Effect of Fruit Volatiles from Native Host Plants on the Sexual Performance of Anastrepha fraterculus sp. 1 Males
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Insects
- Fruits
- Experiments
2.1. Experiment 1. Effect of Native Host Fruit Volatiles on A. fraterculus sp. 1 Male Mating Success
2.2. Experiment 2. Effect of Native Host Fruit Volatiles on A. fraterculus sp. 1 Male Sexual Calling Behavior
- Data Analysis
3. Results
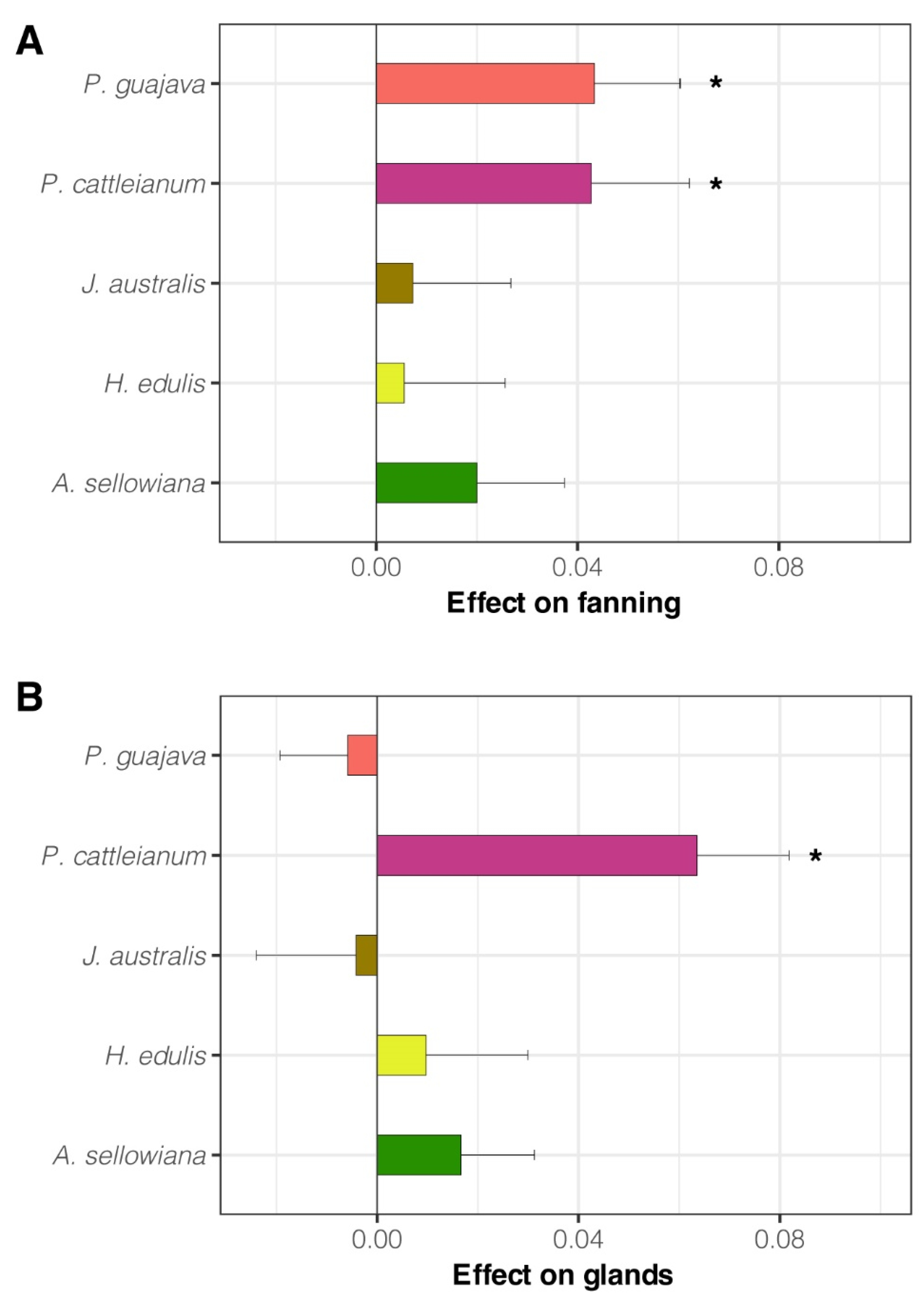
3.1. Experiment 1. Effect of Native Host Fruit Volatiles on A. fraterculus sp. 1 Male Mating Success
3.2. Experiment 2. Effect of Native Host Fruit Volatiles on A. fraterculus sp. 1 Male Sexual Calling Behavior
4. Discussion
5. Conclusions
- Exposure to fruit volatiles of two native host species of A. fraterculus sp. 1 stimulated male sexual behavior.
- The two plant species that triggered this response belong to the Psidium genus. This phenomenon could result from an evolutionary association between A. fraterculus sp. 1 and host plants of this genus, or similarity in the aroma of its fruit, or both.
- Fruit volatiles from the other three native hosts did not enhance male mating success or calling behavior, which shows that males do not respond to any native host fruit, even though they are important (i.e., heavily infested) hosts in nature.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, G.V.P.; Guerrero, A. Interactions of Insect Pheromones and Plant Semiochemicals. Trends Plant Sci. 2004, 9, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Landolt, P.J.; Phillips, T.W. Host plant influences on sex pheromone behavior of phytophagous insects. Annu. Rev. Entomol. 1997, 42, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Landolt, P.J.; Heath, R.R.; Millar, J.G.; Davis-Hernandez, K.M.; Dueben, B.D.; Ward, K.E. Effects of host plant Gossypium hirsutum L. on sexual attraction of cabbage looper moths, Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae). J. Chem. Ecol. 1994, 20, 2959–2974. [Google Scholar] [CrossRef] [PubMed]
- Nishida, R. Chemical ecology of insect-plant interactions: Ecological significance of plant secondary metabolites. Biosci. Biotechnol. Biochem. 2014, 78, 1–13. [Google Scholar] [CrossRef]
- Moreau, J.; Desouhant, E.; Louâpre, P.; Goubault, M.; Rajon, E.; Jarrige, A.; Menu, F.; Thiéry, D. How host plant and fluctuating environments affect insect reproductive strategies? Adv. Bot. Res. 2017, 81, 259–287. [Google Scholar]
- Christenson, L.D.; Foote, R.H. Biology of fruit flies. Annu. Rev. Entomol. 1960, 5, 171–192. [Google Scholar] [CrossRef]
- White, I.M.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Bionomics; CAB International: Wallingford, UK, 1992; p. 610. ISBN 9780851987903. [Google Scholar]
- Segura, D.F.; Belliard, S.A.; Vera, M.T.; Bachmann, G.E.; Ruiz, M.J.; Jofre-Barud, F.; Fernández, P.C.; López, M.L.; Shelly, T.E. Plant Chemicals and the Sexual Behavior of Male Tephritid Fruit Flies. Ann. Entomol. Soc. Am. 2018, 111, 239–264. [Google Scholar] [CrossRef]
- Nishida, R.; Tan, K.H.; Serit, M.; Lajis, N.H.; Sukari, A.M.; Takahashi, S.; Fukami, H. Accumulation of phenylpropanoids in the rectal glands of males of the Oriental fruit fly, Dacus dorsalis. Experientia 1988, 44, 534–536. [Google Scholar] [CrossRef]
- Shelly, T.E.; Dewire, A.M. Chemically mediated mating success in male oriental fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1994, 87, 375–382. [Google Scholar] [CrossRef]
- Hee, A.K.W.; Tan, K.H. Attraction of female and male Bactrocera papayae to conspecific males fed with methyl eugenol and attraction of females to male sex pheromone components. J. Chem. Ecol. 1998, 24, 753–764. [Google Scholar] [CrossRef]
- Wee, S.L.; Tan, K.H.; Nishida, R. Pharmacophagy of methyl eugenol by males enhances sexual selection of Bactrocera carambolae. J. Chem. Ecol. 2007, 33, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.L.; Munir, M.Z.A.; Hee, A.K.W. Attraction and consumption of methyl eugenol by male Bactrocera umbrosa Fabricius (Diptera: Tephritidae) promotes conspecific sexual communication and mating performance. Bull. Entomol. Res. 2018, 108, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, N.; Balagawi, S.; Schutze, M.K.; Clarke, A.R. Evolution of lure response in tephritid fruit flies: Phytochemicals as drivers of sexual selection. Anim. Behav. 2013, 85, 781–789. [Google Scholar] [CrossRef]
- Shelly, T.E.; Nishimoto, J.I. Does female mate choice confer direct fitness benefits? Results from a tephritid fruit fly. Ann. Entomol. Soc. Am. 2016, 110, 204–211. [Google Scholar] [CrossRef]
- Akter, H.; Taylor, P.W. Sexual inhibition of female Queensland fruit flies mated by males treated with raspberry ketone supplements as immature adults. J. Appl. Entomol. 2018, 142, 380–387. [Google Scholar] [CrossRef]
- Shelly, T.E.; Dang, C.; Kennelly, S. Exposure to orange (Citrus sinensis L.) trees, fruit, and oil enhances mating success of male Mediterranean fruit flies (Ceratitis capitata [Wiedemann]). J. Insect Behav. 2004, 17, 303–315. [Google Scholar] [CrossRef]
- Shelly, T.E. Exposure to grapefruits and grapefruit oil increases male mating success in the Mediterranean fruit fly (Diptera: Tephritidae). Proc. Hawaii. Entomol. Soc. 2009, 41, 31–36. [Google Scholar]
- Kouloussis, N.A.; Katsoyannos, B.I.; Papadopoulos, N.T.; Ioannou, C.S.; Iliadis, I.V. Enhanced mating competitiveness of Ceratitis capitata males following exposure to citrus compounds. J. Appl. Entomol. 2013, 137, 30–38. [Google Scholar] [CrossRef]
- Morató, S.; Shelly, T.E.; Rull, J.; Aluja, M. Sexual competitiveness of Anastrepha ludens (Diptera: Tephritidae) males exposed to Citrus aurantium and Citrus paradisi essential oils. J. Econ. Entomol. 2015, 108, 621–628. [Google Scholar] [CrossRef]
- Raghu, S. Functional significance of phytochemical lures to dacine fruit flies (Diptera: Tephritidae): An ecological and evolutionary synthesis. Bull. Entomol. Res. 2004, 94, 385–399. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Kouloussis, N.A.; Katsoyannos, B.I. Effect of plant chemicals on the behavior of the Mediterranean fruit fly. In Proceedings of the 7th International Symposium on Fruit Flies of Economic Importance: From Basic to Applied Knowledge, Salvador, Brazil, 10–15 September 2006; Sugayama, R.L., Zucchi, R.A., Ovruski, S.M., Sivinski, J., Eds.; Sociedade Entomologica Do Brazil Secretaria da Agricultura Federacao da Agricultura e Pecuária do Estado da Bahia: Salvador, Brazil, 2008; pp. 97–106. [Google Scholar]
- Steck, G.J. Biochemical systematics and population genetic structure of Anastrepha fraterculus and related species (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1991, 84, 10–28. [Google Scholar] [CrossRef]
- Salles, L.A.B. Bioecologia e Controle das Moscas das Frutas Sul-Americanas; Embrapa CPACT: Pelotas, Brazil, 1995; Available online: https://www.infoteca.cnptia.embrapa.br/bitstream/doc/742993/1/digitalizar0042.pdf (accessed on 18 January 2023).
- Malavasi, A.; Zucchi, R.A.; Sugayama, R.L. Biogeografıa. Moscas-das-Frutas de Importância Económica no Brasil. Conhecimento Básico e Aplicado; Malavasi, A., Zucchi, R., Eds.; Holos: Ribeirâo Preto, Brazil, 2000; pp. 93–98. [Google Scholar]
- Norrbom, A. Anastrepha fraterculus (South American Fruit Fly). CABI Compendium. 2008. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.5648 (accessed on 18 January 2023).
- Hernández-Ortiz, V.; Gómez-Anaya, J.A.; Sánchez, A.; McPheron, B.A.; Aluja, M. Morphometric analysis of Mexican and South American populations of the Anastrepha fraterculus complex (Diptera: Tephritidae) and recognition of a distinct Mexican morphotype. Bull. Entomol. Res. 2004, 94, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Guillén, D.; Sánchez, R. Expansion of the national fruit fly control programme in argentina. In Area-Wide Control of Insect Pests; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 653–660. [Google Scholar]
- Ramos-Peña, A.M.; Yábar-Landa, E.; Ramos-Peña, J.C. Diversidad, fluctuación poblacional y hospedantes de moscas de la fruta Anastrepha spp. y Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) en el valle de Abancay, Apurímac, Perú. Acta Zool. Mex. 2019, 35, 1–21. [Google Scholar] [CrossRef]
- Cladera, J.L.; Vilardi, J.C.; Juri, M.; Paulin, L.E.; Giardini, M.C.; Gómez Cendra, P.V.; Segura, D.F.; Lanzavecchia, S.B. Genetics and biology of Anastrepha fraterculus: Research supporting the use of the sterile insect technique (SIT) to control this pest in Argentina. BMC Genet. 2014, 15, 1–14. [Google Scholar] [CrossRef]
- Juárez, M.L.; Devescovi, F.; Břízová, R.; Bachmann, G.E.; Segura, D.F.; Kalinová, B.; Fernández, P.C.; Ruiz, M.J.; Yang, J.; Teal, P.E.A.; et al. Evaluating mating compatibility within fruit fly cryptic species complexes and the potential role of sex pheromones in pre-mating isolation. ZooKeys 2015, 540, 125–155. [Google Scholar]
- Vaníčková, L.; Hernández-Ortiz, V.; Bravo, I.S.J.; Dias, V.; Roriz, A.K.P.; Laumann, R.A.; Mendonça, A.L.; Paranhos, B.A.J.; do Nascimento, R.R. Current knowledge of the species complex Anastrepha fraterculus (Diptera, Tephritidae) in Brazil. ZooKeys 2015, 540, 211–237. [Google Scholar] [CrossRef]
- Hernández-Ortiz, V.; Bartolucci, A.F.; Morales-Valles, P.; Frías, D.; Selivon, D. Cryptic species of the Anastrepha fraterculus complex (Diptera: Tephritidae): A multivariate approach for the recognition of South American morphotypes. Ann. Entomol. Soc. Am. 2012, 105, 305–318. [Google Scholar] [CrossRef]
- Vera, M.T.; Ruiz, M.J.; Oviedo, A.; Abraham, S.; Mendoza, M.; Segura, D.F.; Kouloussis, N.A.; Willink, E. Fruit compounds affect male sexual success in the South American fruit fly, Anastrepha fraterculus (Diptera: Tephritidae). J. Appl. Entomol. 2013, 137, 2–10. [Google Scholar] [CrossRef]
- Bachmann, G.E.; Segura, D.F.; Devescovi, F.; Juárez, M.L.; Ruiz, M.J.; Vera, M.T.; Cladera, J.L.; Teal, P.E.A.; Fernández, P.C. Male sexual behavior and pheromone emission is enhanced by exposure to guava fruit volatiles in Anastrepha fraterculus. PLoS ONE. 2015, 10, e0124250. [Google Scholar]
- Bachmann, G.E.; Devescovi, F.; Nussenbaum, A.L.; Milla, F.H.; Shelly, T.E.; Cladera, J.L.; Fernandez, P.C.; Vera, M.T.; Segura, D.F. Mate choice confers direct benefits to females of Anastrepha fraterculus (Diptera: Tephritidae). PLoS ONE 2019, 14, e0214698. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Juárez, M.L.; Barud, F.J.; Goane, L.; Valladares, G.A.; Bachmann, G.E.; Belliard, S.A.; Jerez, P.P.; Belli, A.L.Z.; Méndez, F.D.G.; et al. Lemon and Schinus polygama essential oils enhance male mating success of Anastrepha fraterculus. Entomol. Exp. Appl. 2021, 169, 172–182. [Google Scholar] [CrossRef]
- Belliard, S.A.; Fernández, P.C.; Vera, M.T.; Segura, D.F. Timing of exposure and nutritional status affect male response to guava volatiles, a known courtship enhancer of Anastrepha fraterculus. J. Pest Sci. 2022, 95, 279–290. [Google Scholar] [CrossRef]
- Belliard, S.A.; Bachmann, G.E.; Fernández, P.C.; Hurtado, J.; Vera, M.T.; Segura, D.F. Identification of host plant volatile stimulants of Anastrepha fraterculus male courtship behavior. Front. Ecol. Evol. 2022, 10, 943260. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Sexual selection, competitive communication and species specific signals in insects. In Insect Communication (Proceedings of the 12th Symposium of the Royal Entomological Society of London); Lewis, T., Ed.; Academic Press: New York, NY, USA, 1984; p. 314. [Google Scholar]
- Ryan, M.J. Sexual selection, sensory systems and sensory exploitation. Oxford Surv. Evol. Biol. 1990, 7, 157–195. [Google Scholar]
- Shelly, T.E.; Edu, J.; Pahio, E.; Nishimoto, J. Scented males and choosy females: Does male odor influence female mate choice in the Mediterranean fruit fly? J. Chem. Ecol. 2007, 33, 2308–2324. [Google Scholar] [CrossRef]
- Weatherhead, P.J.; Robertson, R.J. Offspring quality and the polygyny threshold: “The sexy son hypothesis”. Am. Nat. 1979, 113, 201–208. [Google Scholar] [CrossRef]
- Kumaran, N.; Clarke, A.R. Indirect effects of phytochemicals on offspring performance of Queensland fruit fly, Bactrocera tryoni (Diptera: Tephritidae). J. Appl. Entomol. 2014, 138, 361–367. [Google Scholar] [CrossRef]
- Knipling, E.F. Possibilities of insect control or eradication through the use of sexually sterile males. J. Econ. Entomol. 1955, 48, 459–462. [Google Scholar] [CrossRef]
- Pereira, R.; Yuval, B.; Liedo, P.; Teal, P.E.A.; Shelly, T.E.; McInnis, D.O.; Haq, I.; Taylor, P.W.; Hendrichs, J. Improving post-factory performance of sterile male fruit flies in support of the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Taylor & Francis: Boca Raton, FL, USA; CRC Press: Abingdon, UK, 2021; pp. 631–656. [Google Scholar]
- Jaldo, H.E. Estudios Biológicos y Poblacionales de Anastrepha fraterculus (Wiedemann) (Diptera: Tephritidae). Ph.D. Thesis, Facultad de Agronomía y Zootecnia, Universidad Nacional de Tucumán, San Miguel de Tucumán, Argentina, 2001. [Google Scholar]
- Bachmann, G.E.; Devescovi, F.; Nussenbaum, A.L.; Cladera, J.L.; Fernandez, P.C.; Vera, M.T.; Teal, P.E.A.; Segura, D.F. Male sexual enhancement after methoprene treatment in Anastrepha fraterculus (Diptera: Tephritidae): A sustained response that does not fade away after sexual maturation. J. Insect Physiol. 2017, 101, 7–14. [Google Scholar] [CrossRef]
- Juárez, M.L.; Pimper, L.E.; Bachmann, G.E.; Conte, C.A.; Ruiz, M.J.; Goane, L.; Pereyra, P.M.; Castro, F.; Salgueiro, J.; Cladera, J.L.; et al. Gut bacterial diversity and physiological traits of Anastrepha fraterculus Brazilian-1 morphotype males are affected by antibiotic treatment. BMC Microbiol. 2019, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Segura, D.F.; Petit-Marty, N.; Sciurano, R.; Vera, M.T.; Calcagno, G.; Allinghi, A.; Cendra, P.G.; Cladera, J.L.; Vilardi, J. Lekking behavior of Anastrepha fraterculus (Diptera: Tephritidae). Fla. Entomol. 2007, 90, 154–162. [Google Scholar] [CrossRef]
- Nation, J.L. The role of pheromones in the mating system of Anastrepha fruit flies. In World Crop Pests: Fruit Flies, Their Biology, Natural Enemies and Control; Elsevier: Amsterdam, The Netherlands, 1989; pp. 189–205. [Google Scholar]
- Gomez Cendra, P.; Calcagno, G.; Belluscio, L.; Vilardi, J.C. Male courtship behavior of the South American fruit fly, Anastrepha fraterculus, from an Argentinean laboratory strain. J. Insect Sci. 2011, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 18 January 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Delignette-Muller, M.L.; Dutang, C. fitdistrplus: An R Package for Fitting Distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.6. 2022. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 12 February 2023).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; ISBN 9781544336473. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Aluja, M.; Piñero, J.; Jacome, I.; Díaz-Fleischer, F.; Sivinski, J. Behavior of flies in the genus Anastrepha. In Fruit Flies (Diptera: Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 375–408. [Google Scholar]
- Shelly, T.E. Exposure to alpha-copaene and alpha-copaene-containing oils enhances mating success of male Mediterranean fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2001, 94, 497–502. [Google Scholar] [CrossRef]
- Gerofotis, C.D.; Ioannou, C.S.; Papadopoulos, N.T. Aromatized to find mates: α-pinene aroma boosts the mating success of adult olive fruit flies. PLoS ONE 2013, 8, e81336. [Google Scholar] [CrossRef]
- Shelly, T.E.; McInnis, D.O. Exposure to ginger root oil enhances mating success of irradiated, mass-reared males of Mediterranean fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 2001, 94, 1413–1418. [Google Scholar] [CrossRef]
- Flores, S.; Rivera, J.P.; Hernandez, E.; Montoya, P. The effect of ginger oil on the sexual performance of Anastrepha males (Diptera: Tephritidae). Fla. Entomol. 2011, 94, 916–922. [Google Scholar] [CrossRef]
- Morelli, R.; Paranhos, B.J.; Coelho, A.M.; Castro, R.; Garziera, L.; Lopes, F.; Bento, J.M.S. Exposure of sterile Mediterranean fruit fly (Diptera: Tephritidae) males to ginger root oil reduces female remating. J. Appl. Entomol. 2013, 137, 75–82. [Google Scholar] [CrossRef]
- Mohammed, K.; Agarwal, M.; Li, B.; Newman, J.; Liu, T.; Ren, Y. Evaluation of d-limonene and β-ocimene as attractants of Aphytis melinus (Hymenoptera: Aphelinidae), a parasitoid of Aonidiella aurantii (Hemiptera: Diaspididae) on Citrus spp. Insects 2020, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Conchou, L.; Lucas, P.; Meslin, C.; Proffit, M.; Staudt, M.; Renou, M. Insect odor scapes: From plant volatiles to natural olfactory scenes. Front. Physiol. 2019, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Zeni, V.; Benelli, G.; Campolo, O.; Giunti, G.; Palmeri, V.; Maggi, F.; Rizzo, R.; Lo Verde, G.; Lucchi, A.; Canale, A. Toxics or lures? Biological and behavioral effects of plant essential oils on Tephritidae fruit flies. Molecules. 2021, 26, 5898. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.D.; Pereira, T.; Maio Marques, M.O.; Monteiro, A.R. Volatile and non-volatile chemical composition of the white guava fruit (Psidium guajava) at different stages of maturity. Food Chem. 2007, 100, 15–21. [Google Scholar] [CrossRef]
- FAO; IAEA; USDA. Product Quality Control for Sterile Mass-Reared and Released; IAEA: Vienna, Austria, 2019. [Google Scholar]

| Common Name | Scientific Name | Taxonomic Family | Distribution in Argentina | Fruits Ripening |
|---|---|---|---|---|
| Ubajay | Eugenia myrcianthes | Myrtaceae | Northeast | November–January |
| Argentine walnut (Nogal criollo) | Juglans australis | Juglandaceae | Northwest | December–February |
| Strawberry guava (Arazá rojo) | Psidium cattleianum | Myrtaceae | Northeast | February–March |
| Feijoa (Falso guayabo) | Acca sellowiana | Myrtaceae | North and central east | March–May |
| Guava (Guayaba) | Psidium guajava | Myrtaceae | Northeast | March–May |
| Host | Treatment | Latency | Duration | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SE | ChiSq | p Value | Mean ± SE | ChiSq | p Value | ||
| Acca sellowiana | Exposed | 7.46 ± 0.56 | 0.036 | 0.850 | 65.50 ± 1.35 | 0.00004 | 0.570 |
| Non-exposed | 8.24 ± 0.76 | 65.48 ± 1.38 | |||||
| Eugenia myrcianthes | Exposed | 9.00 ± 0.64 | 0.565 | 0.452 | 53.43 ± 1.37 | 0.067 | 0.796 |
| Non-exposed | 9.55 ± 0.59 | 53.97 ± 1.58 | |||||
| Juglans australis | Exposed | 12.47 ± 0.77 | 0.850 | 0.355 | 70.98 ± 1.29 | 0.059 | 0.808 |
| Non-exposed | 12.85 ± 0.70 | 71.41 ± 1.22 | |||||
| Psidium cattleianum | Exposed | 4.58 ± 0.47 | 0.087 | 0.768 | 61.08 ± 1.24 | 0.322 | 0.995 |
| Non-exposed | 4.59 ± 0.51 | 60.09 ± 1.21 | |||||
| Psidium guajava | Exposed | 10.18 ± 0.65 | 0.0003 | 0.986 | 65.50 ± 1.35 | 0.507 | 0.477 |
| Non-exposed | 9.91 ± 0.67 | 65.48 ± 1.38 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachmann, G.E.; Belliard, S.A.; Devescovi, F.; Nussenbaum, A.L.; Fernández, P.C.; Vera, M.T.; Ruiz, M.J.; Segura, D.F. Effect of Fruit Volatiles from Native Host Plants on the Sexual Performance of Anastrepha fraterculus sp. 1 Males. Insects 2023, 14, 188. https://doi.org/10.3390/insects14020188
Bachmann GE, Belliard SA, Devescovi F, Nussenbaum AL, Fernández PC, Vera MT, Ruiz MJ, Segura DF. Effect of Fruit Volatiles from Native Host Plants on the Sexual Performance of Anastrepha fraterculus sp. 1 Males. Insects. 2023; 14(2):188. https://doi.org/10.3390/insects14020188
Chicago/Turabian StyleBachmann, Guillermo Enrique, Silvina Anahí Belliard, Francisco Devescovi, Ana Laura Nussenbaum, Patricia Carina Fernández, María Teresa Vera, María Josefina Ruiz, and Diego Fernando Segura. 2023. "Effect of Fruit Volatiles from Native Host Plants on the Sexual Performance of Anastrepha fraterculus sp. 1 Males" Insects 14, no. 2: 188. https://doi.org/10.3390/insects14020188
APA StyleBachmann, G. E., Belliard, S. A., Devescovi, F., Nussenbaum, A. L., Fernández, P. C., Vera, M. T., Ruiz, M. J., & Segura, D. F. (2023). Effect of Fruit Volatiles from Native Host Plants on the Sexual Performance of Anastrepha fraterculus sp. 1 Males. Insects, 14(2), 188. https://doi.org/10.3390/insects14020188







