Functional Niche Partitioning Occurs over Body Size but Not Nutrient Reserves nor Melanism in a Polar Carabid Beetle along an Altitudinal Gradient
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
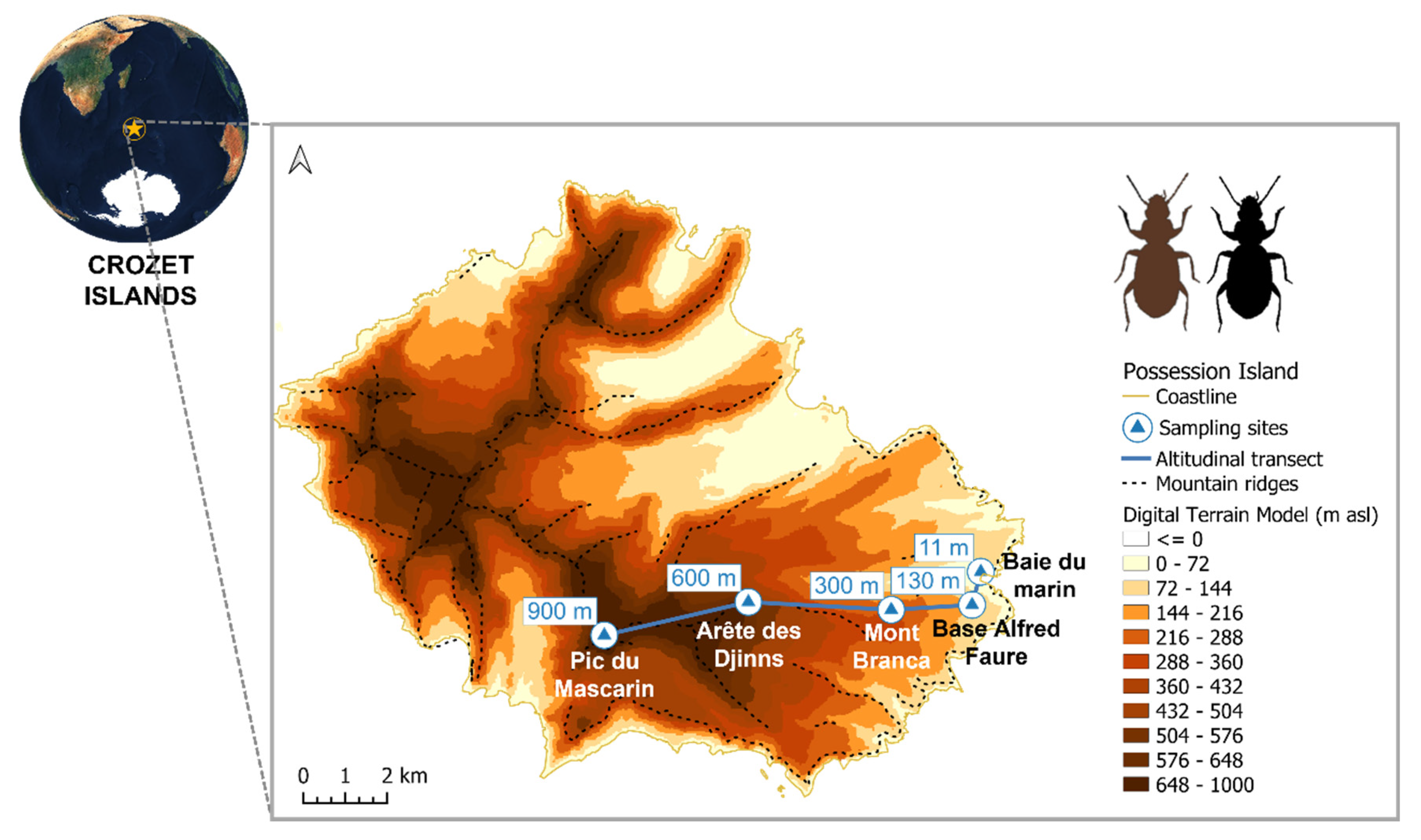
2.1. Study Area
2.2. Field Sampling
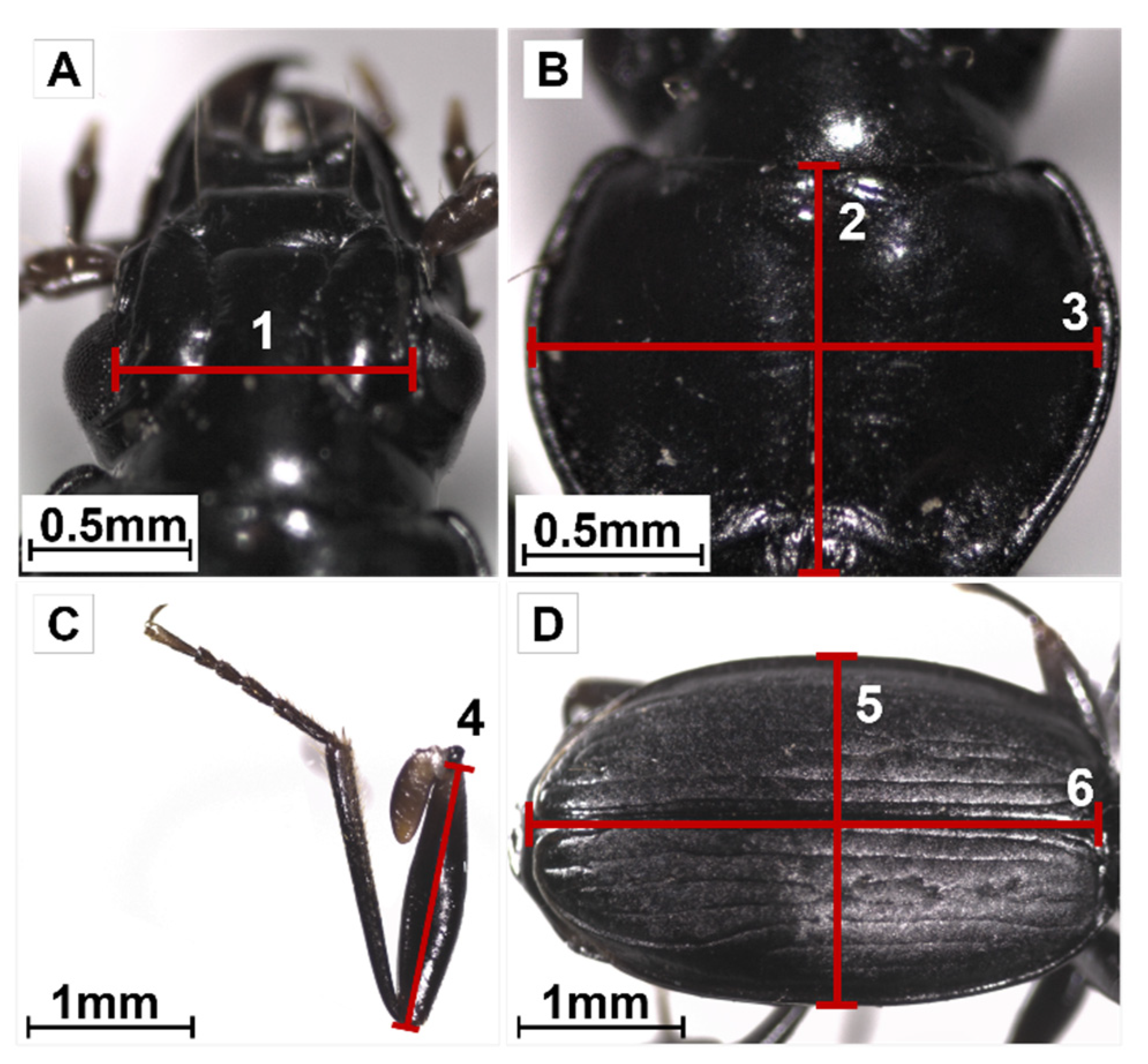
2.3. Functional Trait Measurements
2.4. Statistical Analysis
2.4.1. Univariate Approach: Influence of Altitude, Sex, and Morphotype on Functional Traits
2.4.2. Niche Space of Amblystogenium pacificum: Functional Hypervolumes
3. Results
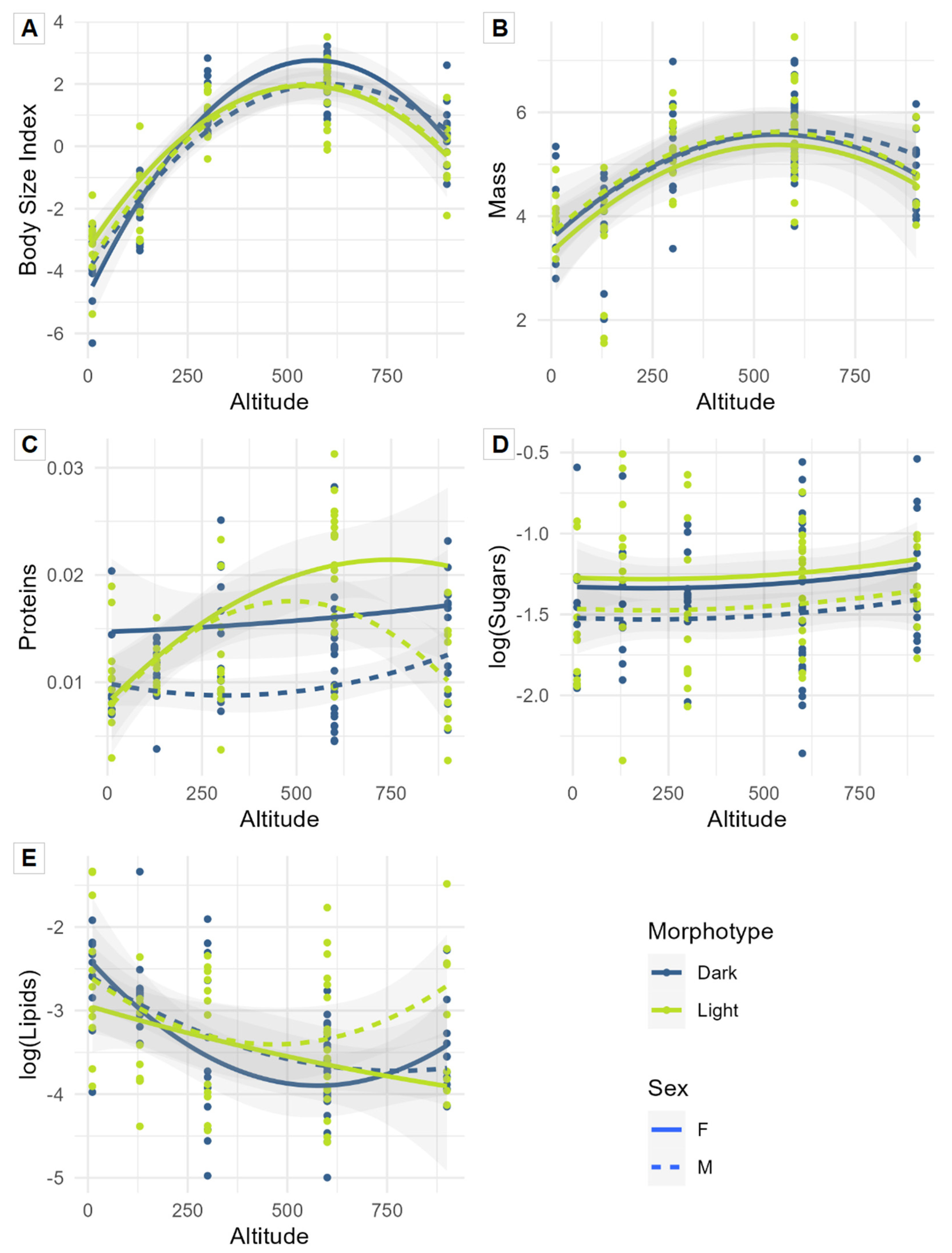
3.1. Univariate Approach: Influence of Altitude, Sex, and Morphotype on Functional Traits
3.2. Niche Space at the Species Scale
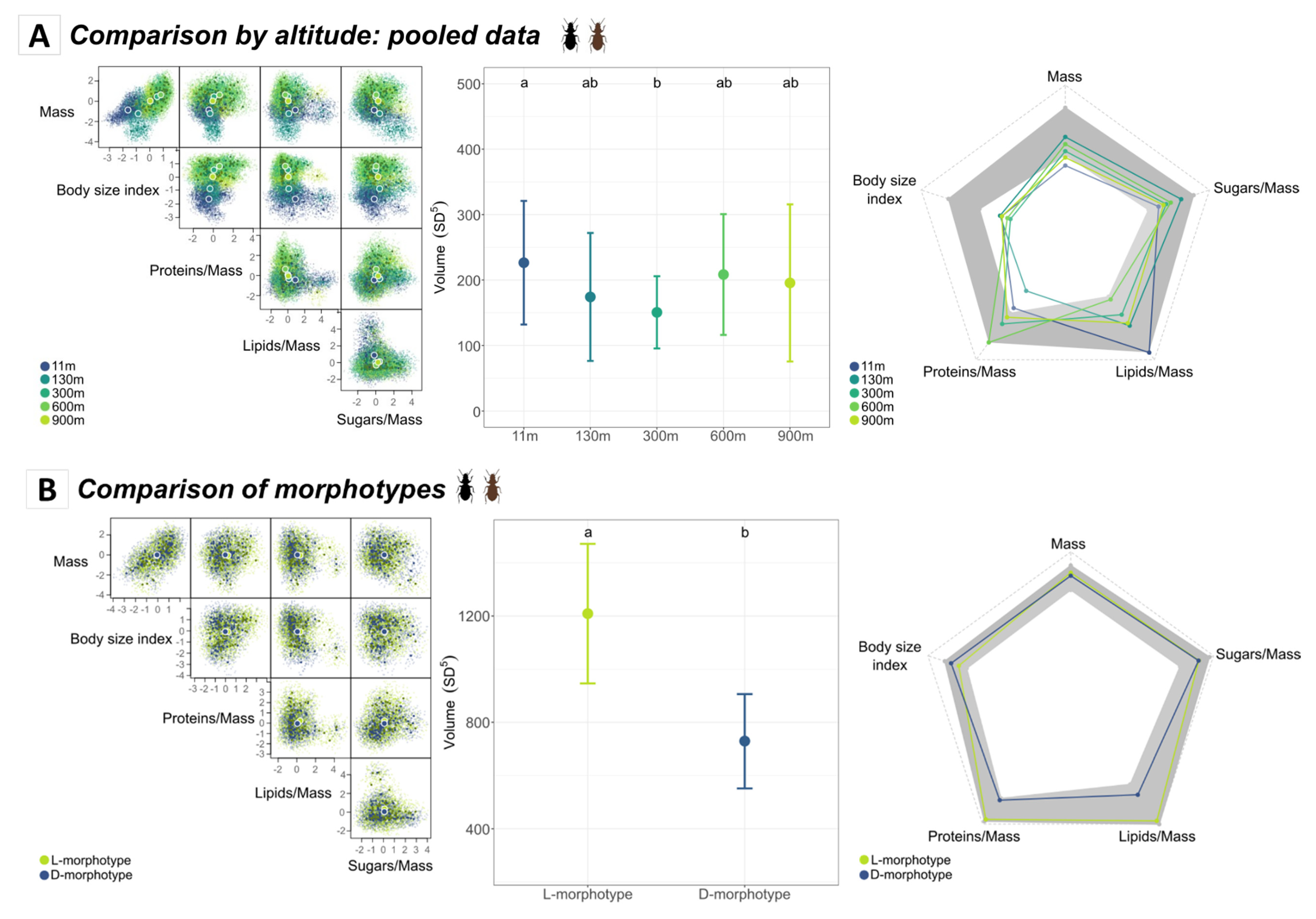
3.2.1. Functional Diversity of Amblystogenium pacificum along the Altitudinal Gradient
3.2.2. Morphotype and Sex Functional Trait Space
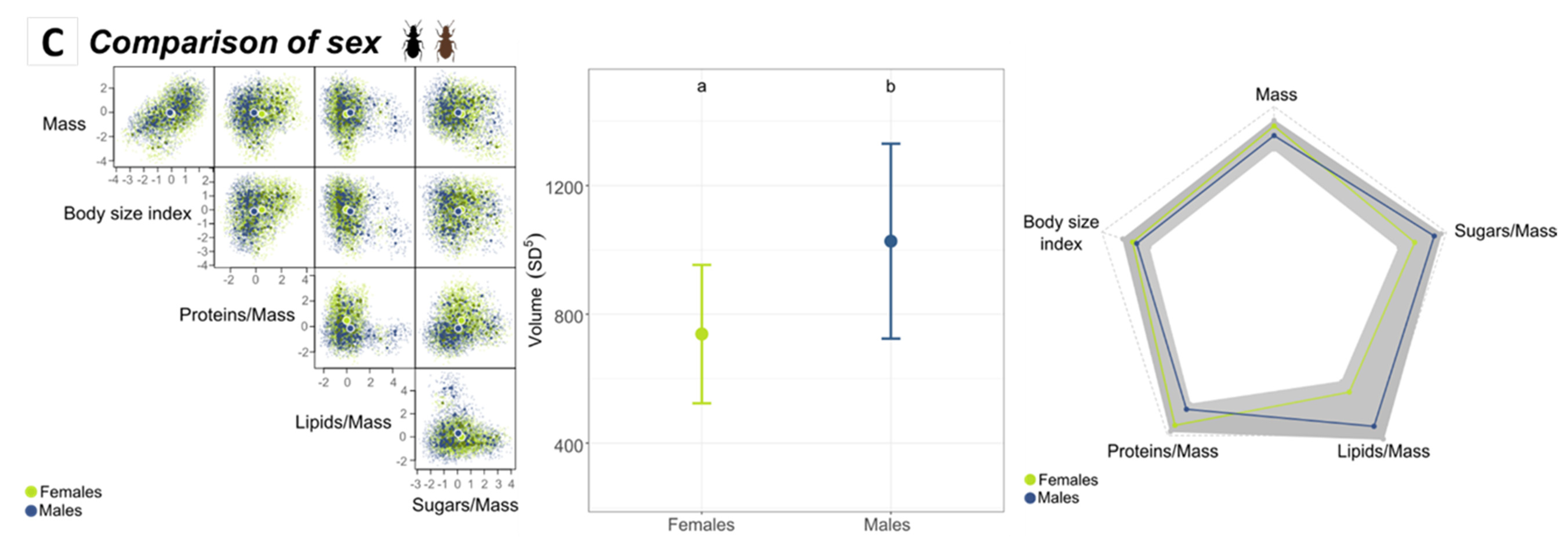
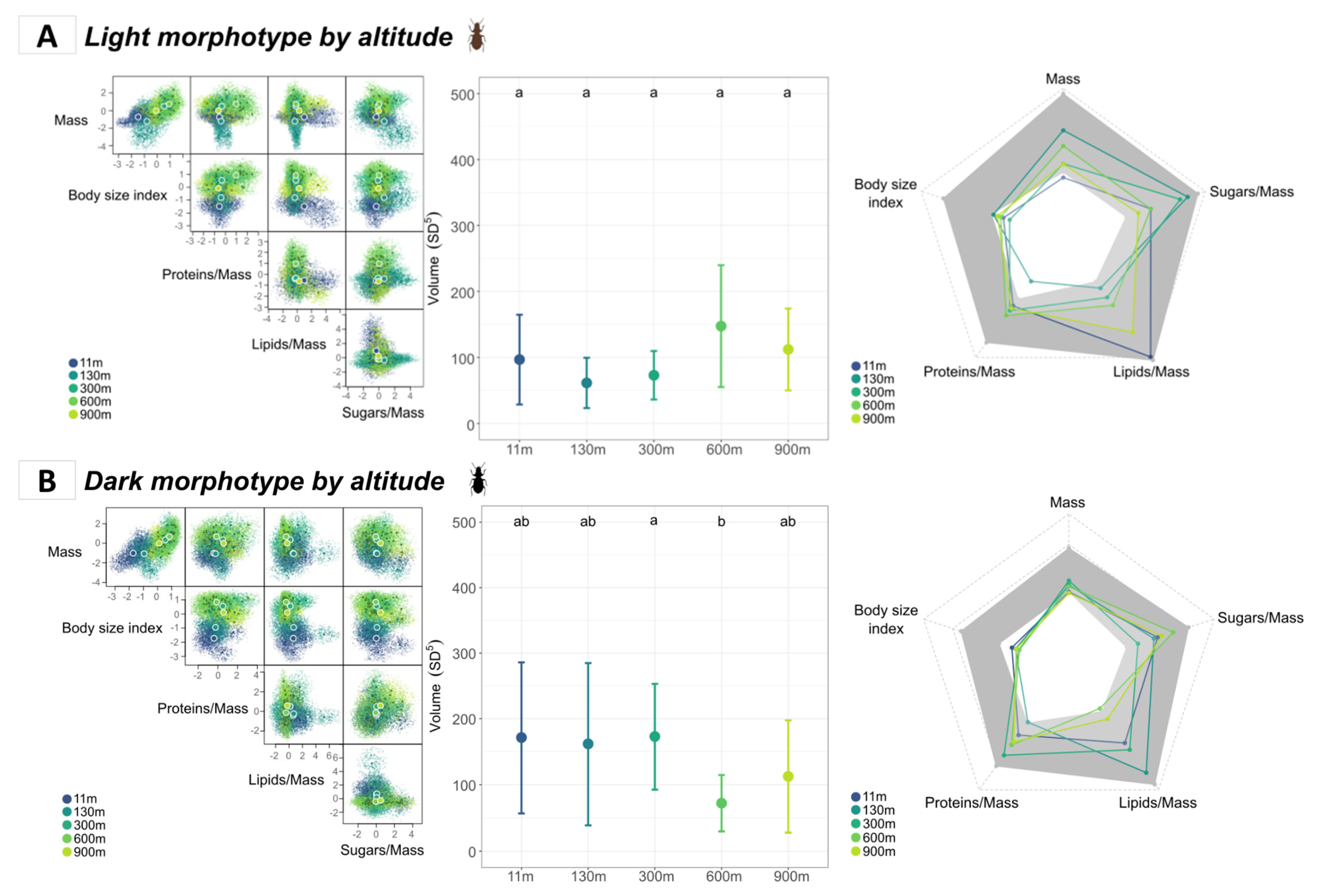
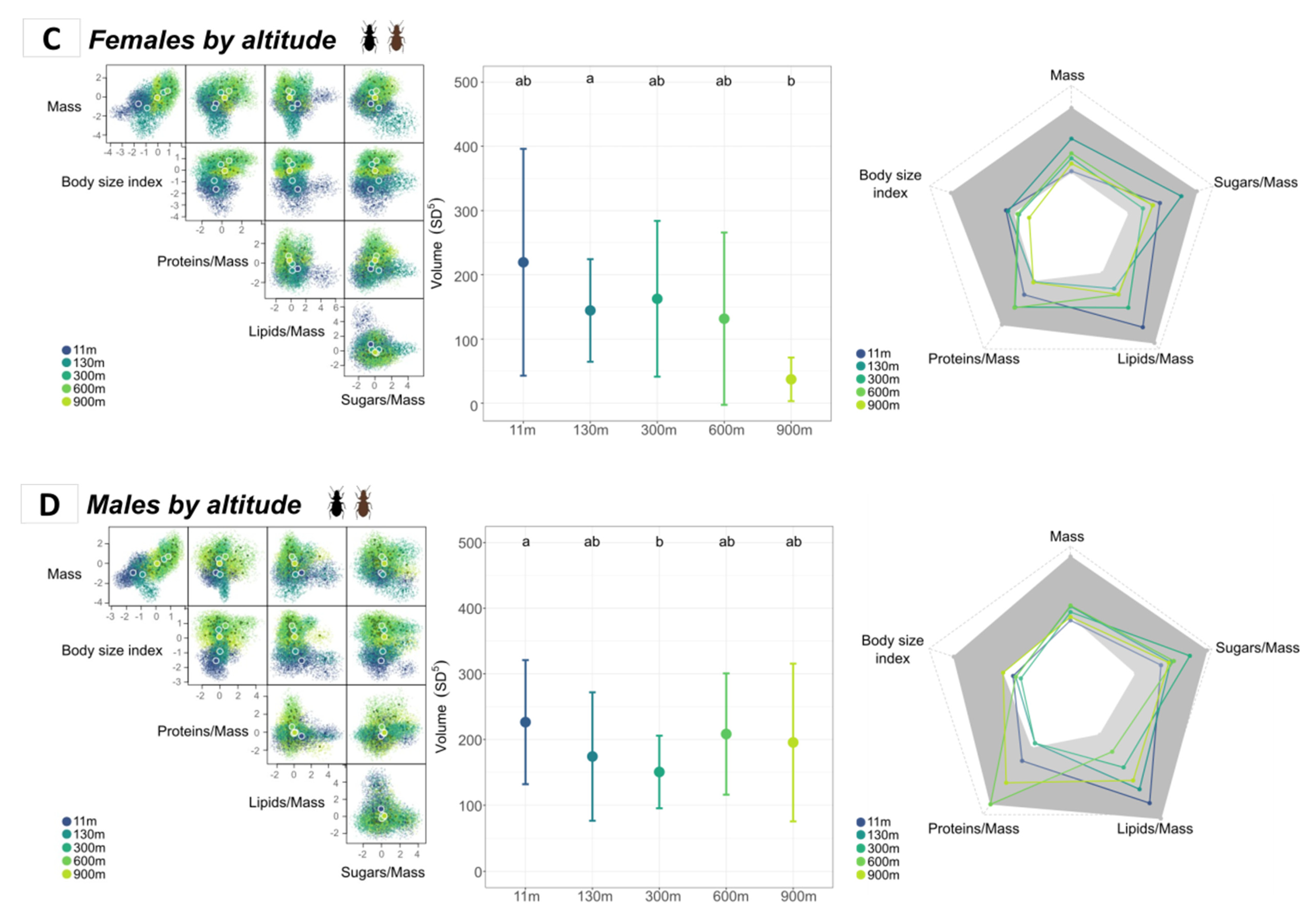
3.3. Intraspecific Niche Comparison
3.3.1. Comparison of Morphotypes According to Altitude
3.3.2. Comparison of Sexes According to Altitude
4. Discussion
4.1. Functional Traits of Amblystogenium pacificum: Influence of Altitude, Sex, and Morphotype
4.2. Functional Diversity and Niche Partitioning of Amblystogenium pacificum
4.2.1. Intraspecific Niche Variations and Elevation Range: Implications for Resilience
4.2.2. Intraspecific Trait Variation: True Polymorphism or Phenotypic Expression?
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huey, R.B.; Stevenson, R.D. Integrating Thermal Physiology and Ecology of Ectotherms: A Discussion of Approaches. Am. Zool. 1979, 19, 357–366. [Google Scholar] [CrossRef]
- Kearney, M.; Shine, R.; Porter, W.P. The Potential for Behavioral Thermoregulation to Buffer “Cold-Blooded” Animals against Climate Warming. Proc. Natl. Acad. Sci. USA 2009, 106, 3835–3840. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.B.; Tewksbury, J.J.; Deutsch, C.A. Can Terrestrial Ectotherms Escape the Heat of Climate Change by Moving? Proc. R. Soc. B Biol. Sci. 2013, 280, 20131149. [Google Scholar] [CrossRef] [PubMed]
- Forsman, A. Rethinking Phenotypic Plasticity and Its Consequences for Individuals, Populations and Species. Heredity 2015, 115, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Leibler, S. Benefits of Phenotypic Plasticity for Population Growth in Varying Environments. Proc. Natl. Acad. Sci. USA 2018, 115, 12745–12750. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, D.W.; Wund, M.A.; Snell-Rood, E.C.; Cruickshank, T.; Schlichting, C.D.; Moczek, A.P. Phenotypic Plasticity’s Impacts on Diversification and Speciation. Trends Ecol. Evol. 2010, 25, 459–467. [Google Scholar] [CrossRef]
- Laparie, M.; Bical, R.; Larvor, V.; Vernon, P.; Frenot, Y.; Renault, D. Habitat Phenotyping of Two Sub-Antarctic Flies by Metabolic Fingerprinting: Evidence for a Species Outside Its Home? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 162, 406–412. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Vive La Différence: Plant Functional Diversity Matters to Ecosystem Processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting Changes in Community Composition and Ecosystem Functioning from Plant Traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional Diversity: Back to Basics and Looking Forward. Ecol. Letters 2006, 9, 741–758. [Google Scholar] [CrossRef]
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A Functional Approach Reveals Community Responses to Disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Mammola, S.; Cardoso, P. Functional Diversity Metrics Using Kernel Density n -dimensional Hypervolumes. Methods Ecol. Evol. 2020, 11, 986–995. [Google Scholar] [CrossRef]
- Fontaine, C.; Dajoz, I.; Meriguet, J.; Loreau, M. Functional Diversity of Plant–Pollinator Interaction Webs Enhances the Persistence of Plant Communities. PLOS Biol. 2005, 4, e1. [Google Scholar] [CrossRef] [PubMed]
- Chillo, V.; Anand, M.; Ojeda, R.A. Assessing the Use of Functional Diversity as a Measure of Ecological Resilience in Arid Rangelands. Ecosystems 2011, 14, 1168–1177. [Google Scholar] [CrossRef]
- Greenop, A.; Woodcock, B.A.; Outhwaite, C.L.; Carvell, C.; Pywell, R.F.; Mancini, F.; Edwards, F.K.; Johnson, A.C.; Isaac, N.J.B. Patterns of Invertebrate Functional Diversity Highlight the Vulnerability of Ecosystem Services over a 45-Year Period. Curr. Biol. 2021, 31, 4627–4634. [Google Scholar] [CrossRef]
- Harris, R.; McQuillan, P.; Hughes, L. Patterns in Body Size and Melanism along a Latitudinal Cline in the Wingless Grasshopper, Phaulacridium vittatum. J. Biogeogr. 2012, 39, 1450–1461. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and Organism Size: A Biological Law for Ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar]
- Chown, S.L.; Gaston, K.J. Body Size Variation in Insects: A Macroecological Perspective. Biol. Rev. 2010, 85, 139–169. [Google Scholar] [CrossRef]
- Bergmann, C. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Größe; Göttingen Studien: Göttingen, Germany, 1847; Volume 3. [Google Scholar]
- Blackburn, T.M.; Gaston, K.J.; Loder, N. Geographic Gradients in Body Size: A Clarification of Bergmann’s Rule. Divers. Distrib. 1999, 5, 165–174. [Google Scholar] [CrossRef]
- Horne, C.R.; Hirst, A.G.; Atkinson, D. Insect Temperature–Body Size Trends Common to Laboratory, Latitudinal and Seasonal Gradients Are Not Found across Altitudes. Funct. Ecol. 2018, 32, 948–957. [Google Scholar] [CrossRef]
- Salomão, R.P.; Arriaga-Jiménez, A.; Kohlmann, B. The Relationship between Altitudinal Gradients, Diversity, and Body Size in a Dung Beetle (Coleoptera: Scarabaeinae: Onthophagus Latreille, 1802) Model System. Can. J. Zool. 2021, 99, 33–43. [Google Scholar] [CrossRef]
- Dillon, M.E.; Frazier, M.R.; Dudley, R. Into Thin Air: Physiology and Evolution of Alpine Insects. Integr. Comp. Biol. 2006, 46, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Shelomi, M.; Day, A.E.T.; Angilletta, E.M. Where Are We Now? Bergmann’s Rule Sensu Lato in Insects. Am. Nat. 2012, 180, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Mani, M.S. Ecology and Biogeography of High Altitude Insects; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 978-940-171-339-9. [Google Scholar]
- Sømme, L. Adaptations of Terrestrial Arthropods to the Alpine Environment. Biol. Rev. 1989, 64, 367–407. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Terrestrial Insects along Elevation Gradients: Species and Community Responses to Altitude. Biol. Rev. 2005, 80, 489–513. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, D.J. Allometry for Sexual Size Dimorphism: Pattern and Process in the Coevolution of Body Size in Males and Females. Annu. Rev. Ecol. Syst. 1997, 28, 659–687. [Google Scholar] [CrossRef]
- Teder, T.; Tammaru, T. Sexual Size Dimorphism within Species Increases with Body Size in Insects. Oikos 2005, 108, 321–334. [Google Scholar] [CrossRef]
- Rensch, B. Die Abhängigkeit Der Relativen Sexualdifferenz von Der Körpergrösse. Bonn. Zool. Beiträge 1950, 1, 58–69. [Google Scholar]
- Eweleit, L.; Reinhold, K. Body Size and Elevation: Do Bergmann’s and Rensch’s Rule Apply in the Polytypic Bushcricket Poecilimon veluchianus? Ecol. Entomol. 2014, 39, 133–136. [Google Scholar] [CrossRef]
- Cohen, N.; Volov, M.; Bodner, L.; Bouchebti, S.; Levin, E. Body Size, Metabolic Rate, and Diapause in the Oriental Hornet (Vespa orientalis), in Two Extreme Climatic Regions. Ecol. Entomol. 2022, 47, 1022–1031. [Google Scholar] [CrossRef]
- Lease, H.M.; Wolf, B.O. Lipid Content of Terrestrial Arthropods in Relation to Body Size, Phylogeny, Ontogeny and Sex. Physiol. Entomol. 2011, 36, 29–38. [Google Scholar] [CrossRef]
- Greenslade, P.J.M. Habitat and Altitude Distribution of Carabidae (Coleoptera) in Argyll, Scotland. Trans. R. Entomol. Soc. Lond. 1968, 120, 39–54. [Google Scholar] [CrossRef]
- Trullas, S.C.; Van Wyk, J.H.; Spotila, J.R. Thermal Melanism in Ectotherms. J. Therm. Biol. 2007, 32, 235–245. [Google Scholar] [CrossRef]
- Sibilia, C.D.; Brosko, K.A.; Hickling, C.J.; Thompson, L.M.; Grayson, K.L.; Olson, J.R. Thermal Physiology and Developmental Plasticity of Pigmentation in the Harlequin Bug (Hemiptera: Pentatomidae). J. Insect Sci. 2018, 18, 4. [Google Scholar] [CrossRef]
- Delhey, K. Revealing the Colourful Side of Birds: Spatial Distribution of Conspicuous Plumage Colours on the Body of Australian Birds. J. Avian Biol. 2019, 51, 1–7. [Google Scholar] [CrossRef]
- Jong, P.; Gussekloo, S.; Brakefield, P. Differences in Thermal Balance, Body Temperature and Activity between Non-Melanic and Melanic Two-Spot Ladybird Beetles (Adalia bipunctata) under Controlled Conditions. J. Exp. Biol. 1996, 199, 2655–2666. [Google Scholar] [CrossRef]
- Hullé, M.; Vernon, P. Terrestrial Macro-Arthropods of the Sub-Antarctic Islands of Possession (Crozet Archipelago) and Kerguelen: Inventory of Native and Non-Native Species. Zoos 2021, 43, 549–561. [Google Scholar] [CrossRef]
- Davies, L.; Bouvet, S.; Vernon, P. All-Year Reproduction and Possible Thermal Melanism in Amblystogenium pacificum (Coleoptera: Carabidae) on the Sub-Antarctic Ile de La Possession (Iles Crozet). Polar Biol. 2007, 30, 253–260. [Google Scholar] [CrossRef]
- Davies, L. Two Amblystogenium Species (Col. Carabidae) Co-Existing on the Subantarctic Possession Island, Crozet Islands. Insect Syst. Evol. 1972, 3, 275–286. [Google Scholar] [CrossRef]
- Davies, L. Ecology of Two Amblystogenium Species (Carabidae) on Ile de La Possession, Iles Crozet. Comp. Nat. Fr. Rech. Antarct. 1982, 51, 167–173. [Google Scholar]
- Davies, L. Long Adult Life, Low Reproduction and Competition in Two Sub-Antarctic Carabid Beetles. Ecol. Entomol. 1987, 12, 149–162. [Google Scholar] [CrossRef]
- Blonder, B.; Lamanna, C.; Violle, C.; Enquist, B.J. The n-Dimensional Hypervolume. Glob. Ecol. Biogeogr. 2014, 23, 595–609. [Google Scholar] [CrossRef]
- Blonder, B. Do Hypervolumes Have Holes? Am. Nat. 2016, 187, E93–E105. [Google Scholar] [CrossRef] [PubMed]
- Mammola, S. Assessing Similarity of N-Dimensional Hypervolumes: Which Metric to Use? J. Biogeogr. 2019, 46, 2012–2023. [Google Scholar] [CrossRef]
- Frenot, Y.; Chown, S.L.; Whinam, J.; Selkirk, P.M.; Convey, P.; Skotnicki, M.; Bergstrom, D.M. Biological Invasions in the Antarctic: Extent, Impacts and Implications. Biol. Rev. 2005, 80, 45–72. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J.; Bridle, J.R.; Montoya, J.M.; Woodward, G. Emerging Horizons in Biodiversity and Ecosystem Functioning Research. Trends Ecol. Evol. 2009, 24, 505–514. [Google Scholar] [CrossRef]
- Luff, M.L. A New Species of Amblystogenium Enderlein (Coleoptera: Carabidae) from the Crozet Islands, with Comparative Notes on A. pacificum (Putzeys). J. Entomol. Ser. B Taxon. 1972, 41, 53–58. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pagès, J. Analyse factorielle multiple appliquée aux variables qualitatives et aux données mixtes. Rev. Stat. Appliquée 2002, 50, 5–37. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Soft. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 2017, 1, 337–354. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Barton, K. MuMIn: Multi-Model Inference. 2009. Available online: http://r-forge.r-project.org/projects/mumin/ (accessed on 1 July 2022).
- Blonder, B. R Package, Version 3.0.2; Hypervolume: High Dimensional Geometry, Set Operations, Projection, and Inference Using Kernel Density Estimation, Support Vector Machines, and Convex Hulls; R Consortium: San Francisco, CA, USA, 2022. [Google Scholar]
- Benavides, R.; Scherer-Lorenzen, M.; Valladares, F. The Functional Trait Space of Tree Species Is Influenced by the Species Richness of the Canopy and the Type of Forest. Oikos 2019, 128, 1435–1445. [Google Scholar] [CrossRef]
- Bittebiere, A.; Saiz, H.; Mony, C. New Insights from Multidimensional Trait Space Responses to Competition in Two Clonal Plant Species. Funct. Ecol. 2019, 33, 297–307. [Google Scholar] [CrossRef]
- Weiss, F.; Linde, A. How to Estimate Carabid Biomass?—An Evaluation of Size-Weight Models for Ground Beetles (Coleoptera: Carabidae) and Perspectives for Further Improvement. J. Insect Conserv. 2022, 26, 537–548. [Google Scholar] [CrossRef]
- Briegel, H. Fecundity, Metabolism, and Body Size in Anopheles (Diptera: Culicidae), Vectors of Malaria. J. Med. Entomol. 1990, 27, 839–850. [Google Scholar] [CrossRef]
- Zhou, G.; Pennington, J.E.; Wells, M.A. Utilization of Pre-Existing Energy Stores of Female Aedes aegypti Mosquitoes during the First Gonotrophic Cycle. Insect Biochem. Mol. Biol. 2004, 34, 919–925. [Google Scholar] [CrossRef]
- Min, K.-J.; Tatar, M. Restriction of Amino Acids Extends Lifespan in Drosophila melanogaster. Mech. Ageing Dev. 2006, 127, 643–646. [Google Scholar] [CrossRef]
- Moya-Laraño, J.; Macías-Ordóñez, R.; Blanckenhorn, W.U.; Fernández-Montraveta, C. Analysing Body Condition: Mass, Volume or Density? J. Anim. Ecol. 2008, 77, 1099–1108. [Google Scholar] [CrossRef]
- Kahiluoto, H.; Kaseva, J.; Hakala, K.; Himanen, S.J.; Jauhiainen, L.; Rötter, R.P.; Salo, T.; Trnka, M. Cultivating Resilience by Empirically Revealing Response Diversity. Glob. Environ. Change 2014, 25, 186–193. [Google Scholar] [CrossRef]
- Cook, L.M.; Saccheri, I.J. The Peppered Moth and Industrial Melanism: Evolution of a Natural Selection Case Study. Heredity 2013, 110, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C.; Richardson, J.L.; Freidenfelds, N.A. Plasticity and Genetic Adaptation Mediate Amphibian and Reptile Responses to Climate Change. Evol. Appl. 2014, 7, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.S.; Thorne, M.A.S.; King, M.; Hipperson, H.; Hoffman, J.I.; Peck, L.S. Life in the Intertidal: Cellular Responses, Methylation and Epigenetics. Funct. Ecol. 2018, 32, 1982–1994. [Google Scholar] [CrossRef]
- de Jong, P.W.; Brakefield, P.M. Climate and Change in Clines for Melanism in the Two–Spot Ladybird, Adalia bipunctata (Coleoptera: Coccinellidae). Proc. R. Soc. Lond. B 1998, 265, 39–43. [Google Scholar] [CrossRef]
- Schindler, D.E.; Hilborn, R.; Chasco, B.; Boatright, C.P.; Quinn, T.P.; Rogers, L.A.; Webster, M.S. Population Diversity and the Portfolio Effect in an Exploited Species. Nature 2010, 465, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Swanson, B.O.; Gibb, A.C.; Marks, J.C.; Hendrickson, D.A. Trophic Polymorphism And Behavioral Differences Decrease Intraspecific Competition in a Cichlid, Herichthys Minckleyi. Ecology 2003, 84, 1441–1446. [Google Scholar] [CrossRef]
- Cloyed, C.S.; Eason, P.K. Niche Partitioning and the Role of Intraspecific Niche Variation in Structuring a Guild of Generalist Anurans. R. Soc. Open Sci. 2017, 4, 170060. [Google Scholar] [CrossRef]
- Whitman, D.; Agrawal, A. What Is Phenotypic Plasticity and Why Is It Important? In Phenotypic Plasticity of Insects; Whitman, D., Ananthakrishnan, T., Eds.; Science Publishers: Rawalpindi, Pakistan, 2009; ISBN 978-157-808-423-4. [Google Scholar]
- Rosel, P.E.; Dizon, A.E.; Heyning, J.E. Genetic Analysis of Sympatric Morphotypes of Common Dolphins (Genus Delphinus). Mar. Biol. 1994, 119, 159–167. [Google Scholar] [CrossRef]
- Herden, T.; Friesen, N. Ecotypes or Phenotypic Plasticity—The Aquatic and Terrestrial Forms of Helosciadium repens (Apiaceae). Ecol. Evol. 2019, 9, 13954–13965. [Google Scholar] [CrossRef]
- Pyszko, P.; Drgová, M.; Ožana, S.; Dorňák, O.; Rožek, D.; Lee Číp, D.; Plášek, V.; Drozd, P. Could Bryophagous Beetles (Coleoptera: Byrrhidae) Help Us Understand Bryophyte Taxonomy? Preferences within the Hypnum Cupressiforme Hedw. Species Complex. Plants 2021, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Morissette, O.; Sirois, P.; Wilson, C.C.; Laporte, M.; Bernatchez, L. The Role of Ecotype-environment Interactions in Intraspecific Trophic Niche Partitioning Subsequent to Stocking. Ecol. Appl. 2019, 29, e01857. [Google Scholar] [CrossRef] [PubMed]
- Skulason, S.; Smith, T.B. Resource Polymorphisms in Vertebrates. Trends Ecol. Evol. 1995, 10, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.E.; Woltering, C.; Alexander, G. Epigenetic Regulation of Trophic Morphology through Feeding Behaviour in Arctic Charr, Salvelinus alpinus. Biol. J. Linn. Soc. 2003, 78, 43–49. [Google Scholar] [CrossRef]
| Estimate | Std. Error | Adjusted SE | Z value | Pr(>|z|) | |
|---|---|---|---|---|---|
| |||||
| (Intercept) | 1.881 | 0.262 | 0.264 | 7.130 | <0.001 |
| Altitude | 1.759 | 0.159 | 0.348 | 5.050 | <0.001 |
| Altitude 2 | −1.712 | 0.167 | 0.367 | 4.660 | <0.001 |
| L-morphotype | −0.079 | 0.163 | 0.164 | 0.480 | 0.629 |
| Males | −0.071 | 0.138 | 0.139 | 0.510 | 0.610 |
| L-morphotype:Altitude | −0.399 | 0.165 | 0.166 | 2.400 | 0.016 |
| |||||
| (Intercept) | 5.373 | 0.279 | 0.281 | 19.090 | <0.001 |
| Altitude | 0.541 | 0.152 | 0.335 | 1.620 | 0.110 |
| Altitude 2 | −0.550 | 0.184 | 0.404 | 1.360 | 0.170 |
| L-morphotype | −0.043 | 0.103 | 0.104 | 0.410 | 0.680 |
| Males | 0.083 | 0.136 | 0.137 | 0.610 | 0.540 |
| |||||
| (Intercept) | 0.016 | 1.109 × 10−3 | 1.119 × 10−3 | 14.450 | <0.001 |
| Altitude | 1.557 × 10−3 | 1.089 × 10−3 | 2.973 × 10−3 | 0.830 | 0.406 |
| Altitude 2 | −5.380 × 10−4 | 7.240 × 10−4 | 1.562 × 10−3 | 0.340 | 0.731 |
| L-morphotype | 5.334 × 10−3 | 1.718 × 10−3 | 1.731 × 10−3 | 3.080 | 2.100 × 10−3 |
| Males | −5.054 × 10−3 | 1.329 × 10−3 | 1.338 × 10−3 | 3.780 | 2.000 × 10−4 |
| Altitude:L-morphotype | 1.470 × 10−3 | 1.100 × 10−3 | 1.100 × 10−3 | 1.330 | 0.183 |
| Altitude:Males | −9.330 × 10−4 | 1.070 × 10−3 | 1.070 × 10−3 | 0.870 | 0.384 |
| L-morphotype:Males | 1.600 × 10−3 | 2.010 × 10−3 | 2.020 × 10−3 | 0.790 | 0.428 |
| L-morphotype:Altitude 2 | −3.390 × 10−3 | 1.780 × 10−3 | 1.78 0× 10−3 | 0.870 | 0.057 |
| |||||
| (Intercept) | −1.291 | 0.059 | 0.060 | 21.650 | <0.001 |
| Altitude | 9.930 × 10−3 | 0.024 | 0.033 | 0.300 | 0.765 |
| Altitude 2 | 3.410 × 10−3 | 0.018 | 0.027 | 0.130 | 0.900 |
| L-morphotype | 9.320 × 10−3 | 0.036 | 0.036 | 0.260 | 0.795 |
| Males | −0.190 | 0.070 | 0.071 | 2.680 | 7.400 × 10−3 |
| |||||
| (Intercept) | −3.689 | 0.140 | 0.141 | 26.130 | <0.001 |
| Altitude | −0.354 | 0.095 | 0.183 | 1.930 | 0.054 |
| Altitude 2 | 0.259 | 0.074 | 0.161 | 1.610 | 0.107 |
| L-morphotype | 0.132 | 0.149 | 0.150 | 0.880 | 0.377 |
| Males | 0.133 | 0.153 | 0.153 | 0.870 | 0.386 |
| Altitude:L-morphotype | 0.180 | 0.157 | 0.158 | 1.140 | 0.256 |
| L-morphotype:Males | 0.035 | 0.130 | 0.131 | 0.270 | 0.790 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espel, D.; Coux, C.; Pertierra, L.R.; Eymar-Dauphin, P.; Lembrechts, J.J.; Renault, D. Functional Niche Partitioning Occurs over Body Size but Not Nutrient Reserves nor Melanism in a Polar Carabid Beetle along an Altitudinal Gradient. Insects 2023, 14, 123. https://doi.org/10.3390/insects14020123
Espel D, Coux C, Pertierra LR, Eymar-Dauphin P, Lembrechts JJ, Renault D. Functional Niche Partitioning Occurs over Body Size but Not Nutrient Reserves nor Melanism in a Polar Carabid Beetle along an Altitudinal Gradient. Insects. 2023; 14(2):123. https://doi.org/10.3390/insects14020123
Chicago/Turabian StyleEspel, Diane, Camille Coux, Luis R. Pertierra, Pauline Eymar-Dauphin, Jonas J. Lembrechts, and David Renault. 2023. "Functional Niche Partitioning Occurs over Body Size but Not Nutrient Reserves nor Melanism in a Polar Carabid Beetle along an Altitudinal Gradient" Insects 14, no. 2: 123. https://doi.org/10.3390/insects14020123
APA StyleEspel, D., Coux, C., Pertierra, L. R., Eymar-Dauphin, P., Lembrechts, J. J., & Renault, D. (2023). Functional Niche Partitioning Occurs over Body Size but Not Nutrient Reserves nor Melanism in a Polar Carabid Beetle along an Altitudinal Gradient. Insects, 14(2), 123. https://doi.org/10.3390/insects14020123







