The Optimal Choice of Trap Type for the Recently Spreading Jewel Beetle Pests Lamprodila festiva and Agrilus sinuatus (Coleoptera, Buprestidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Trap Type
2.2. Experimental Site and Setup
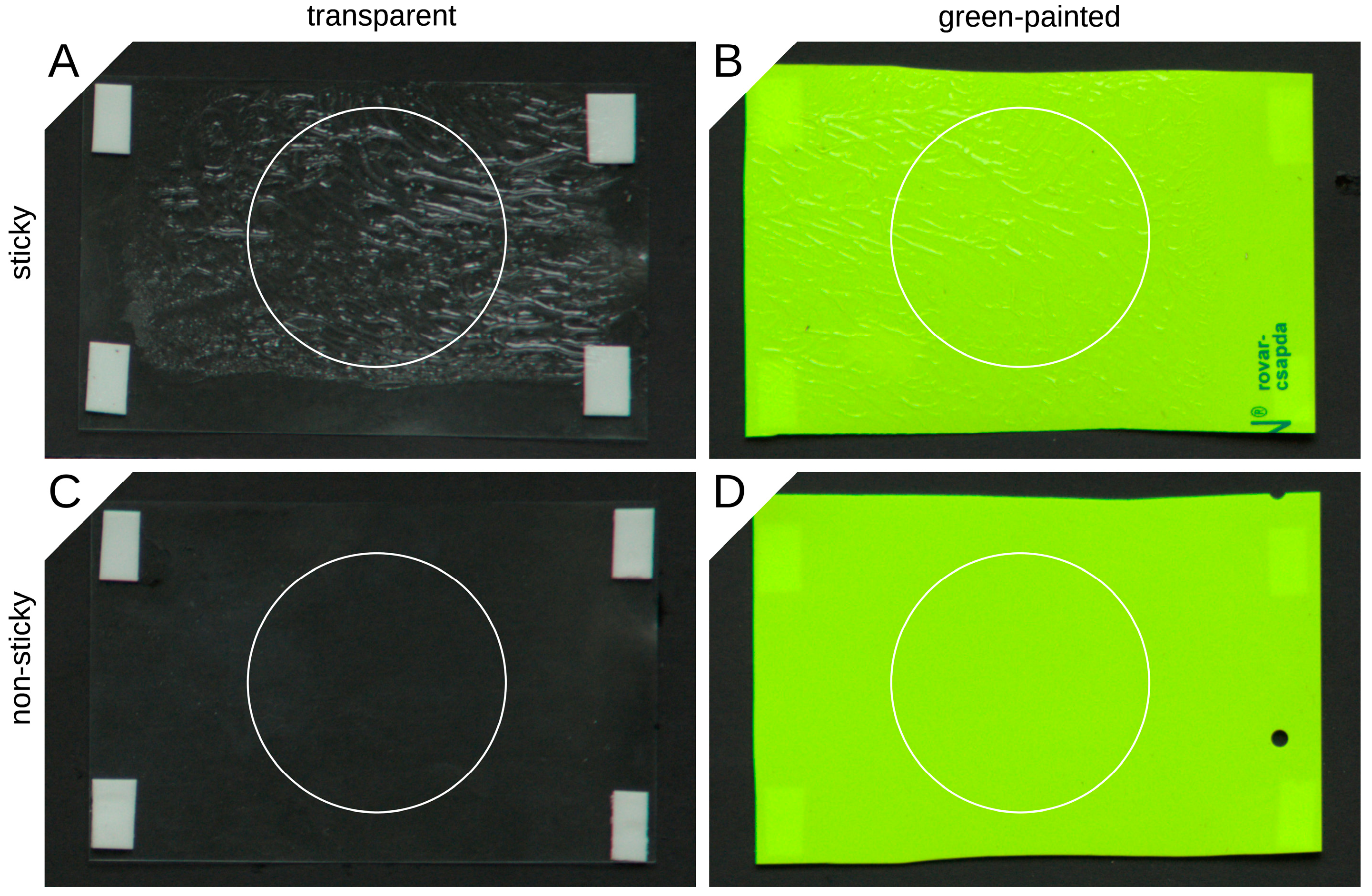
2.3. Reflectance of the Traps
2.4. Statistics
3. Results
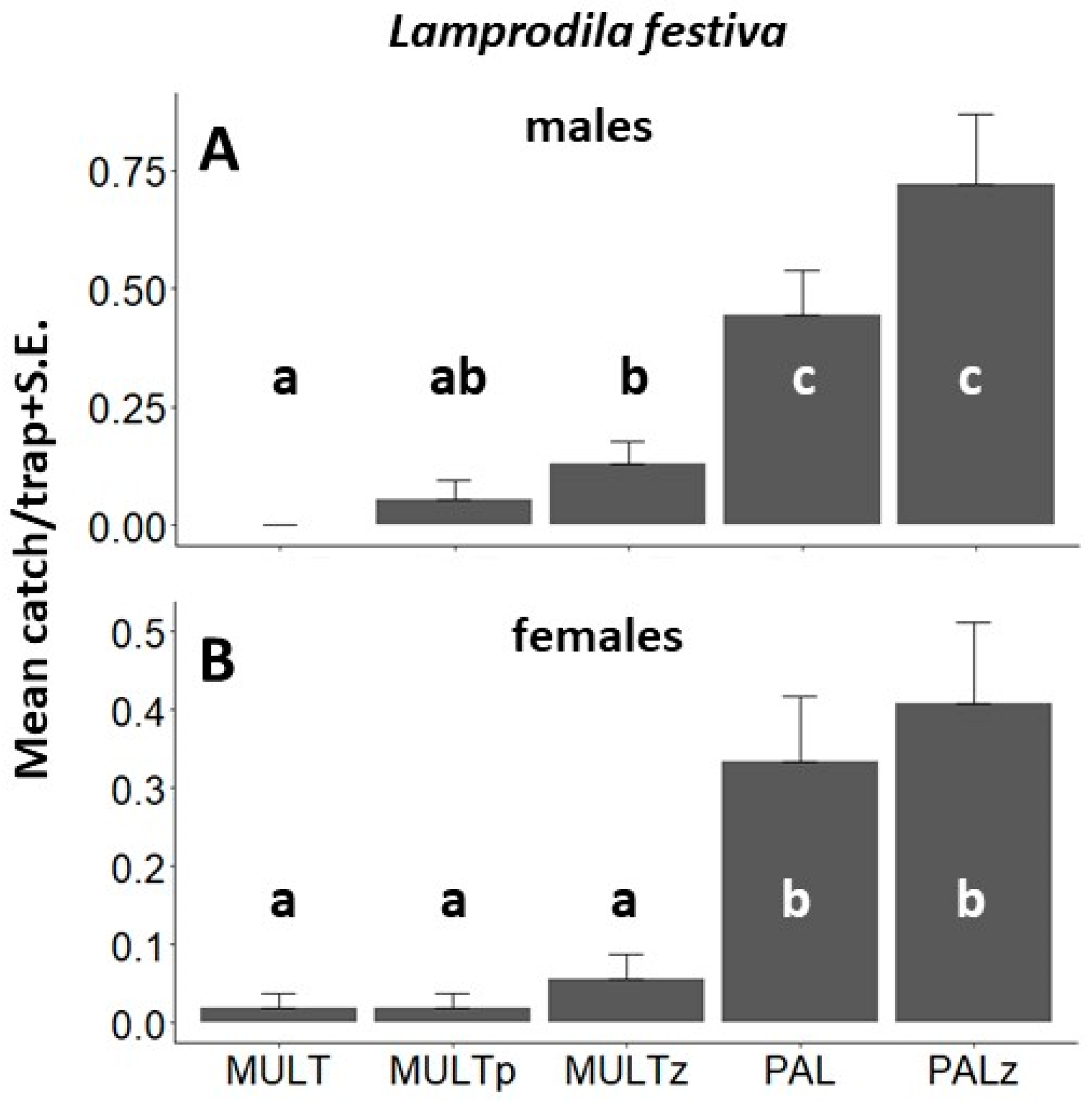
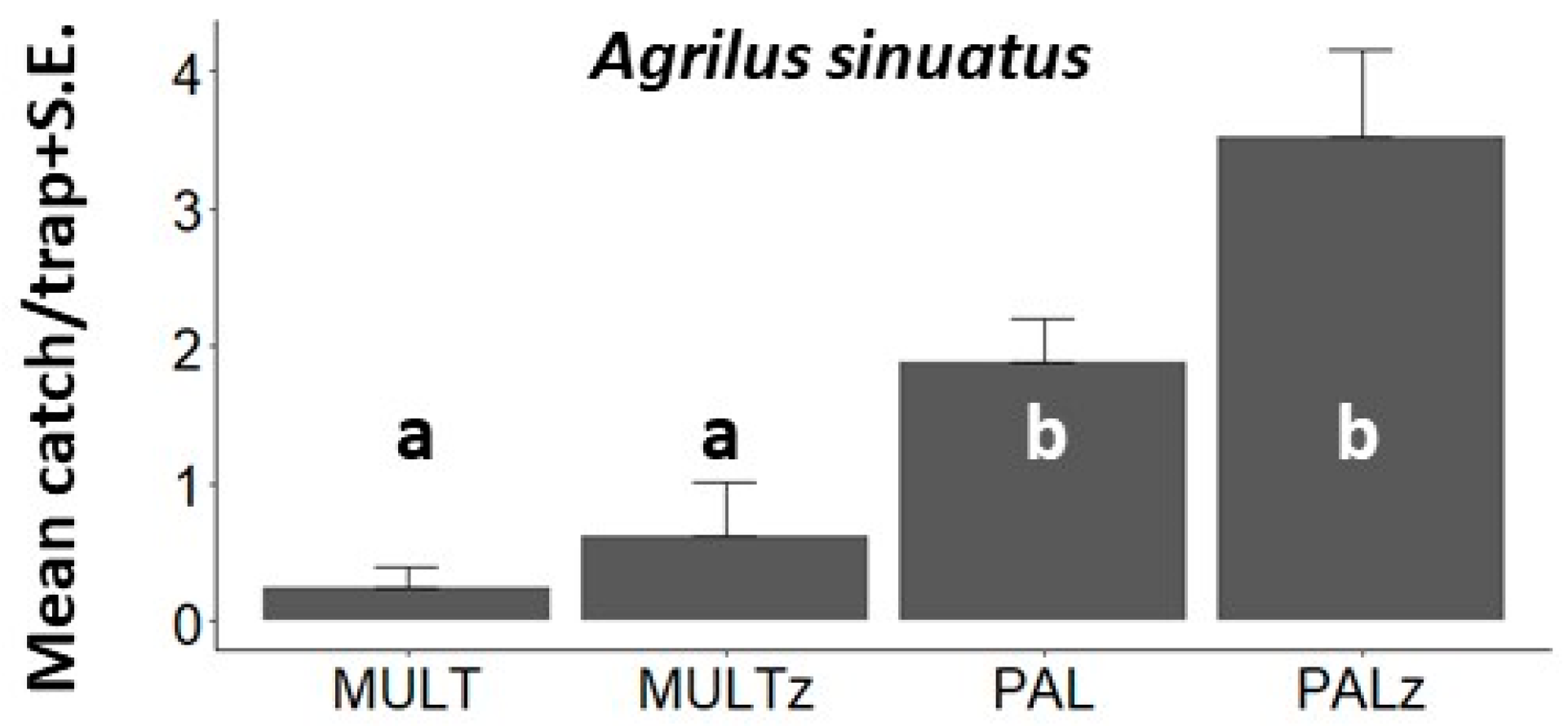
3.1. Behavioral Field Tests
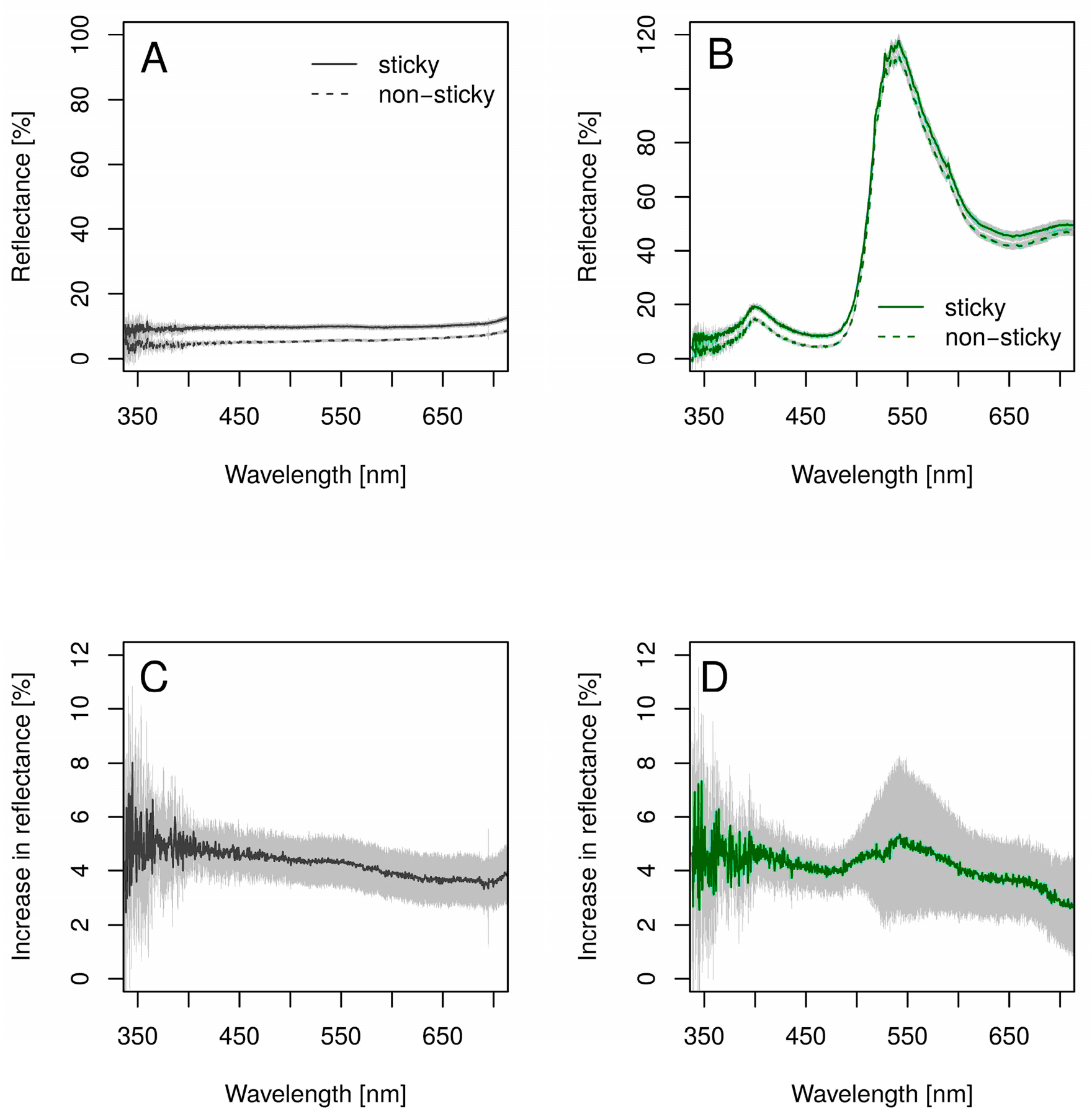
3.2. Reflectance of the Traps
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Species, Sex, Details | Kruskal-Wallis p-Value | Kruskal-Wallis χ2 (df) | MULT vs. MULTp | MULT vs. MULTz | MULT vs. PAL | MULT vs. PALz | MULTp vs. MULTz | MULTp vs. PAL | MULTp vs. PALz | MULTz vs. PAL | MULTz vs. PALz | PAL vs. PALz |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. festiva, EXP1, Tahi, 2019 | <0.001 | 35.7 (4) | 0.084 | 0.255 | 0.001 | 0.014 | <0.001 | <0.001 | <0.001 | 0.004 | 0.077 | 0.196 |
| L. festiva, EXP2, Tahi, 2020 | <0.001 | 78.6 (4) | 0.311 | 0.027 | <0.001 | <0.001 | 0.185 | <0.001 | <0.001 | <0.001 | <0.001 | 0.390 |
| L. festiva, males EXP2, Tahi, 2020 | <0.001 | 55.5 (4) | 0.159 | 0.007 | <0.001 | <0.001 | 0.093 | <0.001 | <0.001 | 0.008 | <0.001 | 0.158 |
| L. festiva, females EXP2, Tahi, 2020 | <0.001 | 35.8 (4) | 1 | 0.315 | <0.001 | <0.001 | 0.315 | <0.001 | <0.001 | 0.002 | 0.002 | 0.866 |
| A. sinuatus, EXP3, Hajdúnánás, 2021 | <0.001 | 28.0 (3) | - | 0.639 | <0.001 | <0.001 | - | - | - | 0.003 | <0.001 | 0.074 |
References
- Ruicănescu, A.; Stoica, A.I. The distribution and behaviour studies on a new invasive Buprestid species, Lamprodila festiva (Coleoptera: Buprestidae) in Romania. Trav. Du Muséum Natl. D’histoire Nat. Grigore Antipa 2019, 62, 43–55. [Google Scholar] [CrossRef]
- Jendek, E.; Poláková, J.; Szopa, R.; Kodada, J. Lamprodila (Palmar) festiva (Coleoptera, Buprestidae) a new adventive jewel beetle pest of Cupressaceae in Slovakia. Entomofauna Carpathica 2018, 30, 13–24. [Google Scholar]
- Ruseva, S.; Todorov, I.; Pencheva, A. Ovalisia (Palmar) festiva (Linnaeus) (Coleoptera: Buprestidae) and its natural enemies reported from Bulgaria. Ecol. Montenegrina 2020, 28, 53–60. [Google Scholar] [CrossRef]
- Fassotte, C.; Lateur, M.; Villette, I.; Cors, R. Le bupreste, Agrilus sinuatus ol., ravageur confirme en vergers de poiriers. Le Fruit Belg. 2004, 512, 181–187. [Google Scholar]
- Volkovitsh, M.; Karpun, N. A new invasive species of buprestid beetles in the Russian fauna: Lamprodila (Palmar) festiva (L.) (Coleoptera, Buprestidae), a pest of Cupressaceae. Entomol. Rev. 2017, 97, 425–437. [Google Scholar] [CrossRef]
- Khachikov, E.A.; Kazeev, K.S.; Poushkova, S.V. The cypress jewel beetle, Lamprodila festiva (linnaeus, 1767) (Coleoptera: Buprestidae)—A real threat to the relict juniper forests of the Black Sea coast of the Caucasus. Ross. Zhurnal Biol. Invazij—Рoссийский Журнал Биoлoгических Инвазий 2022, 15, 101. [Google Scholar] [CrossRef]
- Thoma, J.; Eickermann, M. Erstauftreten des Wacholderprachtkafers Ovalisia festiva (Linnaeus, 1767) in Luxemburg. Bull. Soc. Nat. Luxemb. 2014, 115, 227–229. [Google Scholar]
- Rabl, D.; Rabl, C.; Rabl, S. The Mediterranean distributed Cypress jewel beetle Ovalisia festiva (Linneus, 1767) has riched the east of Austria (Coleoptera: Buprestidae). Entomol. Z. Schwanf. 2017, 127, 109–111. [Google Scholar]
- Nitzu, E.; Dobrin, I.; Dumbravă, M.; Gutue, M. The range expansion of Ovalisia festiva (Linnaeus, 1767) (Coleoptera: Buprestidae) in Eastern Europe and its damaging potential for cupressaceae. Trav. Du Muséum Natl. D’histoire Nat. 2016, 58, 51–57. [Google Scholar] [CrossRef]
- López-Pérez, J.J. Presencia de Lamprodila (Palmar) festiva (Linnaeus, 1767) (Coleoptera: Buprestidae) en la provincia de Huelva (S. O. de Andalucía, España). Rev. Gaditana De Entomol. 2016, 7, 511–513. [Google Scholar]
- Pedersoli, D. Presence of Lamprodila (Palmar) festiva Linneo, 1767 in two sites of the provinces Brescia and Bergamo (Coleoptera Buprestidae Buprestinae). Nat. Brescia. Ann. Del Mus. Civ. De Sci. Nat. Di Brescia 2016, 40, 143–144. [Google Scholar]
- Kereši, T. Recent records of the cypress jewel beetle: Lamprodila (Palmar) festiva (Linnaeus, 1767) (Coleoptera: Buprestidae) in Serbia. Topola 2020, 205, 25–31. [Google Scholar] [CrossRef]
- Fassotte, C. Apprendre a connaitre le bupreste du poirier, Agrilus sinuatus, pour mieux le gerer. In Proceedings of the Journées Techniques Légumes & Cultures Pérennes Biologiques, Avignon, France, 11–13 December 2012; pp. 67–71. [Google Scholar]
- Fassotte, C. Le bupreste du poirier, Agrilus sinuatus Olivier, un ravageur resurgent de nos cultures fruitieres. Le Fruit Belg. 1999, 478, 45–50. [Google Scholar]
- Bosman, J.; Kaljee, H. Schade Door De Larve Van De Perenprachtkever: Meidoorns in De Schaduw Hebben Minder Last Van De Kever. Bomen Vakbl. Voor De Boomverzorging 2021, 56, 21–25. [Google Scholar]
- Bosman, J.; Kaljee, H. Perenprachtkever tast helft Amsterdamse meidoorns aan. Tuin Landsch. 2021, 20, 24–27. [Google Scholar]
- Imrei, Z.; Lohonyai, Z.; Muskovits, J.; Matula, E.; Vuts, J.; Fail, J.; Gould, P.J.L.; Birkett, M.A.; Toth, M.; Domingue, M.J. Developing a non-sticky trap design for monitoring jewel beetles. J. Appl. Entomol. 2020, 144, 224–231. [Google Scholar] [CrossRef]
- Musolin, D.L.; Kirichenko, N.I.; Karpun, N.N.; Aksenenko, E.V.; Golub, V.B.; Kerchev, I.A.; Mandelshtam, M.Y.; Vasaitis, R.; Volkovitsh, M.G.; Zhuravleva, E.N.; et al. Invasive Insect Pests of Forests and Urban Trees in Russia: Origin, Pathways, Damage, and Management. Forests 2022, 13, 521. [Google Scholar] [CrossRef]
- Domingue, M.J.; Imrei, Z.; Lelito, J.P.; Muskovits, J.; Janik, G.; Csóka, G.; Mastro, V.C.; Baker, T.C. Trapping of European buprestid beetles in oak forests using visual and olfactory cues. Entomol. Exp. Appl. 2013, 148, 116–129. [Google Scholar] [CrossRef]
- Imrei, Z.; Lohonyai, Z.; Csoka, G.; Muskovits, J.; Szanyi, S.; Vetek, G.; Fail, J.; Toth, M.; Domingue, M.J. Improving trapping methods for buprestid beetles to enhance monitoring of native and invasive species. Forestry 2020, 93, 254–264. [Google Scholar] [CrossRef]
- Crook, D.J.; Mastro, V.C. Chemical ecology of the emerald ash borer Agrilus planipennis. J. Chem. Ecol. 2010, 36, 101–112. [Google Scholar] [CrossRef]
- Brown, N.; Jeger, M.; Kirk, S.; Williams, D.; Xu, X.M.; Pautasso, M.; Denman, S. Acute oak decline and Agrilus biguttatus: The co-occurrence of stem bleeding and D-shaped emergence holes in Great Britain. Forests 2017, 8, 87. [Google Scholar] [CrossRef]
- Brown, N. Epidemiology of Acute oak Decline in Britain; Imperial College: London, UK, 2013. [Google Scholar]
- Graham, E.E.; Poland, T.M. Efficacy of fluon conditioning for capturing cerambycid beetles in different trap designs and persistence on panel traps over time. J. Econ. Entomol. 2012, 105, 395–401. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 October 2023).
- Wickham, H.; Francois, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. R Package Version 1.1.4. 2017. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 10 October 2023).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1999. [Google Scholar]
- Wall, C. Monitoring and Spray Timing; Wiley: New York, NY, USA, 1989; p. 369. [Google Scholar]
- Francese, J.A.; Rietz, M.L.; Mastro, V.C. Optimization of multifunnel traps for emerald ash borer (Coleoptera: Buprestidae): Influence of size, trap coating, and color. J. Econ. Entomol. 2013, 106, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- IUCN-SSC. Global Innvasive Species Database. CD-ROM. IUCN Species Survival Commission (SSC), Invasive Species Specialist Group, University of Auckland, Centre for Invasive Species Research: Auckland, New Zealand, 2000. [Google Scholar]
- Demidko, D.A.; Demidko, N.N.; Mikhaylov, P.V.; Sultson, S.M. Biological strategies of invasive bark beetles and borers species. Insects 2021, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Baranchikov, Y.; Mozolevskaya, E.; Yurchenko, G.; Kenis, M. Occurence of the emerald ash borer, Agrilus planipennis in Russia and its potential impact on European forestry. OEPP/EPPO Bull. 2008, 38, 233–238. [Google Scholar] [CrossRef]
- Straw, N.A.; Williams, D.T.; Kulinich, O.; Gninenko, Y.I. Distribution, impact and rate of spread of emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae) in the Moscow region of Russia. Forestry 2013, 86, 515–522. [Google Scholar] [CrossRef]
- Chamorro, M.L.; Jendek, E.; Haack, R.A.; Petrice, T.R.; Woodley, N.E.; Konstantinov, A.S.; Volkovitsh, M.G.; Yang, X.K.; Grebennikov, V.V.; Lingafelter, S.W. Illustrated guide to the emerald ash borer Agrilus planipennis Fairmaire and related species (Coleoptera, Buprestidae). Available online: https://www.fs.usda.gov/treesearch/pubs/49163 (accessed on 10 October 2023).
- Haack, R.A.; Jendek, E.; Liu, H.P.; Marchant, K.R.; Petrice, T.R.; Poland, T.M.; Ye, H. The emerald ash borer: A new exotic pest in North America. Newsl. Michigan Entomol. Soc. 2002, 47, 15. [Google Scholar]
- Lelito, J.P.; Fraser, I.; Mastro, V.C.; Tumlinson, J.H.; Böröczky, K.; Baker, T.C. Visually mediated ‘paratrooper copulations’ in the mating behavior of Agrilus planipennis (Coleoptera: Buprestidae), a highly destructive invasive pest of North American ash trees. J. Insect Behav. 2007, 20, 537–552. [Google Scholar] [CrossRef]
- Lelito, J.P.; Domingue, M.J.; Fraser, I.; Mastro, V.C.; Tumlinson, J.H.; Baker, T.C. Field investigation of mating behaviour of Agrilus cyanescens and Agrilus subcinctus. Can. Entomol. 2011, 143, 370–379. [Google Scholar] [CrossRef]
- Domingue, M.J.; Csóka, G.; Tóth, M.; Vétek, G.; Pénzes, B.; Mastro, V.; Baker, T.C. Field observations of visual attraction of three European oak buprestid beetles toward conspecific and heterospecific models. Entomol. Exp. Appl. 2011, 140, 112–121. [Google Scholar] [CrossRef]
- Bartelt, R.J.; Cosse, A.A.; Zilkowski, B.W.; Fraser, I. Antennally active macrolide from the emerald ash borer Agrilus planipennis emitted predominantly by females. J. Chem. Ecol. 2007, 33, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Silk, P.; Ryall, K. Semiochemistry and chemical ecology of the emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae). Can. Entomol. 2015, 147, 277–289. [Google Scholar] [CrossRef]
- Ryall, K.L.; Silk, P.J.; Fidgen, J.; Mayo, P.; Lavallee, R.; Guertin, C.; Scarr, T. Effects of pheromone release rate and trap placement on trapping of Agrilus planipennis (Coleoptera: Buprestidae) in Canada. Environ. Entomol. 2015, 44, 734–745. [Google Scholar] [CrossRef]
- Ryall, K.L.; Silk, P.J.; Mayo, P.; Crook, D.; Khrimian, A.; Cosse, A.A.; Sweeney, J.; Scarr, T. Attraction of Agrilus planipennis (Coleoptera: Buprestidae) to a volatile pheromone: Effects of release rate, host volatile, and trap placement. Environ. Entomol. 2012, 41, 648–656. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matula, E.; Bozsik, G.; Muskovits, J.; Ruszák, C.; Jávorszky, L.; Bonte, J.; Paulin, M.; Vuts, J.; Fail, J.; Tóth, Á.; et al. The Optimal Choice of Trap Type for the Recently Spreading Jewel Beetle Pests Lamprodila festiva and Agrilus sinuatus (Coleoptera, Buprestidae). Insects 2023, 14, 961. https://doi.org/10.3390/insects14120961
Matula E, Bozsik G, Muskovits J, Ruszák C, Jávorszky L, Bonte J, Paulin M, Vuts J, Fail J, Tóth Á, et al. The Optimal Choice of Trap Type for the Recently Spreading Jewel Beetle Pests Lamprodila festiva and Agrilus sinuatus (Coleoptera, Buprestidae). Insects. 2023; 14(12):961. https://doi.org/10.3390/insects14120961
Chicago/Turabian StyleMatula, Eszter, Gábor Bozsik, József Muskovits, Csenge Ruszák, Laura Jávorszky, Jochem Bonte, Márton Paulin, József Vuts, József Fail, Ágoston Tóth, and et al. 2023. "The Optimal Choice of Trap Type for the Recently Spreading Jewel Beetle Pests Lamprodila festiva and Agrilus sinuatus (Coleoptera, Buprestidae)" Insects 14, no. 12: 961. https://doi.org/10.3390/insects14120961
APA StyleMatula, E., Bozsik, G., Muskovits, J., Ruszák, C., Jávorszky, L., Bonte, J., Paulin, M., Vuts, J., Fail, J., Tóth, Á., Egri, Á., Tóth, M., & Imrei, Z. (2023). The Optimal Choice of Trap Type for the Recently Spreading Jewel Beetle Pests Lamprodila festiva and Agrilus sinuatus (Coleoptera, Buprestidae). Insects, 14(12), 961. https://doi.org/10.3390/insects14120961









