Potentially Suitable Geographical Area for Monochamus alternatus under Current and Future Climatic Scenarios Based on Optimized MaxEnt Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
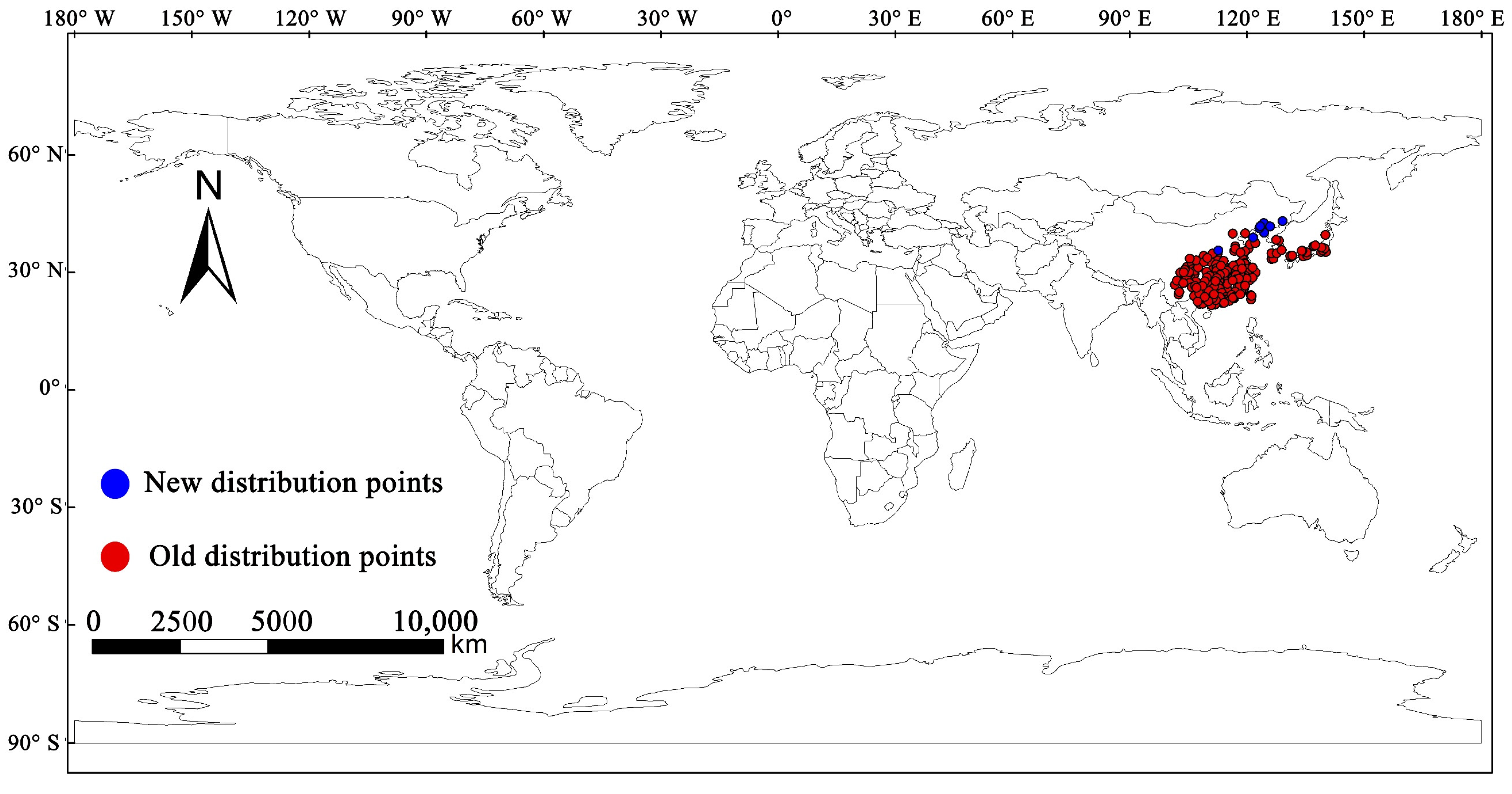
2.1. Global Occurrence Data of M. alternatus
2.2. Bioclimatic Variables
2.3. MaxEnt Model Setting and Selection
2.4. MaxEnt Model Evaluation and Analysis
3. Results
3.1. MaxEnt Model Optimization and Accuracy Evaluation
3.2. Relationships between the Distribution of M. alternatus and Bioclimatic Variables
3.3. Potentially Suitable Distribution Areas of M. alternatus under Current Climate
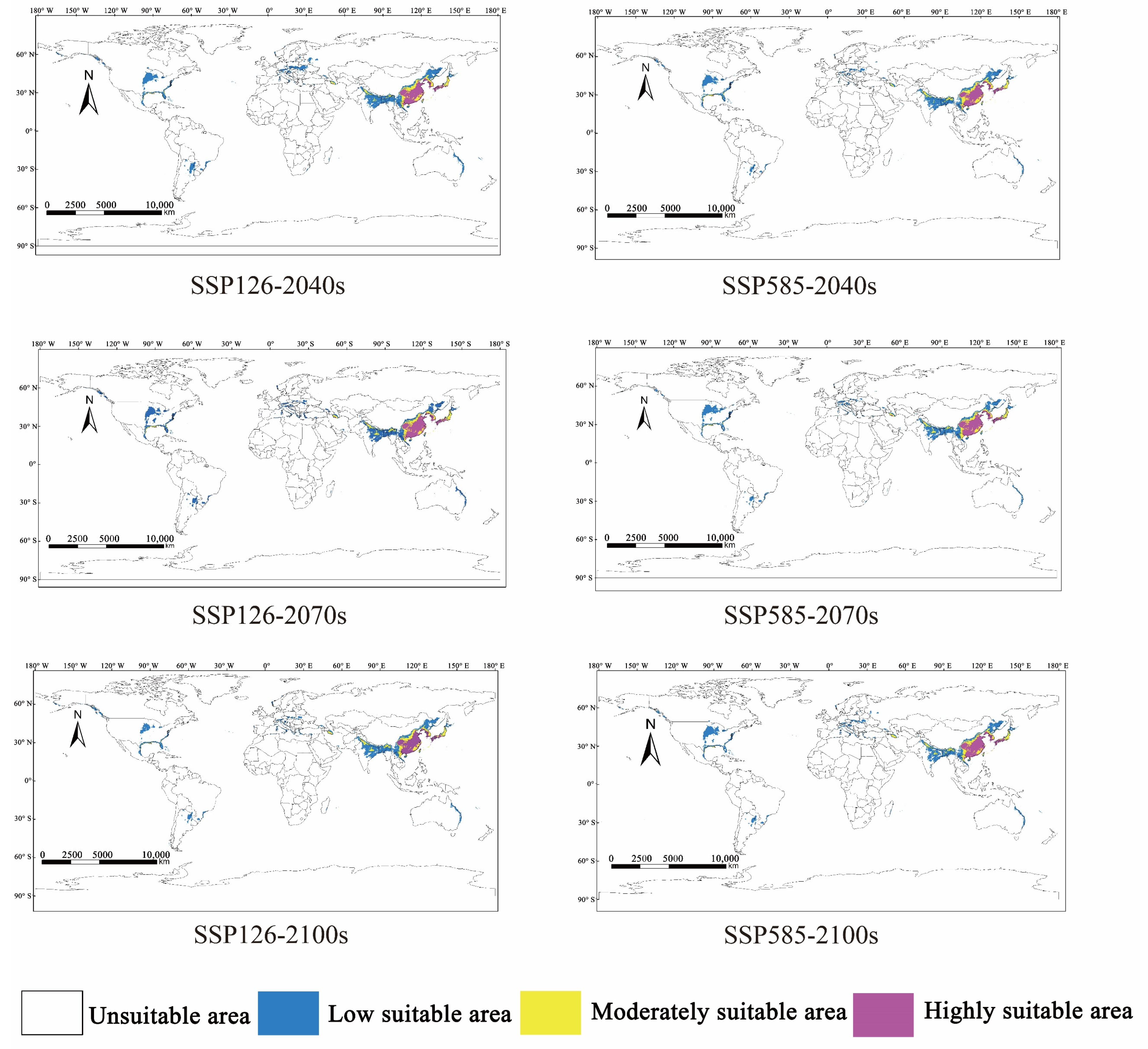
3.4. Change in Potentially Suitable Distribution Areas of M. alternatus under the Future Climate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, causal agent of pine wilt disease on Pinus pinaster in Northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef] [PubMed]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.G.; Futai, K.; Sutherland, J.R.; Takeuchi, Y. (Eds.) Pine wilt disease in China. In Pine Wilt Disease; Springer: Berlin, Germany, 2008; pp. 18–25. [Google Scholar]
- Sousa, E.; Rodrigues, J.M.; Bonifácio, L.F.; Naves, P.M.; Rodrigues, A. Management and control of the pine wood nematode, Bursaphelenchus xylophilus, in Portugal. In Nematodes: Morphology, Functions and Management Strategies; Fasto, B., Jordon, A., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2011; p. 21. [Google Scholar]
- Li, H.; Zhao, X.Y.; Qiao, H.; He, X.Y.; Tan, J.J.; Hao, D.J. Comparative transcriptome analysis of the heat stress response in Monochamus alternatus Hope (Coleoptera: Cerambycidae). Front. Physiol. 2020, 10, 1568. [Google Scholar] [CrossRef]
- Mamiya, Y.; Enda, N. Bursaphelenchus mucronatus n. sp. (Nematoda: Aphelenchoididae) from pine wood and its biology and pathogenicity to pine trees. Nematologica 1972, 25, 353–361. [Google Scholar] [CrossRef]
- Wang, Z.M.; Pi, Z.Q.; Hou, B. Monochamus alternatus were found in Jilin Province. For. Pest Dis. 2006, 3, 35. (In Chinese) [Google Scholar]
- Li, S.W.; Lv, X.L.; Tian, Y.M.; Wang, Y.; Zhang, B. Population dynamics of Monochamus alternatus in a typical occurrence area of Pine Wilt Disease in Dalian City. Liaoning For. Sci. Technol. 2019, 6, 20–22. (In Chinese) [Google Scholar]
- Li, Y.X.; Zhang, X.Y. Analysis of invasion expansion trend of Bursaphelenchus xylophilus. For. Pest Dis. 2018, 37, 1–4. (In Chinese) [Google Scholar]
- Hellmann, J.J.; Byers, J.E.; Bierwagen, B.G.; Dukes, J.S. Five potential consequences of climate change for invasive species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef]
- Kwon, T.S.; Lee, C.M.; Kim, S. Northward range shifts in Korean butterflies. Clim. Chang. 2014, 126, 163–174. [Google Scholar] [CrossRef]
- Beaury, E.M.; Fusco, E.J.; Jackson, M.R.; Laginhas, B.B.; Morelli, T.L.; Allen, J.M.; Pasquarella, V.J.; Bradley, B.A. Incorporating climate change into invasive species management: Insights from managers. Biol. Invasions 2020, 22, 233–252. [Google Scholar] [CrossRef]
- Cornelissen, B.; Neumann, P.; Schweiger, O. Global warming promotes biological invasion of a honey bee pest. Glob. Chang. Biol. 2019, 25, 3571–3994. [Google Scholar] [CrossRef] [PubMed]
- Daniel, G.T.; Alex, C.A.; Wesley, D.; Andrés, L.N.; Sánchez-Guillén Rosa, A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. 2020, 95, 802–821. [Google Scholar]
- Jin, Z.; Yu, W.; Zhao, H.; Xian, X.; Jing, K.; Yang, N.; Lu, X.; Liu, W. Potential Global Distribution of Invasive Alien Species, Anthonomus grandis Boheman, under Current and Future Climate Using Optimal MaxEnt Model. J. Agric. 2022, 12, 1759. [Google Scholar] [CrossRef]
- Rasmann, S.; Pellissier, L.; Defossez, E.; Jactel, H.; Kunstler, G.; Bailey, J.K. Climate-driven change in plant-insect interactions along elevation gradients. Funct. Ecol. 2014, 28, 46–54. [Google Scholar] [CrossRef]
- Iannella, M.; D’Alessandro, P.; Biondi, M. Forecasting the spread associated with climate change in Eastern Europe of the invasive Asiatic flea beetle, Luperomorpha xanthodera (Coleoptera: Chrysomelidae). Eur. J. Entomol. 2020, 117, 130–138. [Google Scholar] [CrossRef]
- Gao, R.H.; Wang, Z.; Wang, H.X.; Hao, Y.P.; Shi, J. Relationship between pine wilt disease outbreaks and climatic variables in the Three Gorges Reservoir Region. Forests 2019, 10, 816. [Google Scholar] [CrossRef]
- Yoon, S.; Jung, J.M.; Hwang, J.; Park, Y.; Hee, W.H. Ensemble evaluation of the spatial distribution of pine wilt disease mediated by insect vectors in South Korea. For. Ecol. Manag. 2023, 529, 120677. [Google Scholar] [CrossRef]
- Zhu, G.P.; Liu, G.Q.; Bu, W.J.; Gao, Y.B. Ecological niche modeling and its applications in biodiversity conservation. Biodivers. Sci. 2013, 21, 90–98. (In Chinese) [Google Scholar]
- Ge, X.Z.; He, S.Y.; Zhu, C.Y.; Wang, T.; Xu, Z.C.; Zong, S.X. Projecting the current and future potential global distribution of Hyphantria cunea (Lepidoptera: Arctiidae) using CLIMEX. Pest Manag. Sci. 2018, 75, 160–169. [Google Scholar] [CrossRef]
- Raffini, F.; Bertorelle, G.; Biello, R.; D’Urso, G.; Russo, D.; Bosso, L. From Nucleotides to Satellite Imagery: Approaches to Identify and Manage the Invasive Pathogen Xylella fastidiosa and Its Insect Vectors in Europe. Sustainability 2020, 12, 4508. [Google Scholar] [CrossRef]
- Lee, C.M.; Lee, D.S.; Kwon, T.S.; Athar, M.; Park, Y.S. Predicting the global distribution of Solenopsis geminata (Hymenoptera: Formicidae) under climate change using the MaxEnt model. Insects 2021, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.; Das, B.; Ramesh, R. Predicting climate change impacts on potential worldwide distribution of fall armyworm based on CMIP6 projections. J. Pest Sci. 2022, 95, 841–854. [Google Scholar] [CrossRef]
- Carpenter, G.; Gillison, A.N.; Winter, J. Domain: A flexible modeling procedure for mapping potential distributions of plants and animals. Biodivers. Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Chejara, V.K.; Kriticos, D.J.; Kristiansen, P.; Sindel, B.M.; Whalley, R.D.B.; Nadolny, C. The current and future potential geographical distribution of Hyparrhenia hirta. Weed Res. 2010, 50, 174–184. [Google Scholar] [CrossRef]
- Bradie, J.; Leung, B. Aquantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeogr. 2017, 44, 1344–1361. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Wang, C.; Hawthorne, D.; Qin, Y.; Pan, X.; Li, Z.; Zhu, S. Impact of climate and host availability on future distribution of Colorado potato beetle. Sci. Rep. 2017, 7, 4489. [Google Scholar] [CrossRef]
- Sultana, S.; Baumgartner, J.B.; Dominiak, B.C.; Royer, J.E.; Beaumont, L.J. Impacts of climate change on high priority fruit fly species in Australia. PLoS ONE 2020, 15, e0213820. [Google Scholar] [CrossRef]
- Wan, J.; Wang, R.; Ren, Y.; McKirdy, S. Potential distribution and the risks of Bactericera cockerelli and its associated plant pathogen Candidatus Liberibacter solanacearum for global potato production. Insects 2020, 11, 298. [Google Scholar] [CrossRef]
- Jackson, C.R.; Robertson, M.P. Predicting the potential distribution of an endangered cryptic subterranean mammal from few occurrence records. J. Nat. Conserv. 2011, 19, 87–94. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Monk, J.; Laurenson, L.; Chisholm, R. Sensitivity of fine-scale species distribution models to locational uncertainty in occurrence data across multiple sample sizes. Methods Ecol. Evol. 2016, 8, 12–21. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, L.; Zhang, X.; Gou, Z.J. Predicting the Potential Distribution of Hylomecon japonica in China under Current and Future Climate Change Based on Maxent Model. Sustainability 2021, 13, 11253. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Xu, C. The potentia geographical distribution of Alsophila spinulosain under climate change in China. Chin. J. Ecol. 2021, 40, 968–979. (In Chinese) [Google Scholar]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2010, 29, 773–785. [Google Scholar] [CrossRef]
- Ramos, R.S.; Kumar, L.; Shabani, F.; Picanco, M.C. Mapping global risk levels of Bemisia tabaci in areas of suitability for open field tomato cultivation under current and future climates. PLoS ONE 2018, 13, e0198925. [Google Scholar] [CrossRef]
- Santana Jr, P.A.; Kumar, L.; Da Silva, R.S.; Pereira, J.L.; Picanco, M.C. Assessing the impact of climate change on the worldwide distribution of Dalbulus maidis (DeLong) using MaxEnt. Pest Manag. Sci. 2019, 75, 2706–2715. [Google Scholar] [CrossRef]
- Yan, H.; He, J.; Xu, X.; Yao, X.; Wang, G.; Tang, L.; Feng, L.; Zou, L.; Gu, X.; Qu, Y.; et al. Prediction of potentially suitable distributions of Codonopsis pilosula in China based on an optimized MaxEnt model. Front. Ecol. Evol. 2021, 9, 821. [Google Scholar] [CrossRef]
- Khanghah, S.S.; Moaneri, M.; Ghorbani, A.; Mostafazadeh, R.; Biswas, A. Modeling potential habitats and predicting habitat connectivity for Leucanthemum vulgare Lam. in northwestern rangelands of Iran. Environ. Monit. Assess. 2022, 194, 1–16. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; McPherson, J. An R package for conducting spatially independent evaluations and estimating optimal model complexity for MaxEnt ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Porfirio, L.L.; Harris, R.M.B.; Lefroy, E.C.; Hugh, S.; Gould, S.F.; Lee, G.; Nathaniel, L.; Bindoff, B.M. Improving the use of species distribution models in conservation planning and management under climate change. PLoS ONE 2014, 9, e113749. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Wang, Z.; Zeng, H.; Shi, J. Tolerance to temperature stresses on Monochamus alternatus and its potential range in China. J. Northwest For. Univ. 2019, 34, 156–161. (In Chinese) [Google Scholar]
- Xu, R.; Zhou, R.; Liu, Q.; Li, W.; Wang, Y. Prediction and Simulation of the Suitable Habitat of Monochamus alternatus under Climate Warming. For. Resour. Manag. 2020, 04, 109–116. (In Chinese) [Google Scholar]
- Kim, J.; Jung, H.; Park, Y.H. Predicting potential distribution of Monochamus alternatus Hope responding to climate change in Korea. Korean J. Appl. Entomol. 2016, 55, 501–511. [Google Scholar] [CrossRef]
- Song, H.M.; Xu, R.M. Global potential geographical distribution of Monochamus alternatus. Chin. Bull. Entomol. 2006, 43, 535–539. (In Chinese) [Google Scholar]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Sillero, N. What does ecological modelling model? A proposed classification of ecological niche models based on their underlying methods. Ecol. Model. 2011, 222, 1343–1346. [Google Scholar] [CrossRef]
- Fotheringham, A.S.; Oshan, T.M. Geographically weighted regression and multicollinearity: Dispelling the myth. J. Geogr. Syst. 2016, 18, 303–329. [Google Scholar] [CrossRef]
- Xu, D.; Zhuo, Z.; Wang, R.; Ye, M.; Pu, B. Modeling the distribution of Zanthoxylum armatum in China with MaxEnt modeling. Glob. Ecol. Conserv. 2019, 19, e00691. [Google Scholar] [CrossRef]
- Li, X.; Xu, D.; Jin, Y.; Zhuo, Z.; Yang, H.; Hu, J.; Wang, R. Predicting the current and future distributions of Brontispa longissima (Coleoptera: Chrysomelidae) under climate change in China. J. Glob. Ecol. Conserv. 2021, 25, e01444. [Google Scholar] [CrossRef]
- Li, A.; Wang, J.; Wang, R.; Yang, H.; Yang, W.; Yang, C.; Jin, Z. MaxEnt modeling to predict current and future distributions of Batocera lineolata (Coleoptera: Cerambycidae) under climate change in China. Ecoscience 2019, 27, 23–31. [Google Scholar] [CrossRef]
- Rutherford, T.A.; Mamiya, Y.; Webster, J.M. Nematode-induced pine wilt disease: Factors influencing its occurrence and distribution. For. Sci. 1990, 36, 145–155. [Google Scholar]
- Evans, H.F.; McNamara, D.G.; Braasch, H.; Chadoeuf, J.; Magnusson, C. Pest risk analysis (PRA) for the territories of the European Union (as PRA area) on Bursaphelenchus xylophilus and its vectors in the genus Monochamus. EPPP Bull. 1996, 26, 199–249. [Google Scholar] [CrossRef]
- Roques, A.; Zhao, L.L.; Sun, J.H.; Robinet, C. Pine wood nematode, pine wilt disease, vector beetle and pine tree: How a multiplayer system could reply to climate change. In Climate Change and Insect Pests; Björkman, C., Niemelä, P., Eds.; CABI Publishing: Oxfordshire, UK, 2015; pp. 220–234. [Google Scholar]
- Wang, Z.; Zhao, L.J.; Liu, J.Q.; Yang, Y.J.; Shi, J.; Wen, J.B.; Gao, R.H. Functional relationship between woody plants and insect communities in response to Bursaphelenchus xylophilus infestation in the Three Gorges Reservoir region. Ecol. Evol. 2021, 11, 8843–8855. [Google Scholar] [CrossRef] [PubMed]
- Calvão, T.; Duarte, C.M.; Pimentel, C.S. Climate and landscape patterns of pine forest decline after invasion by the pinewood nematode. For. Ecol. Manag. 2019, 433, 43–51. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.M.B.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) Climate Change. In The Physical Science Basis: Working Group I Contribution to the 5th Assessment of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Takahashi, D.; Park, Y.S. Spatial heterogeneities of human-mediated dispersal vectors accelerate the range expansion of invaders with source–destination-mediated dispersal. Sci. Rep. 2020, 10, 21410. [Google Scholar] [CrossRef]
- Choi, W.I.; Song, H.J.; Kim, D.S.; Lee, D.S.; Lee, C.Y.; Nam, Y.; Kim, J.B.; Park, Y.S. Dispersal patterns of pine wilt disease in the early stage of its invasion in South Korea. Forests 2017, 8, 411. [Google Scholar] [CrossRef]



| Code | Bioclimatic Variables | Contribution Rate/% |
|---|---|---|
| Bio14 | Precipitation of driest month | 44.5 |
| Bio12 | Annual precipitation | 24.8 |
| Bio10 | Mean temperature of warmest quarter | 19.6 |
| Bio6 | Min. temperature of the coldest month | 3.2 |
| Bio8 | Mean temperature of the wettest quarter | 3.1 |
| Bio4 | Temperature seasonality | 3 |
| Bio2 | Monthly mean temperature difference | 1.3 |
| Bio15 | Precipitation seasonality | 0.6 |
| Default | Optimization | |
|---|---|---|
| β | 1.0 | 1.5 |
| FC | LQHP | LQHP |
| Mean AUC | 0.922 | 0.923 |
| AUCDIFF | 0.020 | 0.018 |
| ORMTP | 0.042 | 0.007 |
| OR10 | 0.153 | 0.146 |
| ΔAICc | 3.539 | 0 |
| Decade | Scenarios | Predicted Area (106 km2) | Comparison with Current Distribution (%) | ||||
|---|---|---|---|---|---|---|---|
| High | Moderate | Low | High | Moderate | Low | ||
| Current | - | 1.95 | 1.31 | 4.26 | - | - | - |
| 2040s | ssp-126 | 2.06 | 1.37 | 5.94 | 5.64 | 4.58 | 39.44 |
| ssp-585 | 1.95 | 1.44 | 4.92 | 0 | 9.92 | 15.49 | |
| 2070s | ssp-126 | 2.13 | 1.39 | 5.32 | 9.23 | 6.11 | 24.88 |
| ssp-585 | 2.10 | 1.24 | 4.97 | 7.69 | −5.34 | 16.67 | |
| 2100s | ssp-126 | 2.01 | 1.43 | 4.96 | 3.08 | 9.16 | 16.43 |
| ssp-585 | 2.08 | 1.33 | 5.31 | 6.67 | 1.53 | 24.65 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Liu, L.; Zhao, L.; Cui, S. Potentially Suitable Geographical Area for Monochamus alternatus under Current and Future Climatic Scenarios Based on Optimized MaxEnt Model. Insects 2023, 14, 182. https://doi.org/10.3390/insects14020182
Gao R, Liu L, Zhao L, Cui S. Potentially Suitable Geographical Area for Monochamus alternatus under Current and Future Climatic Scenarios Based on Optimized MaxEnt Model. Insects. 2023; 14(2):182. https://doi.org/10.3390/insects14020182
Chicago/Turabian StyleGao, Ruihe, Lei Liu, Lijuan Zhao, and Shaopeng Cui. 2023. "Potentially Suitable Geographical Area for Monochamus alternatus under Current and Future Climatic Scenarios Based on Optimized MaxEnt Model" Insects 14, no. 2: 182. https://doi.org/10.3390/insects14020182
APA StyleGao, R., Liu, L., Zhao, L., & Cui, S. (2023). Potentially Suitable Geographical Area for Monochamus alternatus under Current and Future Climatic Scenarios Based on Optimized MaxEnt Model. Insects, 14(2), 182. https://doi.org/10.3390/insects14020182






