Genetically Depauperate and Still Successful: Few Multilocus Genotypes of the Introduced Parthenogenetic Weevil Naupactus cervinus (Coleoptera: Curculionidae) Prevail in the Continental United States
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. DNA Extraction
2.3. DNA Amplification and Sequencing
2.4. Data Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bang, A.; Cuthbert, R.N.; Haubrock, P.J.; Fernandez, R.D.; Moodley, D.; Diagne, C.; Turbelin, A.J.; Renault, D.; Dalu, T.; Courchamp, F. Massive economic costs of biological invasions despite widespread knowledge gaps: A dual setback for India. Biol. Invasions 2022, 24, 2017–2039. [Google Scholar] [CrossRef]
- Capinha, C.; Essl, F.; Seebens, H.; Moser, D.; Pereira, H.M. The dispersal of alien species redefines biogeography in the Anthropocene. Science 2015, 348, 1248–1251. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 2016 12, 20150623. [Google Scholar] [CrossRef]
- Brooks, M.L.; D’Antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of invasive alien plants on fire regimes. BioScience 2004, 54, 677–688. [Google Scholar] [CrossRef]
- Vilà, M.; Hulme, P.E. (Eds.) Impact of Biological Invasions on Ecosystem Services; Springer International Publishing: Cham, Switzerland, 2017; Volume 12. [Google Scholar]
- Ricciardi, A.; Hoopes, M.F.; Marchetti; M. P.; Lockwood; J.L. Progress toward understanding the ecological impacts of nonnative species. Ecol. Monogr. 2013, 83, 263–282. [Google Scholar] [CrossRef]
- Suarez, A.V.; Tsutsui; N. D. The evolutionary consequences of biological invasions. Mol. Ecol. 2008, 17, 351–360. [Google Scholar] [CrossRef]
- Crowl, T.A.; Crist, T.O.; Parmenter, R.R.; Belovsky, G.; Lugo, A.E. The spread of invasive species and infectious disease as drivers of ecosystem change. Front. Ecol. Environ. 2008, 6, 238–246. [Google Scholar] [CrossRef]
- Hatcher, M.J.; Dick, J.T.; Dunn, A.M. Disease emergence and invasions. Funct. Ecol. 2012, 26, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T.; Nakazawa, Y. Environmental data sets matter in ecological niche modelling: An example with Solenopsis invicta and Solenopsis richteri. Glob. Ecol Biogeogr. 2008, 17, 135–144. [Google Scholar] [CrossRef]
- Marvaldi, A.E.; Sequeira, A.S.; O’Brien, C.W.; Farrell, B.D. Molecular and morphological phylogenetics of weevils (Coleoptera, Curculionoidea): Do niche shifts accompany diversification? Syst. Biol. 2002, 51, 761–785. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, A.A.; Marvaldi, A.E.; Suárez, S.M. Gorgojos de la Argentina y sus Plantas Huéspedes; Publicación Especial de la Sociedad Entomológica: Tucuman, Argentina, 2002. [Google Scholar]
- Scataglini, M.A.; Lanteri, A.A.; Confalonieri, V.A. Diversity of boll weevil populations in South America: A phylogeographic approach. Genetica 2002, 126, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, C.F.; de Oliveira, N.C.; Sartório, R.C.; Loureiro, E.B.; Bezerra, N., Jr.; Rosado Neto, G.H. Gonipterus scutellatus Gyllenhal (Coleoptera: Curculionidae) occurrence in eucalyptus plantations in Espírito Santo State, Brazil. Arq. Inst. Biol. 2021, 75, 113–115. [Google Scholar] [CrossRef]
- Guzmán, N.V.; Lanteri, A.A.; Confalonieri, V.A. Colonization ability of two invasive weevils with different reproductive modes. Evol. Ecol. 2012, 26, 1371–1390. [Google Scholar] [CrossRef]
- Infante, F.; Pérez, J.; Vega, F.E. The coffee berry borer: The centenary of a biological invasion in Brazil. Braz. J. Biol. 2014, 74, S125–S126. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, V.A.; Gomez, C.A.; La Manna, L.; Roux, G.; Lanteri, A.A.; Vallejos, N.C.; Marvaldi, A.E. Introduction and establishment of Pissodes castaneus (Coleoptera: Curculionidae) in the Andean Patagonia of Argentina. J. Econ. Entomol. 2016, 109, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Rodriguero, M.S.; Lanteri, A.A.; Guzmán, N.V.; Carús Guedes, J.V.; Confalonieri, V.A. Out of the forest: Past and present range expansion of a parthenogenetic weevil pest, or how to colonize the world successfully. Ecol. Evol. 2016, 6, 5431–5445. [Google Scholar] [CrossRef]
- Rodriguero, M.S.; Guzmán, N.V.; Lanteri, A.A.; Confalonieri, V.A. The effect of reproductive system on invasiveness. Fla. Entomol. 2019, 102, 495–500. [Google Scholar] [CrossRef]
- Logan, D.P.; Maher, B.J.; Dobson, S.S.; Connolly, P.G. Larval survival of Fuller’s rose weevil, Naupactus cervinus, on common groundcover species in orchards of New Zealand kiwifruit. J. Insect Sci. 2008, 8, 51. [Google Scholar] [CrossRef]
- Normark, B.B.; Johnson, N.A. Niche explosion. Genetica 2011, 139, 551–564. [Google Scholar] [CrossRef]
- Lanteri, A.A.; Guedes, J.C.; Parra, J.R. Weevils injurious for roots of citrus in São Paulo State, Brazil. Neotr. Entomol. 2002, 31, 561–569. [Google Scholar] [CrossRef]
- Rodriguero, M.S.; Lanteri, A.A.; Confalonieri, V.A. Mito-nuclear genetic comparison in a Wolbachia infected weevil: Insights on reproductive mode, infection age and evolutionary forces shaping genetic variation. BMC Evol. Biol. 2010, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Rodriguero, M.S.; Wirth, S.A.; Alberghina, J.S.; Lanteri, A.A.; Confalonieri, V.A. A tale of swinger insects: Signatures of past sexuality between divergent lineages of a parthenogenetic weevil revealed by ribosomal intraindividual variation. PLoS ONE 2018, 13, e0195551. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, L.L.F. The Species of Pantomorus of America North of Mexico; US Department of Agriculture: Washington, DC, USA, 1939; No. 341; pp. 1–39. [Google Scholar]
- Monteiro Junqueira, G. Pantomorus godmani (Crotch): Um depredador ocasional do cafeeiro. Solo 1957, 44, 51–58. [Google Scholar]
- Lanteri, A.A. Revisión del género Asynonychus Crotch (Coleoptera: Curculionidae). Nat. Neotrop. 1986, 17, 161–174. [Google Scholar] [CrossRef]
- Lanteri, A.A.; Normark, B.B. Parthenogenesis in the tribe Naupactini (Coleoptera: Curculionidae). Ann. Entomol. Soc. Am. 1995, 88, 722–731. [Google Scholar] [CrossRef]
- Suomalainen, E. Significance of parthenogenesis in the evolution of insects. Annu. Rev. Entomol. 1962, 7, 349–366. [Google Scholar] [CrossRef]
- Mackay-Smith, A.; Dornon, M.K.; Lucier, R.; Okimoto, A.; Mendonca de Sousa, F.; Rodriguero, M.; Confalonieri, V.; Lanteri, A.A.; Sequeira, A.S. Host-specific gene expression as a tool for introduction success in Naupactus parthenogenetic weevils. PLoS ONE 2021, 16, e0248202. [Google Scholar] [CrossRef]
- Chadwick, C.E. A review of Fuller’s Rose Weevil (‘Pantomorus cervinus’ (Boh)) (Col., Curculionidae). J. Aust. Entomol. Soc. 1965, 2, 10–20. [Google Scholar]
- Gyeltshen, J.; Hodges, A. Fuller Rose Beetle, Pantomorus cervinus (Boheman) (Insecta: Coleoptera: Curculionidae). EDIS 2006, 20, 1–5. [Google Scholar] [CrossRef]
- Rodriguero, M.S.; Lanteri, A.A.; Confalonieri, V.A. Speciation in the asexual realm: Is the parthenogenetic weevil Naupactus cervinus a complex of species in statu nascendi? Mol. Phyl. Evol. 2013, 68, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Mander, C.V.; Phillips, C.B.; Glare, T.R.; Chapman, R.B. Preliminary assessment of COI and ITS1 sequence variation in Fullers rose weevil. N. Z. Plant Prot. 2003, 56, 190–193. [Google Scholar] [CrossRef]
- Normark, B.B. Phylogeny and Evolution of Parthenogenesis in the Aramigus tessellatus Complex (Coleoptera: Curculionidae). Ph.D. Dissertation, Cornell University, Ithaca, NY, USA, 1994. [Google Scholar]
- Vrain, T.C.; Wakarchuk, D.A.; Levesque, A.C.; Hamilton, R.I. Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fund. Appl. Nematol. 1992, 15, 563–573. [Google Scholar]
- Cherry, T.; Szalanski, A.L.; Todd, T.C.; Powers, T.O. The internal transcribed spacer region of Belonolaimus (Nemata: Belonolaimidae). J. Nematol. 1997, 29, 23. [Google Scholar] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-Del Barrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Tajima, F. Evolutionary relationship of DNA sequences in finite populations. Genetics 1983, 105, 437–460. [Google Scholar] [CrossRef]
- Nei, M.; Miller, J.C. A simple method for estimating average number of nucleotide substitutions within and between populations from restriction data. Genetics 1990, 125, 873–879. [Google Scholar] [CrossRef]
- Nei, M. Genetic Distance and Molecular Phylogeny; University of Washington Press: Washington, DC, USA, 1987. [Google Scholar]
- Hudson, R.R.; Boos, D.D.; Kaplan, N.L. A statistical test for detecting geographic subdivision. Mol. Biol. Evol. 1992, 9, 138–151. [Google Scholar]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1660. [Google Scholar] [CrossRef]
- Germann, C. First record of the neozoic species Naupactus cervinus Boheman, 1840 (Coleoptera, Curculionidae, Entiminae) for Switzerland with a short review of its spreading and food plants. Bull. Soc. Entomol. Suisse 2016, 89, 1–5. [Google Scholar]
- Chittenden, F.H. Some Insects Injurious to the Violet, Rose, and Other Ornamental Plants: A Collection of Articles Dealing with Insects of This Class; US Department of Agriculture, Division of Entomology: Washington, DC, USA, 1901. [Google Scholar]
- Ney, G.; Frederick, K.; Schul, J. A post-pleistocene calibrated mutation rate from insect museum specimens. PLoS Curr. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; Luoto, M. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 2009, 16, 743–753. [Google Scholar] [CrossRef]
- Gascoigne, J.; Berec, L.; Gregory, S.; Courchamp, F. Dangerously few liaisons: A review of mate-finding Allee effects. Pop. Ecol. 2009, 51, 355–372. [Google Scholar] [CrossRef]
- Hastings, A.; Cuddington, K.; Davies, K.F.; Dugaw, C.J.; Elmendorf, S.; Freestone, A.; Harrison, S.; Holland, M.; Lambrinos, J.; Malvadkar, U.; et al. The spatial spread of invasions: New developments in theory and evidence. Ecol. Lett. 2005, 8, 91–101. [Google Scholar] [CrossRef]
- Suarez, A.V.; Holway, D.A.; Case, T.J. Patterns of spread in biological invasions dominated by long-distance jump dispersal: Insights from Argentine ants. Proc. Natl. Acad. Sci. USA 2001, 98, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.M.; Liebhold, A.M.; Tobin, P.C.; Bjørnstad, O.N. Allee effects and pulsed invasion of the gypsy moth. Nature 2006, 444, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Tobin, P.C.; Whitmire, S.L.; Johnson, D.M.; Bjørnstad, O.N.; Liebhold, A.M. Invasion speed is affected by geographic variation in the strength of Allee effects. Ecol. Lett. 2007, 10, 36–43. [Google Scholar] [CrossRef]
- Tobin, P.C.; Turcotte, R.M.; Snider, D.A. When one is not necessarily a lonely number: Initial colonization dynamics of Adelges tsugae on eastern hemlock, Tsuga canadensis. Biol. Inv. 2013, 15, 1925–1932. [Google Scholar] [CrossRef]
- Schwander, T.; Libbrecht, R.; Keller, L. Supergenes and complex phenotypes. Curr. Biol. 2014, 24, R288–R294. [Google Scholar] [CrossRef] [PubMed]
- Rodriguero, M.S.; Aquino, D.A.; Loiácono, M.S.; Elías Costa, A.J.; Confalonieri, V.A.; Lanteri, A.A. Parasitism of the “Fuller’s rose weevil” Naupactus cervinus by Microctonus sp. in Argentina. BioControl 2014, 59, 547–556. [Google Scholar] [CrossRef]
- Fernandez Goya, L.; Lanteri, A.A.; Confalonieri, V.A.; Rodriguero, M.S. New host-parasitoid interactions in Naupactus cervinus (Coleoptera, Curculionidae) raise the question of Wolbachia horizontal transmission. Symbiosis 2022, 86, 325–336. [Google Scholar] [CrossRef]
- Maynard-Smith, J. The Evolution of Sex; Cambridge University Press: Cambridge, UK, 1978; Volume 4. [Google Scholar]
- Kearney, M. Hybridization, glaciation and geographical parthenogenesis. Trends Ecol. Evol. 2005, 20, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, A.A.; Guzmán, N.V.; Del Río, M.G.; Confalonieri, V.A. Potential geographic distributions and successful invasions of parthenogenetic broad-nosed weevils (Coleoptera: Curculionidae) native to South America. Environ. Entomol. 2013, 42, 677–687. [Google Scholar] [CrossRef]
- Del Río, M.G.; Guzmán, N.V.; Montemayor, S.I.; Confalonieri, V.A.; Lanteri, A.A. Potential geographic distributions of two parthenogenetic weevils (Coleoptera: Curculionidae) associated with citrus in Argentina and Brazil. Fla. Entomol. 2019, 102, 459–463. [Google Scholar] [CrossRef]
- Baker, H.G. Characteristics and modes of origin of weeds. In Genetics of Colonizing Species; Baker, H.G., Stebbins, G.L., Eds.; Academic Press: New York, NY, USA, 1965; pp. 147–172. [Google Scholar]
- Gade, B.; Parker, E.D., Jr. The effect of life cycle stage and genotype on desiccation tolerance in the colonizing parthenogenetic cockroach Pycnoscelus surinamensis and its sexual ancestor P. indicus. J. Evol. Biol. 1997, 10, 479–493. [Google Scholar] [CrossRef]
- Parker, E.D., Jr.; Selander, R.K.; Hudson, R.O.; Lester, L.J. Genetic diversity in colonizing parthenogenetic cockroaches. Evolution 1977, 31, 836–842. [Google Scholar] [CrossRef]
- Lundmark, M. Otiorhynchus sulcatus, an autopolyploid general-purpose genotype species? Hereditas 2010, 147, 278–282. [Google Scholar] [CrossRef]
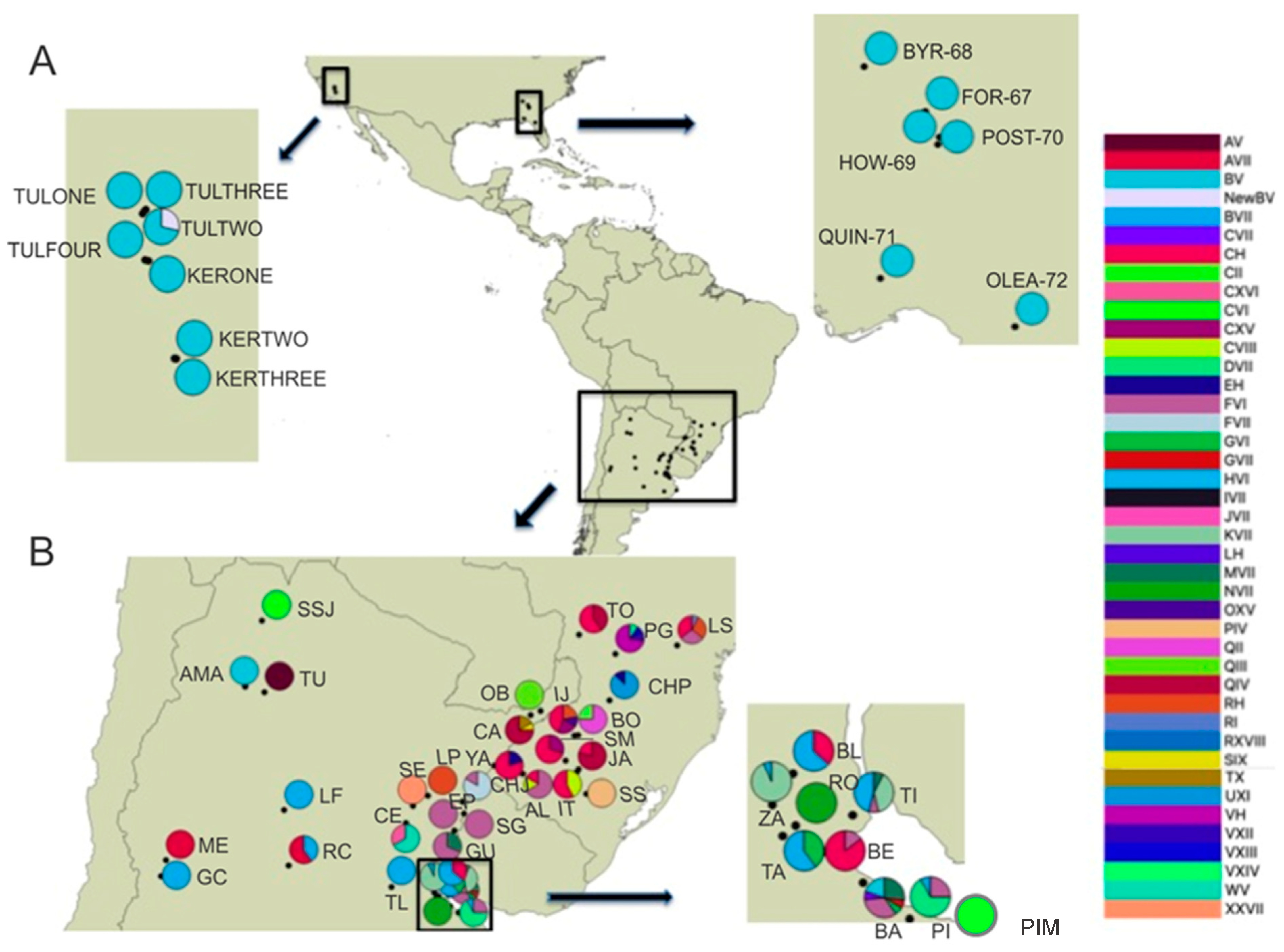
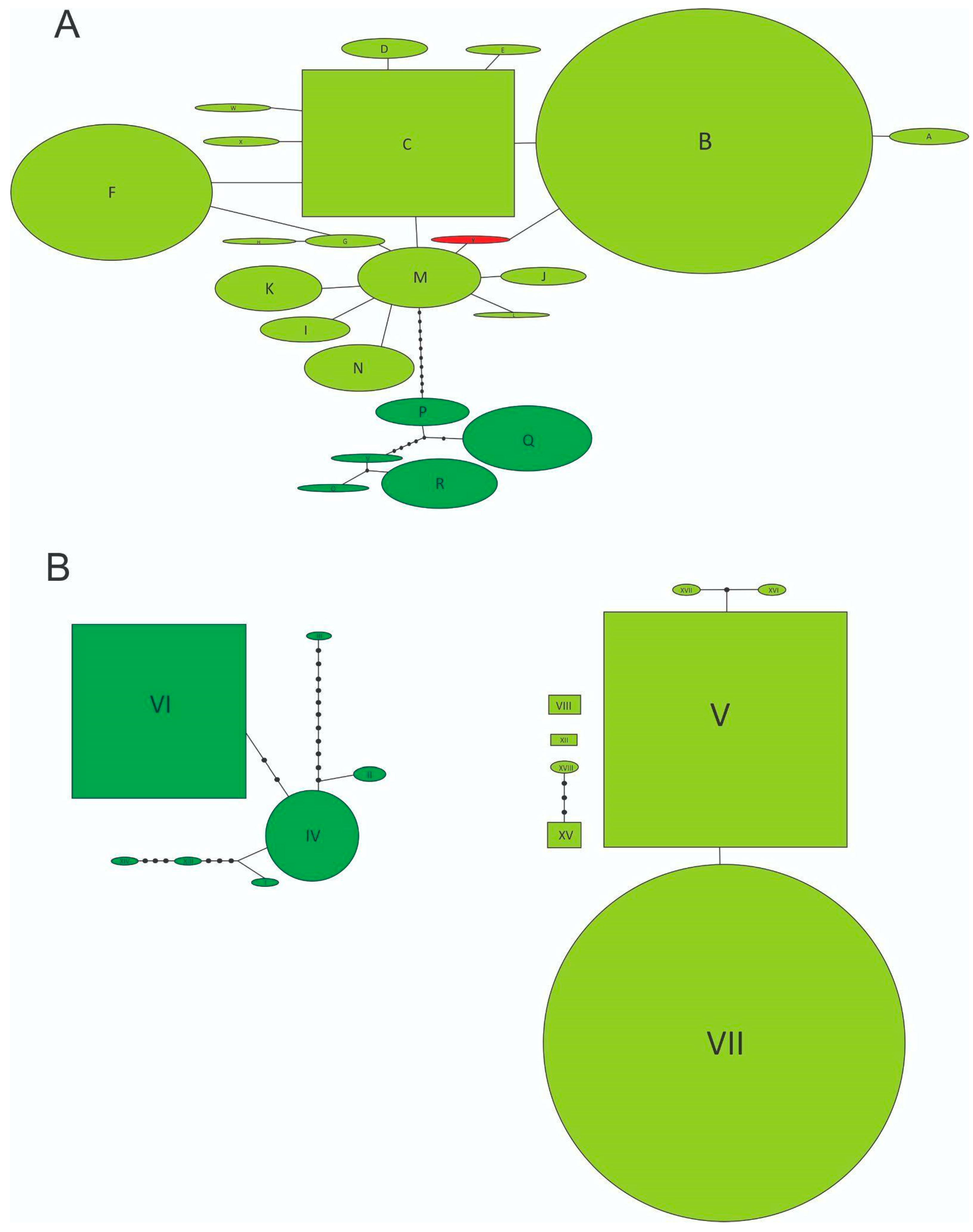
| Locality Group | Locality Details | Code | Coordinates (DDM) | N (CO1/ITS1) | Multilocus Genotype |
|---|---|---|---|---|---|
| Native range (40) | |||||
| AR-Bs. As., Benavídez | BE | 34°24′ S; 58°41′ W | 7/7 | C-H1(6), F-VI(1)b | |
| AR-Bs. As., Buenos Aires | BA | 34°36′ S; 58°26′ W | 16/16 | B-V(3) a,C-VI(1) b,F-VI(5) a, G-VI(1) b,G-VII(1) b, H-VI(1) a, M-VII (4) e | |
| AR-Bs. As., Cardales | CA | 34°18′ S; 58°57′ W | 5/5 | B-VII(3) a,G-VI(2) a | |
| AR-Bs. As., Reserva Otamendi | RO | 34°14′ S; 58°52′ W | 11/11 | N-VII a | |
| AR-Bs. As., Parque Pereyra Iraola | PI | 34°50′ S; 58°8′ W | 12/12 | B-VII(1) a, D-VII(8) a, F-VI(3) a | |
| AR- Bs. As., Pergamino | PE | 33°54′ S; 60°35′ W | 1/1 | B-VII a | |
| AR-Bs. As., Talavera Island | TI | 34°10′ S; 58°30′ W | 17/17 | B-VII(1) a, F-VI(6) a, K-VII(1) a, M-VII (9) a | |
| AR-Bs. As., Tandil | TA | 37°19′ S; 59°08′ W | 7/7 | B-V(5) a, F-VI(2) a | |
| AR-Bs. As., Tres Lomas | TL | 36°28′ S; 62°52′ W | 4/4 | B-VII a | |
| AR-Bs. As., Zárate | ZA | 34°06′ S; 59°01′ W | 15/15 | B-VII(1) a, K-VII(14) a | |
| AR-Bs. As., Pinamar | PIM* | 37°6.57′ S; 56°52.69′ W | 1/1 | C-VI d | |
| AR-Córdoba, La Falda | LF | 31°05′ S; 64°29′ W | 7/7 | B-VII a | |
| AR-Córdoba, Río Cuarto | RC | 33°08′ S; 64°21′ W | 5/5 | A-VII(3) a, B-VII(2) a | |
| AR-Corrientes, Yapeyú | YA | 29°28′ S; 56°50′ W | 5/5 | C-H1(4) a, E-H1(1) a | |
| AR-E. Ríos, Brazo Largo | BL | 33°54′ S, 58°53′ W | 11/11 | M-VII(7) a, C-H4(4) a | |
| AR-E. Ríos, Cerrito | CE | 31°34′ S, 60°03′ W | 3/3 | C-XVI(1) b, W-V(2) b | |
| AR-E. Ríos, Chajarí | CHJ | 30°47′ S, 57°59′ W | 6/6 | F-VII(1) a, F-VI(5) a | |
| AR-E. Ríos, El Palmar | EP | 31°50′ S, 58°17′ W | 5/5 | F-VI a | |
| AR-E. Ríos, Gualeguaychú | GU | 33°01′ S, 58°31′ W | 16/16 | F-VI(11) a, M-VII(5) a | |
| AR-E. Ríos, La Paz | LP | 30°45′ S, 59°38′ W | 6/6 | R-H2b | |
| AR-E. Ríos, Salto Grande | SG | 31°23′ S, 58°01′ W | 4/4 | F-VI a | |
| AR-E. Ríos, Santa Elena | SE | 30°56′ S, 59°48′ W | 2/2 | X-XVII b | |
| AR-Mendoza, Godoy Cruz | GC | 32°56′ S, 68°50′ W | 2/2 | A-VII a | |
| AR-Mendoza, Mendoza | ME | 33°30′ S, 69°W | 3/3 | BVII a | |
| AR-Misiones, Cerro Azul | CA | 27°38′ S, 55°30′ W | 12/12 | QIV(9) a, TX(2) c, SIX(1) c | |
| AR-Misiones, Oberá | OB | 27°29′ S, 55°08′ W | 1/1 | QIII a | |
| AR-Tucumán, San Miguel de Tucumán | TU | 26°46′ S, 65°13′ W | 1/1 | AV b | |
| AR-Tucuman, Amaicha | AMA * | 26°35.74′ S; 65°55.33′ W | 1/1 | BV e | |
| AR-Jujuy, San Salvador de Jujuy | SSJ * | 24°10.82′ S; 65°18.53′ W | 1/1 | CVI e | |
| BR-PR, Laranjeiras do Sul, LS | LS | 25°24′ S, 52°24′ W | 11/11 | VH3(8) b, VXII(1) b, VXIV(1) b, VXIII(1) b | |
| BR-PR, Ponta Grossa, PG | PG | 25°05′ S, 50°09′ W | 14/14 | CH4(5) b, RI(1) a, FVI(4) b, RH2(4) a | |
| BR-PR, Toledo, TO | TO | 24°42′ S, 53°44′ W | 10/10 | CH1(6) a, QIV(4) b | |
| BR-RG do Sul, Alegrete | AL | 29°46′ S, 55°47′ W | 6/6 | CH1(1) a, FVI(4) b, CVIII(1) b | |
| BR-RG do Sul, Bozano | BO | 28°35′ S, 53°59′ W | 4/4 | QII(3) a, CII(1) a | |
| BR-RG do Sul, Ijui | IJ | 28°23′ S, 53°54′ W | 9/9 | RH2(2) b, OXV(1) b, CXV(2) b, CH(4) a | |
| BR-RG do Sul, Itaára | IT | 29°36 S, 53°45 W | 7/7 | CH1(4) a, CVIII(3) b | |
| BR-RG do Sul, Jari | JA | 29°17′ S, 54°13′ W | 17/17 | CXV(5) b, CH1(12) a | |
| BR-RG do Sul, Santa Maria | SM | 29°40′ S, 53°47′ W | 14/14 | QIV(11) a, CH1(3) b | |
| BR-RG do Sul, São Sepé | SS | 30°10′ S, 53°34′ W | 6/6 | PIV a | |
| BR-SC, Chapecó | CHP | 27°03′ S, 52°36′ W | 8/8 | UXI(7) c, EH1(1) a | |
| Introduced range (30) | |||||
| INUSCO1 | US-GA, USDA station, Byron | BYR-68 * | 32°39.22′ N; 083°42.91′ W | 4/3 | 4/3 |
| US-GA, KOA campground, Forsyth | FOR-67 * | 33°02.27′ N; 083°55.55′ W | 3/3 | B-V e | |
| US-GA, Howard North Lake | HOW-69 * | 32°32.61′ N; 083°44.34′ W | 5/7 | B-V e | |
| US-GA, Post Street Park, Douglasville | POST-70 * | 33°42.39′ N; 084°50.56′ W | 5/5 | B-V e | |
| US-FL, Oleno State Park, High Springs | OLEA-72 * | 29°49.44′ N; 082°35.39′ W | 2/2 | B-V e | |
| US-FL, Quincy Research Station | QUIN-71 * | 30°32.71′ N; 84°35.75′ W | 4/7 | B-V e | |
| INUSCO2 | US-CA, Rt. 220, Tulare Co. | TULONE * | 36°21.11′ N; 119°04.84′ W | 9/9 | B-V e |
| US-CA, 7 km. from Lindcove Station, Tulare Co. | TULTWO * | 36°19.99′ N; 119°05.60′ W | 7/8 | B-V(5) e, Y-V(2) e | |
| US-CA, Lindcove Station, Tulare Co. | TULTHREE * | 36°21.22′ N; 119°83.38′ W | 4/5 | B-V e | |
| US-CA, Lally farms, Av. 124 & Rd. 232,Tulare Co. | TULFOUR * | 36°1.39′ N; 119°4.56′ W | 3/7 | B-V e | |
| US-CA, Brekenridge Rd. and Pepper Dr., Kern Co. | KERONE * | 35°21.63′ N; 118°52.44′ W | 4/4 | B-V e | |
| US-CA, Pepper Ranch, Kern Co. | KERTWO * | 35°21.50′ N; 118°51.80′ W | 4/6 | B-V e | |
| US-CA, Valpredo ranch, Kern Co. | KERFOUR * | 36°0.97′ N; 119°3.13′ W | 2/4 | B-V e | |
| INUSI | US-PI, Hawaii, Big Island | BI | 19°36′ N; 155°39′ W | 1/1 | B-V b |
| US-PI, Hawaii, Kauai | KA | 22°07′ N; 159°31′ W | 2/2 | B-V b | |
| US-PI, Hawaii, Maui | MU | 20°50′ N; 156°20′ W | 1/1 | B-V b | |
| US-PI, Hawaii, Oahu | OH | 21°28′ N; 157°59′ W | 1/1 | B-V d | |
| INSA | CHI/PI, Isla de Pascua (Rapa Nui), | IP/EL | 27°08′ S; 109°26′ W | 7/7 | I-VII a |
| CHI, Bio Bio, Chillan, | CHI | 36°36′ S; 72°06′ W | 8/8 | B-VII d | |
| CHI, Santiago | SC | 33°26′ S; 70°29′ W | 6/6 | B-VII a | |
| CHI, Vallenar | VAR | 28°57′ S; 71°15′ W | 11/11 | I-VII(4) a, J-VII(7) a | |
| CHI, La Serena | LSE | 29°50′ S; 71°14′ W | 3/3 | M-VII(2) b, R-XVIII(1) b | |
| INEU | ES, Canary Islands, Tenerife | TE | 27°27′ N; 16°14′ W | 5/5 | B-VII a |
| ES, Valencia | VAL | 39°29′ N; 00°23′ W | 10/10 | B-VII a | |
| INAUN | AU, Victoria, Vermont | VE | 37°50′ S; 145°11′ E | 1/1 | B-V d |
| AU, Victoria, Tatura | TA | 36°26′S; 145°13′ E | 1/1 | B-V d | |
| NZ, Auckland, Awhitu | AW | 37°05′ S; 174°39′ E | 1/1 | B-V d | |
| NZ, Bay of Plenty, Matapihi | MA | 37°41′ S; 176°11′ E | 1/1 | B-V d | |
| INPI | PI, French Polynesia, Rapa Island (Rapa Iti) | RI | 27°32′ S; 144°20′ W | 1/1 | B-VII a |
| PI, French Polynesia, Tahiti | TH | 17°52′ S, 149°56′ W | 3/3 | B-VII a |
| Area | Sequenced Region/Number of Sequences | Within Areas | ||
|---|---|---|---|---|
| Number of Polymorphic Sites/Mutations | k | Pi | ||
| Native | CO1/ 365 | 17/17 | 2.850 | 0.01183 |
| ITS1/ 287 | 27/30 | 6.301 | 0.01011 | |
| Introduced | CO1/ 114 | 7/7 | 0.281 | 0.00117 |
| ITS1/ 114 | 9/9 | 0.158 | 0.00025 | |
| Total | CO1/479 | 18/18 | 2.343 | 0.00972 |
| ITS1/ 401 | 27/30 | 5.586 | 0.00897 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguero, M.S.; Confalonieri, V.A.; Mackay Smith, A.; Dornon, M.K.; Zagoren, E.; Palmer, A.; Sequeira, A.S. Genetically Depauperate and Still Successful: Few Multilocus Genotypes of the Introduced Parthenogenetic Weevil Naupactus cervinus (Coleoptera: Curculionidae) Prevail in the Continental United States. Insects 2023, 14, 113. https://doi.org/10.3390/insects14020113
Rodriguero MS, Confalonieri VA, Mackay Smith A, Dornon MK, Zagoren E, Palmer A, Sequeira AS. Genetically Depauperate and Still Successful: Few Multilocus Genotypes of the Introduced Parthenogenetic Weevil Naupactus cervinus (Coleoptera: Curculionidae) Prevail in the Continental United States. Insects. 2023; 14(2):113. https://doi.org/10.3390/insects14020113
Chicago/Turabian StyleRodriguero, Marcela S., Viviana A. Confalonieri, Ava Mackay Smith, Mary Kate Dornon, Eleanor Zagoren, Alice Palmer, and Andrea S. Sequeira. 2023. "Genetically Depauperate and Still Successful: Few Multilocus Genotypes of the Introduced Parthenogenetic Weevil Naupactus cervinus (Coleoptera: Curculionidae) Prevail in the Continental United States" Insects 14, no. 2: 113. https://doi.org/10.3390/insects14020113
APA StyleRodriguero, M. S., Confalonieri, V. A., Mackay Smith, A., Dornon, M. K., Zagoren, E., Palmer, A., & Sequeira, A. S. (2023). Genetically Depauperate and Still Successful: Few Multilocus Genotypes of the Introduced Parthenogenetic Weevil Naupactus cervinus (Coleoptera: Curculionidae) Prevail in the Continental United States. Insects, 14(2), 113. https://doi.org/10.3390/insects14020113






