Vast Gene Flow among the Spanish Populations of the Pest Bactrocera oleae (Diptera, Tephritidae), Phylogeography of a Metapopulation to Be Controlled and Its Mediterranean Genetic Context
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Methods
2.2.1. DNA Isolation, Amplification and Sequencing
2.2.2. Data Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean Olive Orchards under Climate Change: A Review of Future Impacts and Adaptation Strategies. Agronomy 2021, 11, 56. [Google Scholar] [CrossRef]
- United Nations Educational, Scientific and Cultural Organization, UNESCO. Available online: https://ich.unesco.org/en/RL/mediterranean-diet-00884 (accessed on 28 December 2021).
- MAPA, Ministerio de Agricultura, Pesca y Alimentación. Available online: https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/aceite-oliva-y-aceituna-mesa/ (accessed on 28 December 2021).
- Rodríguez Sousa, A.A.; Parra-López, C.; Sayadi-Gmada, S.; Barandica, J.M.; Rescia, A.J. Multifunctional assessment of integrated and ecological farming in olive agroecosystems in southwestern Spain using the Analytic Hierarchy Process. Ecol. Econ. 2020, 173, 106658. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization (EPPO). Available online: https://gd.eppo.int/taxon/DACUOL/distribution (accessed on 28 December 2021).
- Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02009L0128-20190726 (accessed on 28 December 2021).
- Boletín Oficial del Estado (BOE) A-2012-11605: Real Decreto 1311/2012, de 14 de Septiembre, Por el Que se Establece el Marco de Actuación para Conseguir un uso Sostenible de los Productos Fitosanitarios. Available online: https://www.boe.es/eli/es/rd/2012/09/14/1311/con (accessed on 28 December 2021).
- Ochando, M.D.; Reyes, A.; Callejas, C.; Segura, D.; Fernández, P. Molecular genetic methodologies applied to the study of fly pests. Trends Entomol. 2003, 3, 73–85. [Google Scholar]
- Singh, S.; Mishra, V.K.; Kumar Bhoi, T. Insect molecular markers and its utility. A review. Int. J. Agric. Environ. Biotechnol. 2017, 10, 469–479. [Google Scholar] [CrossRef]
- Nardi, F.; Carapelli, A.; Dallai, R.; Roderick, G.K.; Frati, F. Population structure and colonization history of the olive fly, Bactrocera oleae (Diptera, Tephritidae). Mol. Ecol. 2005, 14, 2729–2738. [Google Scholar] [CrossRef]
- Segura, M.D. Análisis poblacional y evolutivo en Bactrocera oleae (Gmelin) mediante el uso de marcadores moleculares. Ph.D. Thesis, University Complutense of Madrid, Madrid, Spain, 2022. [Google Scholar]
- Segura, M.D.; Callejas, C.; Ochando, M.D. Bactrocera oleae: A single large population in Northern Mediterranean basin. J. Appl. Entomol. 2008, 132, 706–713. [Google Scholar] [CrossRef]
- Lantero, E. Estudio genético de la plaga del olivo Bactrocera oleae (Rossi 1790) y su aplicación al control biológico. Ph.D. Thesis, University Complutense of Madrid, Madrid, Spain, 31 October 2018. [Google Scholar]
- Augustinos, A.A.; Mamuris, Z.; Stratikopoulos, E.E.; D’Amelio, S.; Zacharopoulou, A.; Mathiopoulos, K.D. Microsatellite analysis of olive fly populations in the Mediterranean indicaates a westward expansion of species. Genetica 2005, 125, 231–241. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Stratikopoulos, E.E.; Drosopoulou, E.; Kakani, E.; Mavragani-Tsipidou, P.; Zacharopoulou, A.; Mathiopoulos, K.D. Isolation and characterization of microsatellite markers from the olive fly, Bactrocera oleae, and their cross-species amplification in the Tephritidae family. BMC Genom. 2008, 9, 618. [Google Scholar] [CrossRef] [Green Version]
- Zygouridis, N.E.; Augustinos, A.A.; Zalom, F.G.; Mathiopoulos, K.D. Analysis of olive fly invasion in California based on microsatellite markers. Heredity 2009, 102, 402–412. [Google Scholar] [CrossRef] [Green Version]
- Pentisaari, M.; Salmela, H.; Mutanen, M.; Roslin, T. Molecular evolution of a widely adopted taxonomic marker (COI) across the animal tree of life. Sci. Rep. 2016, 6, 35275. [Google Scholar] [CrossRef]
- Gopurenko, D.; Hughes, J.M.; Keenan, C.P. Mitochondrial DNA evidence for rapid colonisation of the Indo—West Pacific by the mud crab Scylla serrata. Mar. Biol. 1999, 134, 227–233. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The Clustal X window interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 24, 4876–4882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Song, H.; Buhay, J.E.; Whiting, M.F.; Crandall, K.A. Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. Proc. Natl. Acad. Sci. USA 2008, 105, 13486–13491. [Google Scholar] [CrossRef] [Green Version]
- Buhay, J.E. “COI—like” sequences are becoming problematic in molecular systematic and DNA barcoding studies. J. Crust. Biol. 2009, 29, 96–110. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Cassens, I.; Mardulyn, P.; Milinkovitch, M.C. Evaluating Intraspecific “Network” Construction Methods Using Simulated Sequence Data: Do Existing Algorithms Outperform the Global Maximum Parsimony Approach? Syst. Biol. 2005, 54, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Network Software. Available online: www.flexus-engineering.com (accessed on 28 December 2021).
- Nardi, F.; Carapelli, A.; Boore, J.L.; Roderick, G.K.; Dallai, R.; Frati, F. Domestication of olive fly through a multi-regional host shift to cultivated olives: Comparative dating using complete mitochondrial genomes. Mol. Phylogenet. Evol. 2010, 57, 678–686. [Google Scholar] [CrossRef]
- Frey, J.E.; Guillén, L.; Frey, B.J.; Samietz, R.J.; Aluja, M. Developing diagnostic SNP panels for the identification of true fruit flies (Diptera: Tephritidae) within the limits of COI-Based species delimitation. BMC Evol. Biol. 2013, 13, 106. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.J.; Xu, L.; Nardi, F.; Li, J.G.; Zhang, R.J. The complete nucleotide sequence of the mitochondrial genome of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Gene 2007, 396, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.R.; Slatkin, M.; Maddison, W.P. Estimation of levels of geneflow from DNA sequence data. Genetics 1992, 132, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evol. Bioinform. Online 2005, 1, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Rice, W.R. Analysing tables of statistical tests. Ecology 1989, 43, 223–225. [Google Scholar] [CrossRef]
- Sneath, P.H.; Sokal, R.R. Numerical Taxonomy: The Principles and Practice of Numerical Classification, 1st ed.; W.H. Freeman: San Francisco, CA, USA, 1973. [Google Scholar]
- Rohlf, F.J. NTSYSpc: Numerical Taxonomy System, version. 2.10q; Exeter Publishing, Ltd.: Setauket, NY, USA, 2000. [Google Scholar]
- Wright, S. Evolution in Mendelian Populations. Genetics 1931, 16, 290. [Google Scholar] [CrossRef]
- Dupanloup, I.; Schneider, S.; Excoffier, L. A simulated annealing approach to define the genetic structure of populations. Mol. Ecol. 2002, 11, 2571–2581. [Google Scholar] [CrossRef]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef]
- Ramos-Onsins, S.E.; Rozas, J. Statistical properties of new neutrality tests against population growth. Mol. Biol. Evol. 2002, 19, 2092–2100. [Google Scholar] [CrossRef] [Green Version]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Hudson, R.R. Gene genealogies and the coalescent processing. In Oxford Surveys in Evolutionary Biology; Futuyama, D., Antonovics, J., Eds.; Oxford University Press: Oxford, UK, 1990; Volume 7, pp. 1–42. [Google Scholar]
- Rogers, A.R.; Harpending, H. Population growth makes waves in the distribution of pairwise genetic differences. Mol. Biol. Evol. 1992, 9, 552–569. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Anastasiou, I.; Vogler, A.P. Revisiting the Insect Mitochondrial Molecular Clock: The Mid-Aegean Trench Calibration. Mol. Biol. Evol. 2010, 27, 1659–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Asch, B.; Pereira-Castro, I.; Rei, F.; Teixeira da Costa, L. Marked genetic differentiation between western Iberian and Italic populations of olive fly: Southern France as intermediate area. PLoS ONE 2015, 10, e0126702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matallanas, B.; Lantero, E.; M’Saad, M.; Callejas, C.; Ochando, M.D. Genetic polymorphism at the cytochrome oxidase I gene in mediterranean populations of Bactrocera oleae (Diptera: Tephritidae). J. Appl. Entomol. 2013, 137, 624–630. [Google Scholar] [CrossRef]
- Koohkanzadeh, M.; Pramual, P.; Fekrat, L. Genetic Analysis of Populations of the Peach Fruit Fly, Bactrocera zonata (Diptera: Tephritidae), in Iran. Neotrop. Entomol. 2019, 48, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Kunprom, C.; Sopaladawan, P.N.; Pramual, P. Population genetics and demographic history of guava fruit fly Bactrocera correcta (Diptera: Tephritidae) in northeastern Thailand. Eur. J. Entomol. 2015, 112, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Garzón-Orduña, I.J.; Scott, M.G.; Norman, B.B. The Genetic Diversity of Bactrocera dorsalis (Diptera: Tephritidae) in China and Neighboring Countries: A Review from Published Studies. J. Econ. Entomol. 2019, 112, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.S.; Song, S.L.; Lim, P.E.; Eamsobhana, P. Complete mitochondrial genome of Zeugodacus tau (Insecta: Tephritidae) and differentiation of Z. tau species complex by mitochondrial Cytochrome C Oxidase subunit I gene. PLoS ONE 2017, 12, e0189325. [Google Scholar] [CrossRef] [Green Version]
- Berlocher, S.H.; Bush, G.L. An electrophoretic analysis of Rhagoletis (Diptera: Tephritidae) phylogeny. Sys. Zool. 1982, 31, 136–155. [Google Scholar] [CrossRef]
- Jiménez. Tephritidae-Dacus oleae/Bactrocera oleae (Gmelin) Madrid 1ª edición. 915–917. In Entomología Agroforestal: Insectos y ácaros que Dañan a los Montes, Cultivos, Jardines e Invernaderos; Agrotécnicas, E., Ed.; 1988; ISBN 9788487480546. Available online: https://www.agapea.com/libros/Entomologia-agroforestal-insectos-y-acaros-que-danan-a-los-montes-cultivos-jardines-e-invernaderos-9788487480546-i.htm (accessed on 15 July 2022).
- Terral, J.; Arnold-Simard, G. Beginnings of Olive Cultivation in Eastern Spain in Relation to Holocene Bioclimatic Changes. Quat. Res. 1996, 46, 176–185. [Google Scholar] [CrossRef]
- Vernet, J.L.; Badal Garcñia, E.; Grau Almero, E. La végétation néolithique du sud-est de l’Espagne (Valencia, Alicante) d’après l’analyse anthracologique. C. R. L’acad. Des Sci. 1983, 296, 669–672. [Google Scholar]
- Lantero, E.; Matallanas, B.; Pascual, S.; Ochando, M.D.; Callejas, C. Phylogeography of organophosphate resistant ace alleles in Spanish olive fruit fly populations: A Mediterranean perspective in global change context. Insects 2020, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Doorenweerd, C.; San Jose, M.; Leblanc, L.; Barr, N.; Geib, S.; Arthur, Y.C.; Rubinoff, D. DNA barcodes and reliable molecular identifications in a diverse group of invasive pests: Lessons from Bactrocera fruit flies on variation across the COI gene, introgression, and standardization. BioRxiv 2020. [Google Scholar] [CrossRef]
- Skouras, P.J.; Margaritopoulos, J.; Seraphides, N.; Ioannides, I.; Kakani, E.; Mathiopoulos, K.; Tsitsipis, J. Organophosphate resistance in the olive fruit fly Bactrocera oleae populations in Greece and Cyprus. J. Pest. Sci. 2008, 63, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Economopoulos, A.P.; Haniotakis, G.E.; Mathioudis, J.; Missis, N.; Kinigakis, P. Long-distaance flight of wild and artificially reared Dacus oleae (Gmelin) (Diptera: Tephritidae). J. App. Entomol. 1978, 87, 101–108. [Google Scholar] [CrossRef]
- El-hajj, A.K.; Nemer, N.; Chhadeh, S.; Dandashi, F.; Yosef, H.; Nasrallah, M.; Moussa, Z. Status, Distribution and Parasitism Rate of Olive Fruit Fly (Bactrocera oleae. Rossi) Natural Enemies in Lebanon. JAS 2017, 6, 246–260. [Google Scholar]
- Rice, R.E. Bionomics of the olive fruit fly Bactrocera (Dacus) oleae. UC Plant Prot. Q. 2000, 10, 1–5. [Google Scholar]
- Rubio de Casas, R.; Besnard, G.; Schonswetter, P.; Balaguer, L.; Vargas, P. Extensive gene flow blurs phylogeographic but not phylogenetic signal in Olea europaea L. Theor. Appl. Genet. 2006, 113, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Besnard, G.; Rubio de Casas, R.; Vargas, P. Plastid and Nuclear DNA polymorphism reveals historical processes of isolation and reticulation in the olive tree complex (Olea europaea). J. Biogeogr. 2007, 34, 736–752. [Google Scholar] [CrossRef]
- Besnard, G.; Rubio de Casas, R.; Christin, P.A.; Vargas, P. Phylogenetics of Olea (Oleaceae) based on plastid and nuclear ribosomal DNA sequences: Tertiary climatic shifts and lineage differentiation times. Ann. Bot. 2009, 104, 143–160. [Google Scholar] [CrossRef] [Green Version]
- Nardi, F.; Frati, F. The olive fly Bactrocera oleae keeping up in an ever changing environment. Team News Lett. 2011, 10, 3–8. [Google Scholar]
- White, I.M. Morphological features of the tribe Dacini (Dacinae): Their significance to behaviour and classification. In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behaviour; CRC Press: Boca Raton, FL, USA, 1999; pp. 505–533. [Google Scholar] [CrossRef]
- Segura, M.D.; Callejas, C.; Fernández, M.P.; Ochando, M.D. New contributions towards the understanding of the phylogenetic relationships among economically important fruit flies (Diptera: Tephritidae). Bull. Entomol. Res. 2006, 96, 279–288. [Google Scholar] [CrossRef]
- Corrales, C.; Pavlovska, M.; Höglund, J. Phylogeography and subespecies status of Black Grouse. J. Ornithol. 2013, 155, 13–25. [Google Scholar] [CrossRef] [Green Version]
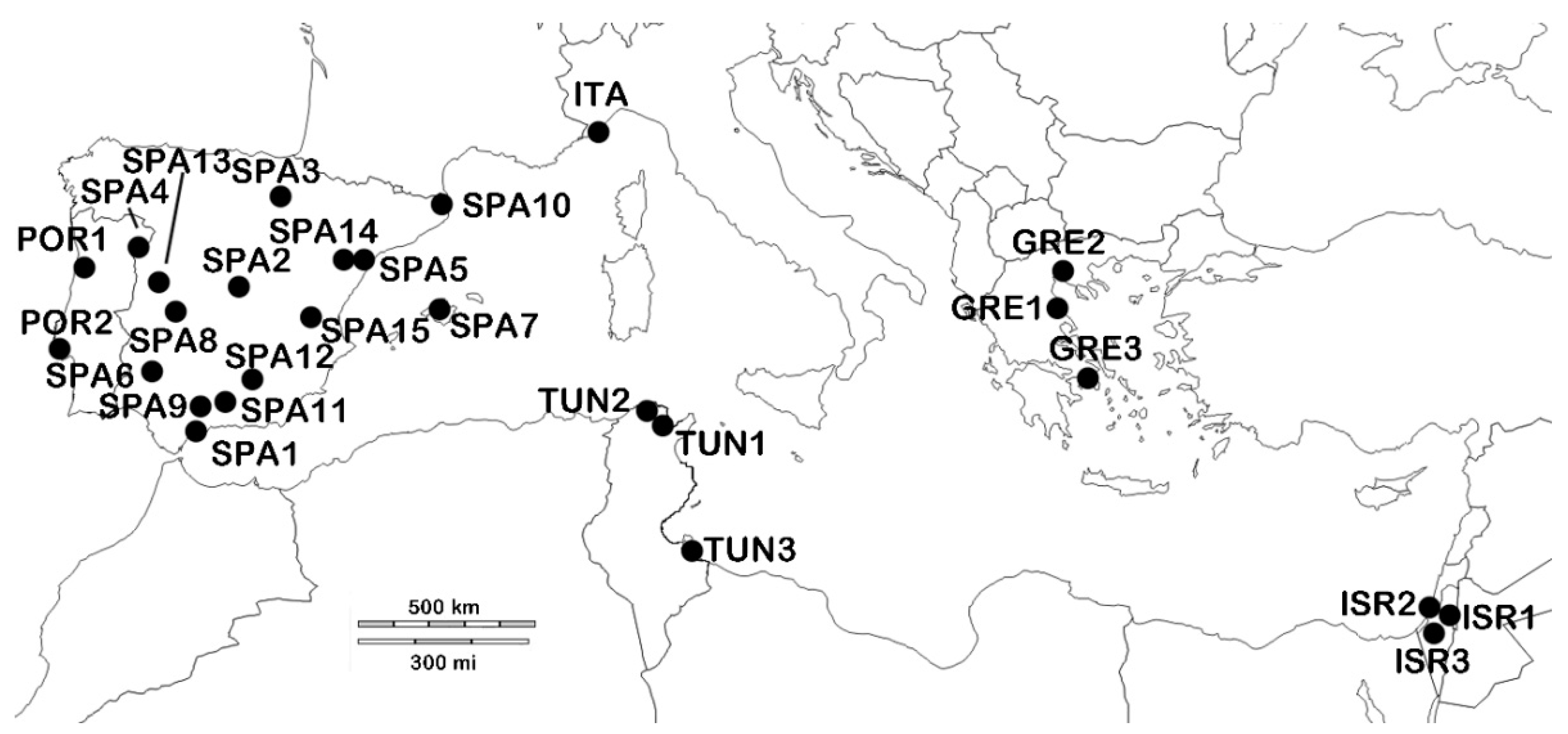
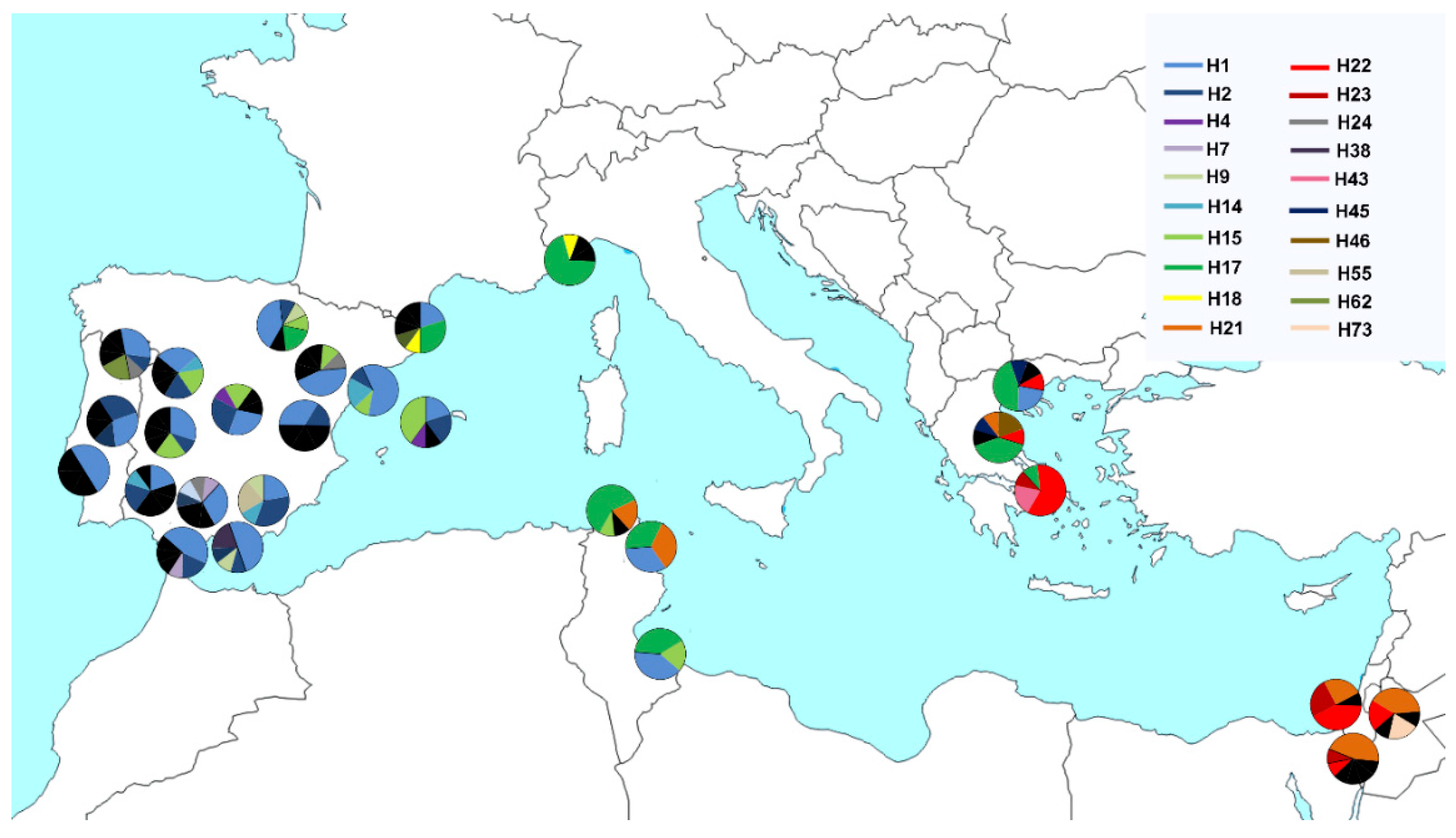

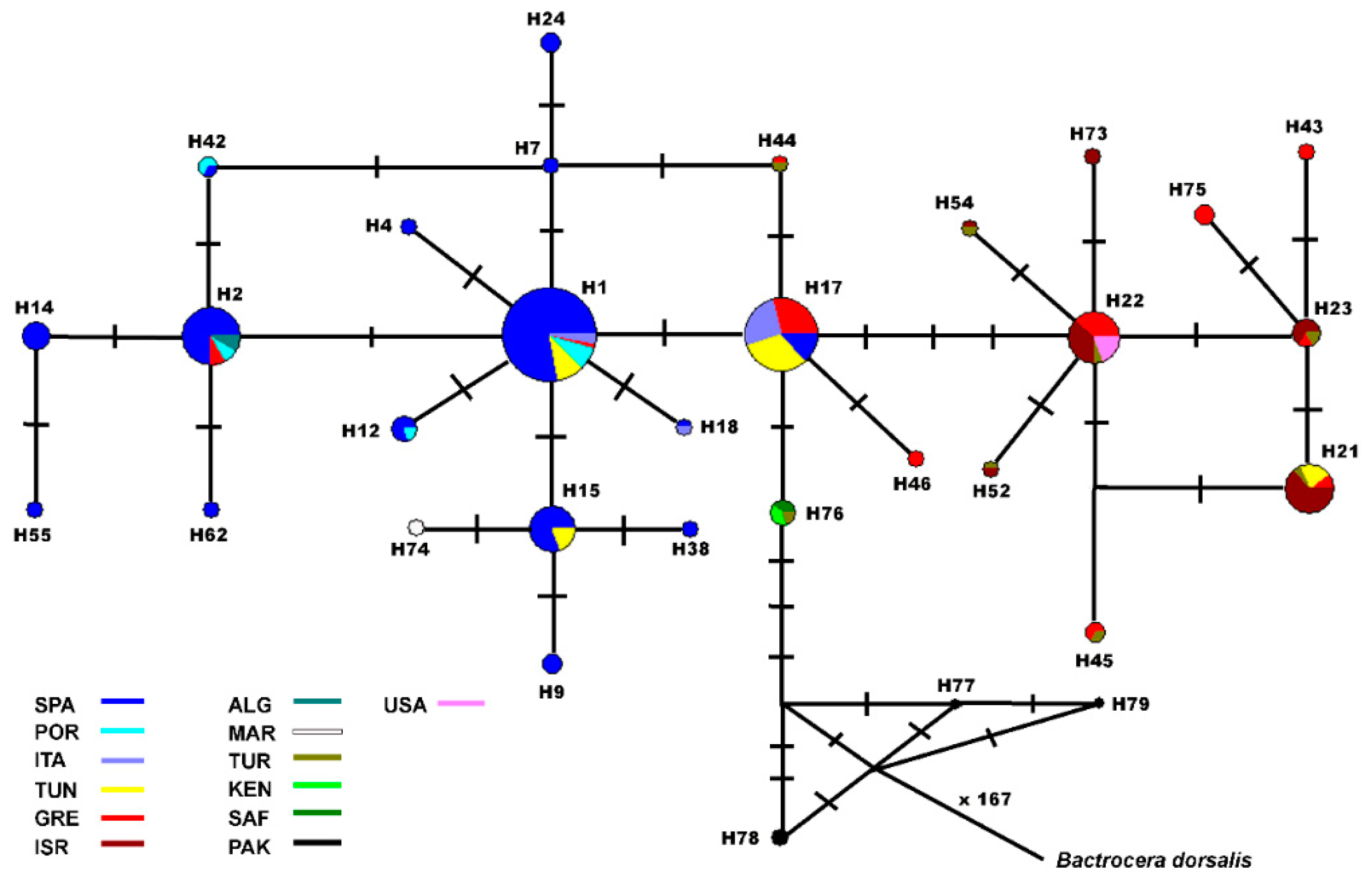
| CODE | Locality, Country | LAT | LONG | N |
|---|---|---|---|---|
| SPA1 | Ronda, Málaga, ES | 36.6587 | −4.7603 | 11 |
| SPA2 | Morata de Tajuña, Madrid, ES | 40.2275 | −3.4369 | 11 |
| SPA3 | Arróniz, Navarra, ES | 42.4222 | −2.0913 | 10 |
| SPA4 | Aldeadávila de la Ribera, Salamanca, ES | 41.2183 | −6.62 | 10 |
| SPA5 | Tortosa, Tarragona, ES | 40.811 | 0.5209 | 10 |
| SPA6 | Montemolín, Badajoz, ES | 38.1552 | −6.2069 | 10 |
| SPA7 | Mallorca, Islas Baleares, ES | 39.6952 | 3.0175 | 10 |
| SPA8 | Castañar de Ibor, Cáceres, ES | 39.6277 | −5.4166 | 10 |
| SPA9 | Campus de Rabanales, Córdoba, ES | 37.2647 | −4.6327 | 10 |
| SPA10 | El Cortalet, Gerona, ES | 42.2253 | 3.0970 | 10 |
| SPA11 | Íllora, Granada, ES | 37.3461 | −3.8727 | 9 |
| SPA12 | La Iruela, Jaén, ES | 37.9469 | −2.9583 | 10 |
| SPA13 | Lagunilla, Salamanca, ES | 40.3246 | −5.9687 | 10 |
| SPA14 | La Portellada, Teruel, ES | 40.89 | −0.0336 | 9 |
| SPA15 | Requena, Valencia, ES | 39.4878 | −1.1003 | 6 |
| POR1 | Fundao, PT | 40.1369 | −7.4994 | 7 |
| POR2 | Lisboa, PT | 38.7069 | −9.1356 | 6 |
| ITA | Diana Marina, Liguria, IT | 43.9098 | 8.0818 | 10 |
| TUN1 | Sidi Thabet, TN | 36.9081 | 10.0222 | 10 |
| TUN2 | Tunisia, TN | 36.7916 | 10.0634 | 6 |
| TUN3 | Zarzis, TN | 33.523 | 11.0852 | 10 |
| GRE1 | Agia, GR | 39.7188 | 22.7550 | 10 |
| GRE2 | Tesalonica, GR | 40.6393 | 22.9446 | 9 |
| GRE3 | Atenas, GR | 37.9791 | 23.7166 | 10 |
| ISR1 | Jerusalem, IL | 31.7383 | 35.2137 | 10 |
| ISR2 | Rehovot, IL | 31.8927 | 34.8112 | 12 |
| ISR3 | Lahav Forest, IL | 31.3725 | 34.8408 | 11 |
| POP | s | h | Hd | π |
|---|---|---|---|---|
| SPA1 | 6 | 6 | 0.800 | 0.00117 |
| SPA2 | 6 | 6 | 0.872 | 0.00130 |
| SPA3 | 5 | 6 | 0.844 | 0.00114 |
| SPA4 | 8 | 6 | 0.888 | 0.00176 |
| SPA5 | 3 | 4 | 0.644 | 0.00089 |
| SPA6 | 9 | 8 | 0.955 | 0.00209 |
| SPA7 | 4 | 5 | 0.822 | 0.00112 |
| SPA8 | 7 | 7 | 0.911 | 0.00158 |
| SPA9 | 9 | 8 | 0.933 | 0.00170 |
| SPA10 | 8 | 7 | 0.911 | 0.00162 |
| SPA11 | 5 | 5 | 0.861 | 0.00164 |
| SPA12 | 6 | 5 | 0.755 | 0.00154 |
| SPA13 | 6 | 7 | 0.911 | 0.00151 |
| SPA14 | 8 | 6 | 0.833 | 0.00154 |
| SPA15 | 6 | 5 | 0.933 | 0.00174 |
| SPAIN | 45 | 49 | 0.864 | 0.00151 |
| POR1 | 5 | 5 | 0.904 | 0.00157 |
| POR2 | 4 | 2 | 1.000 | 0.00348 |
| ITA | 5 | 4 | 0.533 | 0.00114 |
| TUN1 | 8 | 4 | 0.644 | 0.00207 |
| TUN2 | 6 | 3 | 0.800 | 0.00278 |
| TUN3 | 2 | 3 | 0.711 | 0.00077 |
| GRE1 | 8 | 6 | 0.844 | 0.00236 |
| GRE2 | 7 | 5 | 0.805 | 0.00193 |
| GRE3 | 5 | 4 | 0.644 | 0.00124 |
| ISR1 | 5 | 5 | 0.822 | 0.00160 |
| ISR2 | 3 | 4 | 0.757 | 0.00096 |
| ISR3 | 7 | 7 | 0.818 | 0.00171 |
| SPECIES | 60 | 73 | 0.908 | 0.00259 |
| Source of Variation | Variance Components | Percentage of Variation | p-Value |
|---|---|---|---|
| Spanish and Portuguese populations (Iberian Peninsula) | |||
| Among populations | 0.01160 | 1.31 | >0.0500 |
| Within populations | 0.87064 | 98.69 | |
| Mediterranean populations | |||
| Among populations | 0.625 | 41.38 | <0.0001 |
| Within populations | 0.886 | 58.62 | |
| 2 genetic groups: group I: Iberian Peninsula, Italy, Tunisia and Greece 1/2; and group II: Greece 3 and Israel | |||
| Among groups | 1.707 | 62.26 | <0.001 |
| Among populations | 0.133 | 4.87 | <0.001 |
| Within populations | 0.901 | 32.87 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lantero, E.; Matallanas, B.; Ochando, M.D.; Callejas, C. Vast Gene Flow among the Spanish Populations of the Pest Bactrocera oleae (Diptera, Tephritidae), Phylogeography of a Metapopulation to Be Controlled and Its Mediterranean Genetic Context. Insects 2022, 13, 642. https://doi.org/10.3390/insects13070642
Lantero E, Matallanas B, Ochando MD, Callejas C. Vast Gene Flow among the Spanish Populations of the Pest Bactrocera oleae (Diptera, Tephritidae), Phylogeography of a Metapopulation to Be Controlled and Its Mediterranean Genetic Context. Insects. 2022; 13(7):642. https://doi.org/10.3390/insects13070642
Chicago/Turabian StyleLantero, Esther, Beatriz Matallanas, M. Dolores Ochando, and Carmen Callejas. 2022. "Vast Gene Flow among the Spanish Populations of the Pest Bactrocera oleae (Diptera, Tephritidae), Phylogeography of a Metapopulation to Be Controlled and Its Mediterranean Genetic Context" Insects 13, no. 7: 642. https://doi.org/10.3390/insects13070642
APA StyleLantero, E., Matallanas, B., Ochando, M. D., & Callejas, C. (2022). Vast Gene Flow among the Spanish Populations of the Pest Bactrocera oleae (Diptera, Tephritidae), Phylogeography of a Metapopulation to Be Controlled and Its Mediterranean Genetic Context. Insects, 13(7), 642. https://doi.org/10.3390/insects13070642






