scholar] [CrossRef]
- Beers, E.H.; Hull, L.A. Timing of Mite Injury Affects the Bloom and Fruit Development of Apple. J. Econ. Entomol. 1990, 83, 547–551. [Google Scholar] [CrossRef]
- Marini, R.P.; Pfeiffer, D.G.; Sowers, D.S. Influence of European Red Mite (Acari: Tetranychidae) and Crop Density on Fruit Size and Quality and on Crop Value of “Delicious” Apples. J. Econ. Entomol. 1994, 87, 1302–1311. [Google Scholar] [CrossRef]
- Lakso, A.N.; Mattii, G.B.; Nyrop, J.P.; Denning, S.S. Influence of European Red Mite on Leaf and Whole-Canopy Carbon Dioxide Exchange, Yield, Fruit Size, Quality, and Return Cropping in ‘Starkrimson Delicious’ Apple Trees. J. Am. Soc. Hortic. Sci. 1996, 121, 954–958. [Google Scholar] [CrossRef]
- van Leeuwen, T.; Tirry, L.; Yamamoto, A.; Nauen, R.; Dermauw, W. The Economic Importance of Acaricides in the Control of Phytophagous Mites and an Update on Recent Acaricide Mode of Action Research. Pestic. Biochem. Physiol. 2015, 121, 12–21. [Google Scholar] [CrossRef]
- Martinson, T.E.; Nyrop, J.P.; Dennehy, T.J.; Reissig, W.H. Field Measurements of Selection for European Red Mite (Acari: Tetranychidae) Resistance to Dicofol in Apple Orchards. J. Econ. Entomol. 1991, 84, 1–6. [Google Scholar] [CrossRef]
- Reissig, W.H.; Hull, L.A. Hexythiazox Resistance in a Field Population of European Red Mite (Acari: Tetranychidae) on Apples. J. Econ. Entomol. 1991, 84, 727–735. [Google Scholar] [CrossRef]
- Pree, D.J.; Whitty, K.J.; van Driel, L. Baseline Susceptibility and Cross-Resistances of Some New Acaricides in the European Red Mite, Panonychus Ulmi. Exp. Appl. Acarol. 2005, 37, 165–171. [Google Scholar] [CrossRef]
- Rameshgar, F.; Khajehali, J.; Nauen, R.; Dermauw, W.; van Leeuwen, T. Characterization of Abamectin Resistance in Iranian Populations of European Red Mite, Panonychus Ulmi Koch (Acari: Tetranychidae). Crop Prot. 2019, 125, 104903. [Google Scholar] [CrossRef]
- Badieinia, F.; Khajehali, J.; Nauen, R.; Dermauw, W.; van Leeuwen, T. Metabolic Mechanisms of Resistance to Spirodiclofen and Spiromesifen in Iranian Populations of Panonychus Ulmi. Crop Prot. 2020, 134, 105166. [Google Scholar] [CrossRef]
- Yaghoobi, R.; Khajehali, J.; Alavijeh, E.S.; Nauen, R.; Dermauw, W.; van Leeuwen, T. Fenpyroximate Resistance in Iranian Populations of the European Red Mite Panonychus Ulmi (Acari: Tetranychidae). Exp. Appl. Acarol. 2021, 83, 69–79. [Google Scholar] [CrossRef]
- Biddinger, D.J.; Hull, L.A. Effects of Several Types of Inseciticides on the Mite Predator, Stethorus Punctum (Coleoptera: Coccinellidae), Including Insect Growth Regulators and Abamectin. J. Econ. Entomol. 1995, 88, 358–366. [Google Scholar] [CrossRef]
- Biddinger, D.J.; Weber, D.C.; Hull, L.A. Coccinellidae as Predators of Mites: Stethorini in Biological Control. Biol. Control 2009, 51, 268–283. [Google Scholar] [CrossRef]
- Biddinger, D.J.; Butler, B.; Joshi, N.K. Biological Control of Mites in Pennsylvania and Maryland Apple Orchards. PA Fruit News 2014, 91, 41–51. [Google Scholar]
- Biddinger, D.J.; Hull, L.A. Survey of Pennsylvania Apple Orchards for a Mite Predator to Give Effective and Sustainable Control of Spider Mites. Penn Fruit News 2005, 85, 23–28. [Google Scholar]
- Tollerup, K.; Higbee, B. Evaluation of a “preventative” Strategy to Manage Spider Mites on Almond. Insects 2020, 11, 772. [Google Scholar] [CrossRef] [PubMed]
- Hamby, K.A.; Alifano, J.A.; Zalom, F.G. Total Effects of Contact and Residual Exposure of Bifenthrin and λ-Cyhalothrin on the Predatory Mite Galendromus Occidentalis (Acari: Phytoseiidae). Exp. Appl. Acarol. 2013, 61, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Simko, I.; Piepho, H.P. The Area under the Disease Progress Stairs: Calculation, Advantage, and Application. Anal. Theor. Plant Pathol. 2012, 102, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Chant, D.A. Phytoseiid Mites (Acarina: Phytoseiidae). Mem. Entomol. Soc. Can. 1959, 91, 5–166. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Prentice Hall: Hoboken, NJ, USA, 1996; ISBN 0130845426. [Google Scholar]
- Abro, G.H.; Dybas, R.A.; Green, A.S.J.; Wright, D.J. Translaminar and Residual Activity of Avermectin B1 against Plutella Xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 1989, 82, 385–388. [Google Scholar] [CrossRef]
- Hoy, M.A.; Cave, F.E. Laboratory Evaluation of Avermectin as a Selective Acaricide for Use with Metaseiulus Occidentalis (Nesbitt) (Acarina: Phytoseiidae). Exp. Appl. Acarol. 1985, 1, 139–152. [Google Scholar] [CrossRef]
- Lasota, J.A.; Dybas, R.A. Avermectins, a Novel Class of Compounds: Implications for Use in Arthropod Pest Control. Annu. Rev. Entomol. 1991, 36, 91–117. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Sanderson, J.P. Relative Toxicity of Abamectin to the Predatory Mite Phytoseiulus Persimilis (Acari: Phytoseiidae) and Twospotted Spider Mite (Acari: Tetranychidae). J. Econ. Entomol. 1990, 83, 1783–1790. [Google Scholar] [CrossRef]
- van der Werf, W.; Nyrop, J.P.; Hardman, J.M. Sampling Predator/Prey Ratios to Predict Cumulative Pest Density in the Mite-Predatory Mite System Panonychus Ulmi—Typhlodromus Pyri in Apples. Asp. Appl. Biol. 1994, 37, 41–51. [Google Scholar]
- Lester, P.J.; Thistlewood, H.M.A.; Harmsen, R. The Effects of Refuge Size and Number on Acarine Predator–Prey Dynamics in a Pesticide-Disturbed Apple Orchard. J. Appl. Ecol. 1998, 35, 323–331. [Google Scholar] [CrossRef]
- Aliniazee, M.T.; Cranham, J.E. Effect of Four Synthetic Pyrethroids on a Predatory Mite, Typhlodromus Pyri, and Its Prey, Panonychus Ulmi, on Apples in Southeast England. Environ. Entomol. 1980, 9, 436–439. [Google Scholar] [CrossRef]
- Marshall, D.B.; Pree, D.J. Effects of Miticides on the Life Stages of the European Red Mite, Panonychus Ulmi (Koch) (Acari: Tetranychidae). Can. Entomol. 1991, 123, 77–87. [Google Scholar] [CrossRef]
- Ay, R.; Kara, F.E. Toxicity, Inheritance of Fenpyroximate Resistance, and Detoxification-Enzyme Levels in a Laboratory-Selected Fenpyroximate-Resistant Strain of Tetranychus Urticae Koch (Acari: Tetranychidae). Crop Prot. 2011, 30, 605–610. [Google Scholar] [CrossRef]
- Schmidt-Jeffris, R.A.; Beers, E.H.; Sater, C. Meta-Analysis and Review of Pesticide Non-Target Effects on Phytoseiids, Key Biological Control Agents. Pest Manag. Sci. 2021, 77, 4848–4862. [Google Scholar] [CrossRef]
- Agnello, A.M.; Atanassov, A.; Bergh, J.C.; Biddinger, D.J.; Gut, L.J.; Haas, M.J.; Harper, J.K.; Hogmire, H.W.; Hull, L.A.; Kime, L.F.; et al. Reduced-Risk Pest Management Programs for Eastern U.S. Apple and Peach Orchards: A 4-Year Regional Project. Am. Entomol. 2009, 55, 184–197. [Google Scholar] [CrossRef]
- Hunter, M.D.; Biddinger, D.J.; Carlini, E.J.; McPheron, B.A.; Hull, L.A. Effects of Apple Leaf Allelochemistry on Tufted Apple Bud Moth (Lepidoptera: Tortricidae) Resistance to Azinphosmethyl. J. Econ. Entomol. 1994, 87, 1423–1429. [Google Scholar] [CrossRef]
- Jones, V.P.; Steffan, S.A.; Hull, L.A.; Brunner, J.F.; Biddinger, D.J. Effects of the Loss of Organophosphate Pesticides in the US: Opportunities and Needs to Improve IPM Programs. Outlooks Pest Manag. 2010, 21, 161–166. [Google Scholar] [CrossRef]
- Joshi, N.K.; Hull, L.A.; Krawczyk, G.; Rajotte, E. Field Results of Mating Disruption Technologies for the Control of Codling Moth, Cydia Pomonella (L.), and Oriental Fruit Moth, Grapholita Molesta (Busck) in Pennsylvania Apple Orchards. Asp. Appl. Biol. 2008, 84, 153–161. [Google Scholar]
- Joshi, N.K.; Hull, L.A.; Rajotte, E.G.; Krawczyk, G.; Bohnenblust, E. Evaluating Sex-Pheromone- and Kairomone-Based Lures for Attracting Codling Moth Adults in Mating Disruption versus Conventionally Managed Apple Orchards in Pennsylvania. Pest Manag. Sci. 2011, 67, 1332–1337. [Google Scholar] [CrossRef]
- Hull, L.A.; Joshi, N.K.; Zaman, F.U. Management of Internal Feeding Lepidopteran Pests in Apple. Arthropod Manag. Tests 2009, 34, A8. [Google Scholar] [CrossRef]
- Beers, E.H.; Hull, L.A.; Grimm, J.W. Relationships between Leaf: Fruit Ratio and Varying Levels of European Red Mite Stress on Fruit Size and Return Bloom of Apple. J. Am. Soc. Hortic. Sci. 1987, 112, 608–612. [Google Scholar] [CrossRef]




| Trmt # | Treatment | Manufacturer | Active Ingredient | Amount/Acre | Grams Active Ingredient/Acre | Date of Application |
|---|---|---|---|---|---|---|
| 1 | Nealta 200SC | BASF | cyflumetofen | 405 mL | 81 g | 11 Jul |
| +Tactic | Loveland Products, Inc | organo-silicone surfactant | 473 mL | -- | ||
| 2 | Nealta 200SC | BASF | cyflumetofen | 405 mL | 81 g | 11 Jul |
| +Cohere | Helena Agri-Enterprises, LLC | surfactant | 473 mL | -- | ||
| 3 | DPX-RDS63 200SC | DuPont Crop Protection | dicloromezotiaz | 610 mL | 121.5 g | 11 Jul |
| 4 | Delegate 25WG | Corteva Agriscience | spinetoram | 147.4 g | 36.9 g | 11 Jul |
| 5 | Bifenture 2EC | United Phosphorus, Inc. | bifenthrin | 379 mL | 90.8 g | 11 Jul |
| 6 | Portal 0.4EC | Nichino America, Inc. | fenpyroximate | 946 mL | 45.4 g | 11 Jul |
| 7 | Zeal 72WP | Valent USA, LLC | etoxazole | 85.1 g | 61.2 | 11 Jul |
| +LI-700 | Loveland Products, Inc | non-ionic surfactant | 473 mL (0.25% v/v) | -- | ||
| 8 | Gladiator 0.25EC | FMC Corporation | zeta-cypermethrin + avermectin B1 | 533 mL | 78.0 g + 5.0 g | 22 May |
| +JMS Stylet Oil | JMS Flower Farms, Inc | mineral oil | 3785 mL (1% v/v) | -- | ||
| 9 | Agri-Mek 0.15EC | Syngenta Crop Protection, LLC | abamectin | 355 mL | 6.4 g | 22 May |
| +JMS Stylet Oil | JMS Flower Farms, Inc | mineral oil | 3785 mL (1% v/v) | -- | ||
| 10 | Envidor 2SC | Bayer CropScience | spirodiclofen | 533 mL | 127.8 g | 11 Jul |
| 11 | Untreated Control | -- | -- | -- | -- | -- |
| Predator/Prey Ratios | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trmt # | 21 May | 3 Jun | 11 Jun | 18 Jun | 24 Jun | 1 Jul | 8 Jul | 15 Jul | 22 Jul | 29 Jul | 5 Aug | 12 Aug | 19 Aug | 26 Aug |
| 1 | 1.0 | 0.1 | 0.1 | 0.0 | 0.2 | 0.0 | 0.1 | 0.1 | 0.3 | 9.0 | 8.0 | 18.0 | ∞ | 7.5 |
| 2 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.0 | 0.3 | ∞ | ∞ | 2.7 | ∞ | ∞ | 2.7 |
| 3 | 0.0 | 0.0 | 0.3 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.3 | 0.6 | 2.1 | 11.0 | 2.8 |
| 4 | 0.0 | 0.0 | 0.3 | 0.0 | 0.1 | 0.0 | 0.0 | 0.2 | 0.0 | 0.2 | 0.6 | 0.4 | 0.7 | 9.0 |
| 5 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 1.3 | 0.0 | 0.3 | 0.1 | 0.4 | 0.5 | 0.6 |
| 6 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.3 | 0.2 | 0.9 | 2.0 | 1.4 |
| 7 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.1 | 0.1 | 0.5 | 0.7 | 0.3 | 0.1 |
| 8 | 0.0 | 0.0 | 0.8 | 0.0 | 0.0 | 0.2 | 0.3 | 0.3 | 0.1 | 13.0 | 2.5 | 2.0 | ∞ | 18.0 |
| 9 | 0.0 | 0.0 | 1.2 | 0.0 | 0.0 | 0.0 | 0.0 | 3.0 | 1.0 | 9.0 | 2.2 | 2.3 | 5.3 | ∞ |
| 10 | 0.0 | 0.0 | 0.1 | 0.5 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 5.0 | 0.4 | 1.0 | 0.4 | 1.3 |
| 11 | ∞ | 0.0 | 0.4 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.3 | 0.9 | 0.6 | 3.5 | 0.5 | 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, N.K.; Phan, N.T.; Biddinger, D.J. Management of Panonychus ulmi with Various Miticides and Insecticides and Their Toxicity to Predatory Mites Conserved for Biological Mite Control in Eastern U.S. Apple Orchards. Insects 2023, 14, 228. https://doi.org/10.3390/insects14030228
Joshi NK, Phan NT, Biddinger DJ. Management of Panonychus ulmi with Various Miticides and Insecticides and Their Toxicity to Predatory Mites Conserved for Biological Mite Control in Eastern U.S. Apple Orchards. Insects. 2023; 14(3):228. https://doi.org/10.3390/insects14030228
Chicago/Turabian StyleJoshi, Neelendra K., Ngoc T. Phan, and David J. Biddinger. 2023. "Management of Panonychus ulmi with Various Miticides and Insecticides and Their Toxicity to Predatory Mites Conserved for Biological Mite Control in Eastern U.S. Apple Orchards" Insects 14, no. 3: 228. https://doi.org/10.3390/insects14030228
APA StyleJoshi, N. K., Phan, N. T., & Biddinger, D. J. (2023). Management of Panonychus ulmi with Various Miticides and Insecticides and Their Toxicity to Predatory Mites Conserved for Biological Mite Control in Eastern U.S. Apple Orchards. Insects, 14(3), 228. https://doi.org/10.3390/insects14030228








