Effects of the Entomopathogenic Fungus Mucor hiemalis BO-1 on the Physical Functions and Transcriptional Signatures of Bradysia odoriphaga Larvae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Materials
2.2. Entomopathogenic Fungi Strain
2.3. Pathogenicity Assays
2.4. Effects of M. hiemalis BO-1 on Food Consumption
2.5. Biochemical Assay of Nutrient Contents in Larvae
2.6. Enzyme Activity Assay
2.7. Transcriptome Analysis
2.8. Statistical Analysis
3. Results
3.1. Pathogenicity of M. hiemalis BO-1
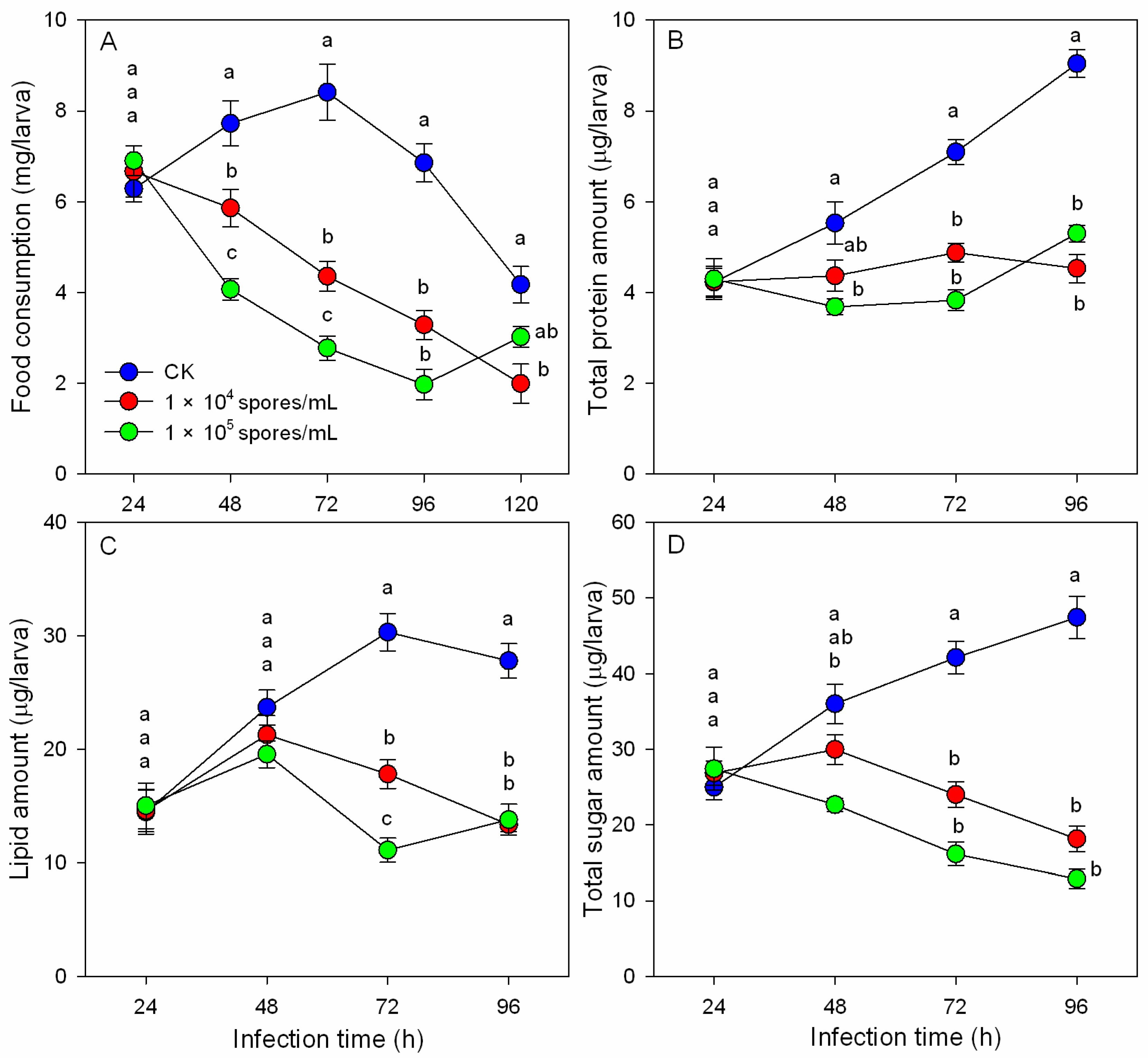
3.2. Effects of M. hiemalis Infection on Food Consumption and Nutrient Contents of B. odoriphaga
3.3. Effects of M. hiemalis Infection on Digestive Enzyme Activities
3.4. Effects of M. hiemalis Infection on Antioxidant Enzyme Activities
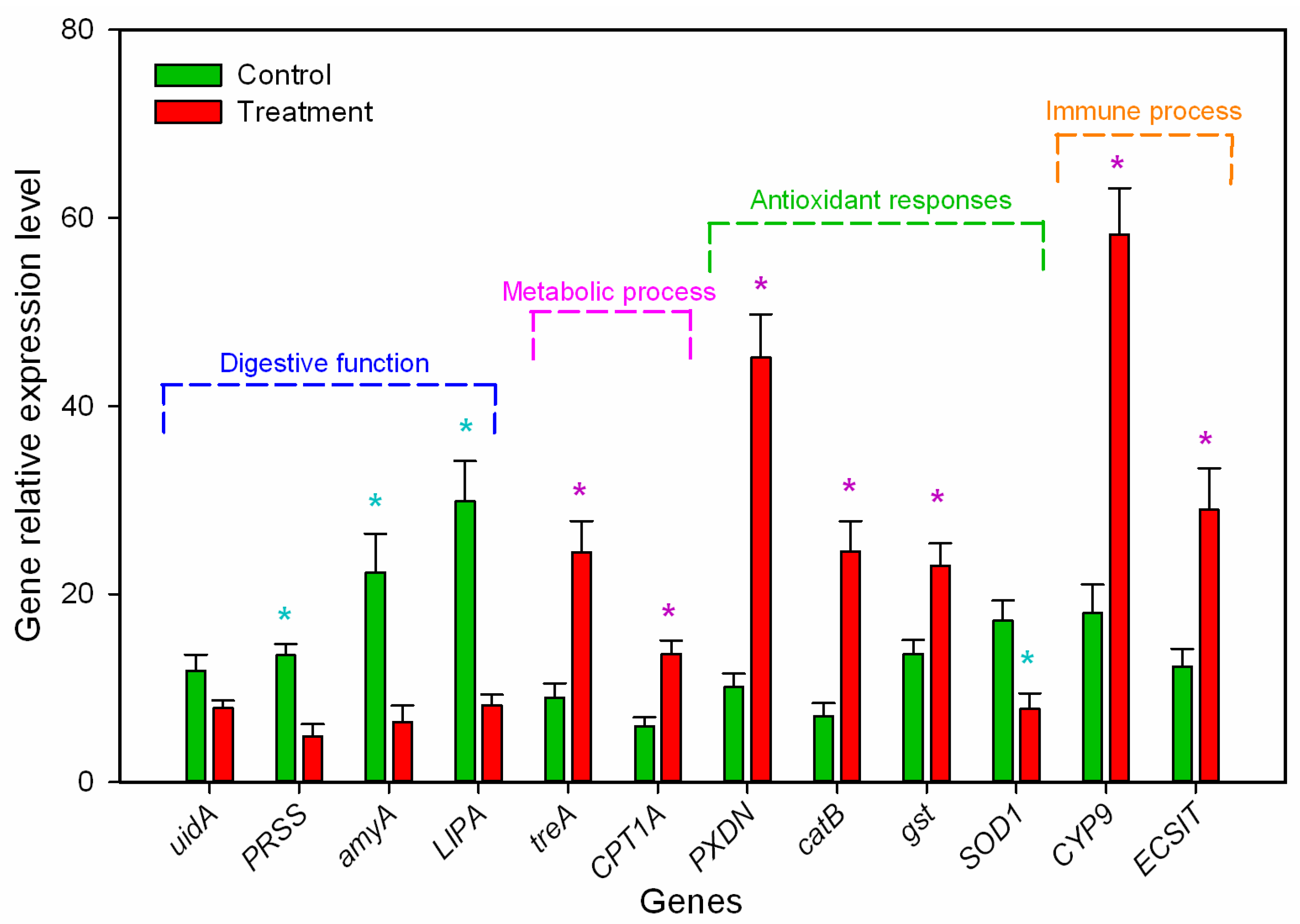
3.5. Transcriptome Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paulitz, T.C.; Bélanger, R.R. Biological control in greenhouse systems. Annu. Rev. Phytopathol. 2001, 39, 103–133. [Google Scholar] [PubMed]
- Chailleux, A.; Bearez, P.; Pizzol, J.; Amiens-Desneux, E.; Ramirez-Romero, R.; Desneux, N. Potential for combined use of parasitoids and generalist predators for biological control of the key invasive tomato pest Tuta absoluta. J. Pest Sci. 2013, 86, 533–541. [Google Scholar] [CrossRef]
- Lewis, M.W.; Robalino, I.V.; Keyhani, N.O. Uptake of the fluorescent probe FM4–64 by hyphae and haemolymph-derived in vivo hyphal bodies of the entomopathogenic fungus Beauveria bassiana. Microbiology 2009, 155, 3110–3120. [Google Scholar] [CrossRef] [PubMed]
- Pelizza, S.A.; Mariottini, Y.; Russo, L.M.; Vianna, M.F.; Scorsetti, A.C.; Lange, C.E. Beauveria bassiana (Ascomycota: Hypocreales) introduced as an endophyte in corn plants and its effects on consumption, reproductive capacity, and food preference of Dichroplus maculipennis (Orthoptera: Acrididae: Melanoplinae). J. Insect Sci. 2017, 17, 53. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, M.; Zhang, Y.; Du, G.; Cao, G.; Nong, X.; Liu, C.; Zhang, Z. Effects of different inhibitors on digestive enzyme and detoxification enzyme in midgut of locusta Migratoria manilensis during Metarhizium anisopliae infection. Chin. J. Biol. Control 2016, 32, 756–761. [Google Scholar] [CrossRef]
- Ekesi, S.; Maniania, N.; Ampong-Nyarko, K. Effect of temperature on germination, radial growth and virulence of Metarhizium anisopliae and Beauveria bassiana on Megalurothrips sjostedti. Biocontrol Sci. Technol. 1999, 9, 177–185. [Google Scholar] [CrossRef]
- Fargues, J.; Vidal, C.; Smits, N.; Rougier, M.; Boulard, T.; Mermier, M.; Nicot, P.; Reich, P.; Jeannequin, B.; Ridray, G. Climatic factors on entomopathogenic hyphomycetes infection of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) in Mediterranean glasshouse tomato. Biol. Control 2003, 28, 320–331. [Google Scholar] [CrossRef]
- Mwamburi, L.A.; Laing, M.D.; Miller, R.M. Effect of surfactants and temperature on germination and vegetative growth of Beauveria bassiana. Braz. J. Microbiol. 2015, 46, 67–74. [Google Scholar] [CrossRef]
- Fargues, J.; Goettel, M.; Smits, N.; Ouedraogo, A.; Rougier, M. Effect of temperature on vegetative growth of Beauveria bassiana isolates from different origins. Mycologia 1997, 89, 383–392. [Google Scholar] [CrossRef]
- Shimazu, M. Effects of temperature on growth of Beauveria bassiana F-263, a strain highly virulent to the Japanese pine sawyer, Monochamus alternatus, especially tolerance to high temperatures. Appl. Entomol. Zool. 2004, 39, 469–475. [Google Scholar] [CrossRef]
- Mannino, M.C.; Huarte-Bonnet, C.; Davyt-Colo, B.; Pedrini, N. Is the insect cuticle the only entry gate for fungal infection? Insights into alternative modes of action of entomopathogenic fungi. J. Fungi 2019, 5, 33. [Google Scholar] [CrossRef]
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H.; Abubakar, Y.S.; Qasim, M.; Ali, H. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microb. Pathog. 2021, 159, 105122. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, X.; Keyhani, N.O.; Tang, G.; Pei, Y.; Zhang, W.; Tang, S. Regulatory cascade and biological activity of Beauveria bassiana oosporein that limits bacterial growth after host death. Proc. Natl. Acad. Sci. USA 2017, 114, E1578–E1586. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.N.; Zhang, H.H.; Chi, D.F. Effects of a pathogenic Beauveria bassiana (Hypocreales: Cordycipitaceae) strain on detoxifying and protective enzyme activities in Xylotrechus rusticus (Coleoptera: Cerambycidae) larvae. Fla. Entomol. 2015, 98, 1148–1156. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan-Haq, M.; Al-Ayedh, H.; Ahmed, S.; Al-Jabr, A. Effect of Beauveria bassiana infection on the feeding performance and antioxidant defence of red palm weevil, Rhynchophorus ferrugineus. BioControl 2015, 60, 849–859. [Google Scholar] [CrossRef]
- Bitencourt, R.; Salcedo-Porras, N.; Umana-Diaz, C.; Costa Angelo, I.; Lowenberger, C. Antifungal immune responses in mosquitoes (Diptera: Culicidae): A review. J. Invertebr. Pathol. 2021, 178, 107505. [Google Scholar] [CrossRef]
- Karthi, S.; Vaideki, K.; Shivakumar, M.S.; Ponsankar, A.; Thanigaivel, A.; Chellappandian, M.; Senthil-Nathan, S. Effect of Aspergillus flavus on the mortality and activity of antioxidant enzymes of Spodoptera litura Fab. (Lepidoptera: Noctuidae) larvae. Pestic. Biochem. Phys. 2018, 149, 54–60. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Dunlap, C.A.; Muturi, E.J.; Barletta, A.B.; Rooney, A.P. Entomopathogenic fungal infection leads to temporospatial modulation of the mosquito immune system. PLoS Neglect. Trop. D 2018, 12, e0006433. [Google Scholar] [CrossRef]
- Rafaluk-Mohr, C.; Wagner, S.; Joop, G. Cryptic changes in immune response and fitness in Tribolium castaneum as a consequence of coevolution with Beauveria bassiana. J. Invertebr. Pathol. 2018, 152, 1–7. [Google Scholar] [CrossRef]
- Cabral, S.; de Paula, A.; Samuels, R.; da Fonseca, R.; Gomes, S.; Silva, J.R.; Mury, F. Aedes aegypti (Diptera: Culicidae) immune responses with different feeding regimes following infection by the entomopathogenic fungus Metarhizium anisopliae. Insects 2020, 11, 95. [Google Scholar] [CrossRef]
- Chen, C.; Wang, C.; Guo, J.; Xu, G.; Shi, X.; Song, D. Neonicotinoid insecticide resistance in Bradysia odoriphaga Yang et Zhang. Chin. J. Appl. Entomol. 2016, 53, 1250–1254. [Google Scholar] [CrossRef]
- Zhu, G.; Luo, Y.; Xue, M.; Zhao, H.; Sun, X.; Wang, X. Effects of feeding on different host plants and diets on Bradysia odoriphaga population parameters and tolerance to heat and insecticides. J. Econ. Entomol. 2017, 110, 2371–2380. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Ding, J.; Wang, Y.; Zhang, Z.; Liu, F.; Mu, W. Sublethal effects of chlorfenapyr on the life table parameters, nutritional physiology and enzymatic properties of Bradysia odoriphaga (Diptera: Sciaridae). Pestic. Biochem. Phys. 2018, 148, 93–102. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, L.; Tian, L.; Zhao, C.; Niu, H.; Hu, Y.; Shi, C.; Xie, W.; Zhang, Y. Function and characterization analysis of BodoOBP8 from Bradysia odoriphaga (Diptera: Sciaridae) in the recognition of plant volatiles and sex pheromones. Insects 2021, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.D.; Xue, M.; Li, Z.X.; Zhao, H.P.; Ji, G.X. Toxic effects of five insect growth regulators on chive gnat Bradysia odoriphaga. J. Plant Prot. 2015, 42, 271–277. [Google Scholar] [CrossRef]
- Wu, H.; Gong, Q.; Fan, K.; Sun, R.; Xu, Y.; Zhang, K. Synergistic effect of entomopathogenic nematodes and thiamethoxam in controlling Bradysia odoriphaga Yang and Zhang (Diptera: Sciaridae). Biol. Control 2017, 111, 53–60. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, T.; Zhuang, Q.; Zhang, A.; Zhang, S.; Yu, Y. Indoor bioassay of Beauveria bassiana against Bradysia odoriphaga and evaluation on control effect in field. Shandong Agric. Sci. 2014, 46, 117–120. [Google Scholar] [CrossRef]
- Zhu, G.; Ding, W.; Xue, M.; Zhao, Y.; Li, M.; Li, Z. Identification and pathogenicity of a new entomopathogenic fungus, Mucor hiemalis (Mucorales: Mucorales), on the root maggot, Bradysia odoriphaga (Diptera: Sciaridae). J. Insect Sci. 2022, 22, 2. [Google Scholar] [CrossRef]
- Bibbs, C.S.; Vitoreli, A.M.; Benny, G.; Harmon, C.L.; Baldwin, R.W. Susceptibility of Latrodectus geometricus (Araneae: Theridiidae) to a Mucor strain discovered in north central Florida, USA. Fla. Entomol. 2013, 96, 1052–1061. [Google Scholar] [CrossRef]
- Ma, Y.H.; Lai, Y.P. Identification and pathogenicity evaluation of pathogenic fungi from Evergestis extimalis. Acta Agric. Univ. Jiangxiensis 2022, 44, 1112–1121. [Google Scholar] [CrossRef]
- Chen, J.X.; Wei, J.L.; Qian, Y.H.; Ma, H.C.; Wu, J.R. Identification of Nemouroidea (Plecoptera) pathogen in Shangri-La, Yunnan. J. Environ. Entomol. 2021, 43, 122–129. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, Y.; Wang, Q.; Liu, F.; Mu, W. Toxic characters and toxicity of hexaflumuron against Bradysia odoriphaga Yang et Zhang (Diptera: Sciaridae) at different developmental stages. J. Plant Prot. 2016, 43, 670–676. [Google Scholar] [CrossRef]
- Ma, J.; Chen, S.; Moens, M.; Han, R.; De Clercq, P. Efficacy of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) against the chive gnat, Bradysia odoriphaga. J. Pest Sci. 2013, 86, 551–561. [Google Scholar] [CrossRef]
- Ondiaka, S.N.; Masinde, E.W.; Koenraadt, C.J.; Takken, W.; Mukabana, W.R. Effects of fungal infection on feeding and survival of Anopheles gambiae (Diptera: Culicidae) on plant sugars. Parasites Vectors 2015, 8, 35. [Google Scholar] [CrossRef]
- Shaurub, E.S.H.; Reyad, N.F.; Mohamed, A.A. Pathogen-mediated modulation of host metabolism and trophic interactions in Spodoptera littoralis larvae. Entomol. Exp. Appl. 2020, 168, 956–966. [Google Scholar] [CrossRef]
- Vinayaga Moorthi, P.; Balasubramanian, C.; Selvarani, S.; Radha, A. Efficacy of sub lethal concentration of entomopathogenic fungi on the feeding and reproduction of Spodoptera litura. SpringerPlus 2015, 4, 681. [Google Scholar] [CrossRef]
- Zhang, L.B.; Feng, M.G. Antioxidant enzymes and their contributions to biological control potential of fungal insect pathogens. Appl. Microbiol. Biotechnol. 2018, 102, 4995–5004. [Google Scholar] [CrossRef]
- Li, L.J.; Liu, X.M.; Guo, Y.P.; Ma, E.B. Activity of the enzymes of the antioxidative system in cadmium-treated Oxya chinensis (Orthoptera Acridoidae). Environ. Toxicol. Pharm. 2005, 20, 412–416. [Google Scholar] [CrossRef]
- Noskov, Y.A.; Polenogova, O.V.; Yaroslavtseva, O.N.; Belevich, O.E.; Yurchenko, Y.A.; Chertkova, E.A.; Glupov, V.V. Combined effect of the entomopathogenic fungus Metarhizium robertsii and avermectins on the survival and immune response of Aedes aegypti larvae. PeerJ 2019, 7, e7931. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, V.; Kryukova, N.A.; Tomilova, O.G.; Vorontsova, Y.; Chertkova, E.; Pervushin, A.L.; Yaroslavtseva, O.N. Comparative analysis of the immune response of the wax moth Galleria mellonella after infection with the fungi Cordyceps militaris and Metarhizium robertsii. Microb. Pathog. 2020, 141, 103995. [Google Scholar] [CrossRef]
- Shamakhi, L.; Zibaee, A.; Karimi-Malati, A.; Hoda, H. Simultaneous effects of thermal stress and fungal infection on lipid peroxidation and antioxidant system of rice-striped stem borer, Chilo suppressalis Walker (Lepidoptera: Crambidae). Biol. Rhythm Res. 2020, 51, 225–237. [Google Scholar] [CrossRef]
- Chaurasia, A.; Lone, Y.; Wani, O.; Gupta, U.S. Effect of certain entomopathogenic fungi on oxidative stress and mortality of Periplaneta americana. Pestic. Biochem. Phys. 2016, 127, 28–37. [Google Scholar] [CrossRef]
- Gillespie, J.P.; Bailey, A.M.; Cobb, B.; Vilcinskas, A. Fungi as elicitors of insect immune responses. Arch. Insect Biochem. 2000, 44, 49–68. [Google Scholar] [CrossRef]
- Cui, K.; Zhao, Y.; He, L.; Ding, J.; Li, B.; Mu, W.; Liu, F. Comparison of transcriptome profiles of the fungus Botrytis cinerea and insect pest Bradysia odoriphaga in response to benzothiazole. Front. Microbiol. 2020, 11, 1043. [Google Scholar] [CrossRef]
- Chen, H.; Lin, L.; Xie, M.; Zhang, G.; Su, W. De novo sequencing and characterization of the Bradysia odoriphaga (Diptera: Sciaridae) larval transcriptome. Comp. Biochem. Phys. D 2015, 16, 20–27. [Google Scholar] [CrossRef]
- Cheng, J.; Su, Q.; Xia, J.; Yang, Z.; Zhang, Y. Comparative transcriptome analysis of differentially expressed genes in Bradysia odoriphaga Yang et Zhang (Diptera: Sciaridae) at different acute stress temperatures. Genomics 2020, 112, 3739–3750. [Google Scholar] [CrossRef]
- Hou, C.; Qin, G.; Liu, T.; Geng, T.; Gao, K.; Pan, Z.; Guo, X. Transcriptome analysis of silkworm, Bombyx mori, during early response to Beauveria bassiana challenges. PLoS ONE 2014, 9, e91189. [Google Scholar] [CrossRef]
- Black, J.L.; Clark, M.K.; Sword, G.A. Physiological and transcriptional immune responses of a non-model arthropod to infection with different entomopathogenic groups. PLoS ONE 2022, 17, e0263620. [Google Scholar] [CrossRef]
- Qasim, M.; Xiao, H.; He, K.; Omar, M.A.A.; Hussain, D.; Noman, A.; Li, F. Host-pathogen interaction between Asian citrus psyllid and entomopathogenic fungus (Cordyceps fumosorosea) is regulated by modulations in gene expression, enzymatic activity and HLB-bacterial population of the host. Comp. Biochem. Phys. C 2021, 248, 109112. [Google Scholar] [CrossRef]
- Shen, D.; Tang, Z.; Wang, C.; Wang, J.; Dong, Y.; Chen, Y.; Xia, A. Infection mechanisms and putative effector repertoire of the mosquito pathogenic oomycete Pythium guiyangense uncovered by genomic analysis. PloS Genet. 2019, 15, e1008116. [Google Scholar] [CrossRef] [PubMed]
- Daquila, B.V.; Scudeler, E.L.; Dossi, F.C.; Moreira, D.R.; Pamphile, J.A.; Conte, H. Action of Bacillus thuringiensis (Bacillales: Bacillaceae) in the midgut of the sugarcane borer Diatraea saccharalis (Fabricius, 1794) (Lepidoptera: Crambidae). Ecotox. Environ. Safe 2019, 184, 109642. [Google Scholar] [CrossRef]
- Bawin, T.; Seye, F.; Boukraa, S.; Zimmer, J.Y.; Raharimalala, F.N.; Ndiaye, M.; Francis, F. Histopathological effects of Aspergillus clavatus (Ascomycota: Trichocomaceae) on larvae of the southern house mosquito, Culex quinquefasciatus (Diptera: Culicidae). Fungal Biol.-UK 2016, 120, 489–499. [Google Scholar] [CrossRef]
- Xu, L.; Deng, J.; Zhou, F.; Cheng, C.; Lu, M. Gut microbiota in an invasive bark beetle infected by a pathogenic fungus accelerates beetle mortality. J. Pest Sci. 2019, 92, 343–351. [Google Scholar] [CrossRef]
- Wei, G.; Lai, Y.L.; Wang, G.D.; Chen, H.; Li, F.; Wang, S.B. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl. Acad. Sci. USA 2017, 114, 5994–5999. [Google Scholar] [CrossRef]
- Bai, J.; Xu, Z.; Li, L.; Ma, W.; Ma, L. Temporospatial modulation of Lymantria dispar immune system against an entomopathogenic fungal infection. Pest Manag. Sci. 2020, 76, 3982–3989. [Google Scholar] [CrossRef]



| Infection Time (d) | Regression Equation (y = ax + b) | LC50 Median Lethal Concentration (spores/mL) | 95% Confidence Interval (spores/mL) | R2 |
| 3 | y = −10.802x + 8.663 | 2.180 × 106 | 1.099~4.736 × 106 | 0.892 |
| 4 | y = −9.888x + 7.143 | 1.891 × 105 | 1.001~3.836 × 105 | 0.983 |
| 5 | y = −11.187x + 7.804 | 0.965 × 105 | 0.551~1.729 × 105 | 0.940 |
| 6 | y = −7.384x + 4.798 | 2.914 × 104 | 1.438~6.742 × 104 | 0.999 |
| Spore concentration (spores/mL) | Regression equation (y = ax + b) | LT50 Median lethal time (d) | 95% Confidence interval (d) | R2 |
| 1.00 × 104 | y = −7.149x + 4.661 | 4.787 | 4.476~5.146 | 0.995 |
| 1.00 × 105 | y = −7.004x + 8.663 | 3.791 | 3.518~4.085 | 0.988 |
| 1.00 × 106 | y = −7.672x + 3.845 | 2.986 | 2.753~3.207 | 0.887 |
| 1.00 × 107 | y = −8.143x + 3.605 | 2.618 | 2.407~2.816 | 0.938 |
| 1.00 × 108 | y = −7.796x + 3.209 | 2.432 | 2.223~2.627 | 0.898 |
| Samples | Total Reads | Clean Reads | Q30(%) | GC Content (%) | Mapped Reads | Mapped Ratio (%) |
|---|---|---|---|---|---|---|
| Control-1 | 7,485,207,667 | 50,812,192 | 94.22 | 41.92 | 45,067,128 | 88.69 |
| Control-2 | 7,558,357,480 | 51,677,542 | 94.33 | 42.02 | 46,192,515 | 89.39 |
| Treatment-1 | 7,141,260,179 | 49,670,662 | 94.19 | 42.21 | 43,655,545 | 87.89 |
| Treatment-2 | 6,615,063,328 | 44,925,166 | 94.27 | 42.01 | 39,841,706 | 88.68 |
| Gene ID | Gene Name | GO Biological Process | GO Function | KEGG Pathways | log2 Ratio (T/C) | p-Value |
|---|---|---|---|---|---|---|
| HA402_000028 | uidA | GO0005975: Carbohydrate metabolic process | Beta-glucosidase activity | ko04973: Carbohydrate digestion and absorption | −2.05 | 3.96 × 10-4 |
| HA402_001933 | PRSS | GO0007586: Digestion | Serine-type endopeptidase activity | ko04974: Protein digestion and absorption | −1.85 | 8.96 × 10-6 |
| HA402_001429 | amyA | GO0005975: Carbohydrate metabolic process | Alpha-amylase activity | ko04973: Carbohydrate digestion and absorption | −2.40 | 5.38 × 10-6 |
| HA402_001695 | LIPA | GO0016042: Lipid digestion process | Lipase activity | ko00100: Steroid biosynthesis | −3.08 | 1.53 × 10-6 |
| HA402_003401 | treA | GO0005991: Trehalose metabolic process | Trehalase activity | ko01100: Metabolic pathways | 1.96 | 2.97 × 10-13 |
| HA402_014097 | CPT1A | GO0006635: Fatty acid beta-oxidation | Palmitoleoyltransferase activity | ko01212: Fatty acid metabolism | 2.54 | 2.97 × 10-13 |
| HA402_012831 | PRDX | GO0006979: Response to oxidative stress | Peroxidase activity | ko04146: Peroxisome | 1.56 | 4.69 × 10-4 |
| HA402_001366 | catB | GO0042744: Hydrogen peroxide catabolic process | Catalase activity | ko04146: Peroxisome | 2.31 | 4.24 × 10-3 |
| HA402_005674 | gst | GO0006979: Response to oxidative stress | Glutathione transferase activity | ko00480: Glutathione metabolism | −2.32 | 2.18 × 10-7 |
| HA402_011746 | SOD1 | GO0019430: Removal of superoxide radicals | Superoxide dismutase activity | ko04146: Peroxisome | −1.59 | 1.11 × 10-5 |
| HA402_008470 | CYP9 | GO0017144: Drug metabolic process | cytochrome P450 activity | ko00982: Drug metabolism—cytochrome P450 | 2.67 | 4.25 × 10-6 |
| HA402_001791 | ECSIT | GO0045087: Innate immune response | Hydrolase activity | ko04624: Toll and Imd signaling pathway | 1.27 | 5.32 × 10-4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.; Ding, W.; Zhao, H.; Xue, M.; Chu, P.; Jiang, L. Effects of the Entomopathogenic Fungus Mucor hiemalis BO-1 on the Physical Functions and Transcriptional Signatures of Bradysia odoriphaga Larvae. Insects 2023, 14, 162. https://doi.org/10.3390/insects14020162
Zhu G, Ding W, Zhao H, Xue M, Chu P, Jiang L. Effects of the Entomopathogenic Fungus Mucor hiemalis BO-1 on the Physical Functions and Transcriptional Signatures of Bradysia odoriphaga Larvae. Insects. 2023; 14(2):162. https://doi.org/10.3390/insects14020162
Chicago/Turabian StyleZhu, Guodong, Wenjuan Ding, Haipeng Zhao, Ming Xue, Pengfei Chu, and Liwei Jiang. 2023. "Effects of the Entomopathogenic Fungus Mucor hiemalis BO-1 on the Physical Functions and Transcriptional Signatures of Bradysia odoriphaga Larvae" Insects 14, no. 2: 162. https://doi.org/10.3390/insects14020162
APA StyleZhu, G., Ding, W., Zhao, H., Xue, M., Chu, P., & Jiang, L. (2023). Effects of the Entomopathogenic Fungus Mucor hiemalis BO-1 on the Physical Functions and Transcriptional Signatures of Bradysia odoriphaga Larvae. Insects, 14(2), 162. https://doi.org/10.3390/insects14020162






