The Sex Ratio Indicates the Conclusion and Onset of Population Cycles in the Beet Webworm Loxostege sticticalis L. (Lepidoptera: Pyralidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Testing the Mating Potential of Male Beet Webworms
2.3. Testing the Mating Potential of Female Beet Webworms
2.4. Effects of Sex Ratio on Reproduction and Longevity of Adults
2.5. Reproductive Parameters
2.6. Data Analysis
3. Results
3.1. Mating Potential
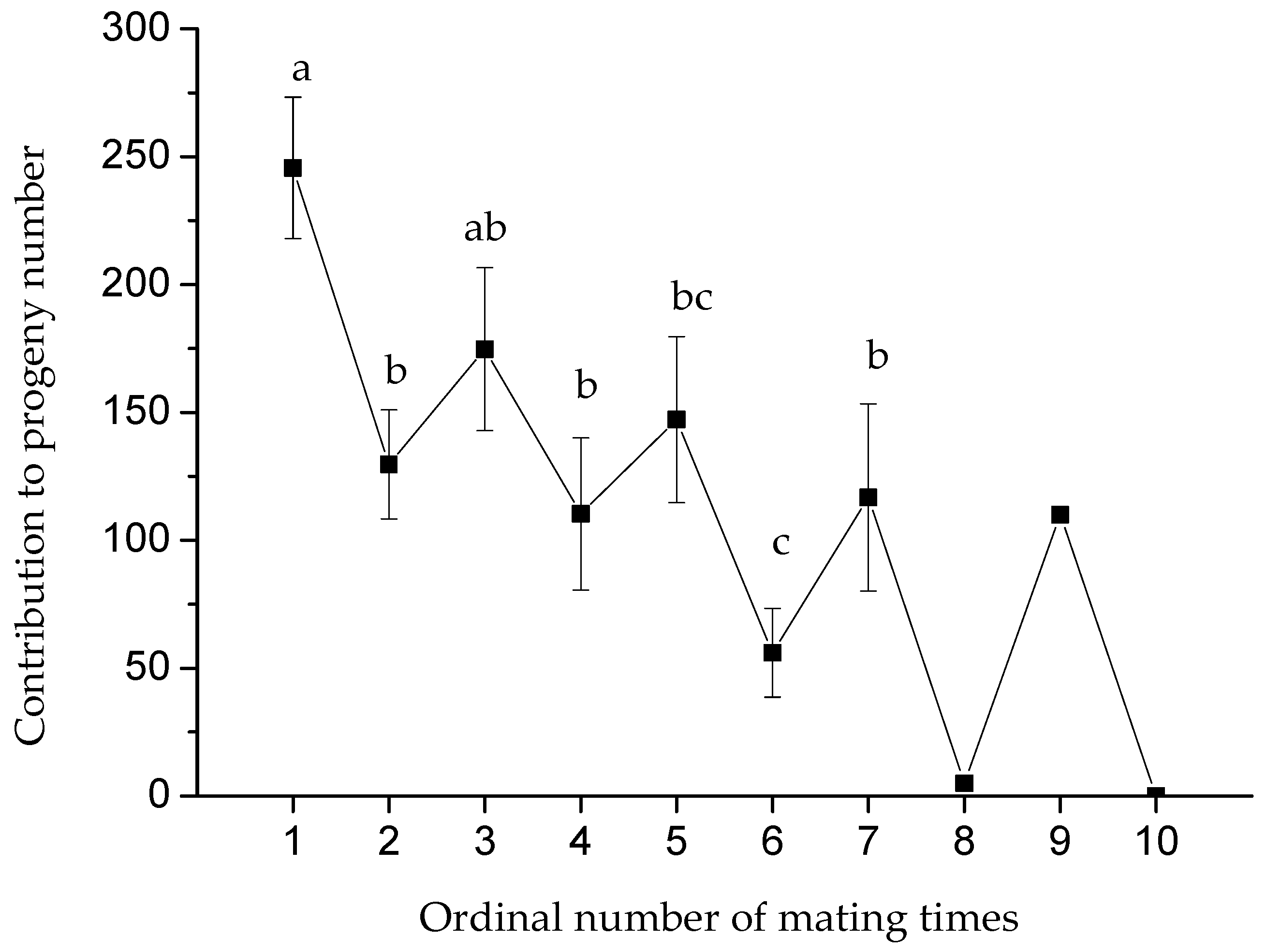
3.2. Effect of Maximum Mating Frequency on the Number of Progenies
3.3. Effect of the Sex Ratio on the Reproduction of Beet Webworms
4. Discussion
4.1. Mating Potential and Multiple Mating in Beet Webworms
4.2. Sex Ratio and Population Outbreaks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Hardy, I.C.W. Sex Ratios: Concepts and Research Methods; Cambridge University Press: New York, USA, 2002. [Google Scholar]
- Traut, W.; Sahara, K.; Marec, F. Sex chromosomes and sex determination in Lepidoptera. Sex Dev. 2007, 1, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Hake, L.; O’Connor, C. Genetic mechanisms of sex determination. Nat. Educ. 2008, 1, 25. [Google Scholar]
- Sanchez, L. Sex-determining mechanisms in insects. Int. J. Dev. Biol. 2008, 52, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Kwan, L.; Bedhomme, S.; Prasad, N.G.; Chippindale, A.K. Sexual conflict and environmental change: Trade-offs within and between the sexes during the evolution of desiccation resistance. J. Genet. 2008, 87, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, K.; Morse, S.; Gruwell, M.; Mayberry, J.; DiBlasi, E. Spatial and temporal complexities of reproductive behavior and sex ratios: A case from parasitic insects. PLoS ONE 2011, 6, e19438. [Google Scholar] [CrossRef]
- Lachowsky, L.E.; Reid, M.L. Developmental mortality increases sex-ratio bias of a size-dimorphic bark beetle. Ecol. Entomol. 2014, 39, 300–308. [Google Scholar] [CrossRef]
- Atlan, A.; Joly, D.; Capillon, C.; Montchamp-Moreau, C. Sex-ratio distorter of Drosophila simulans reduces male productivity and sperm competition ability. J. Evol. Biol. 2004, 17, 744–751. [Google Scholar] [CrossRef]
- Kageyama, D.; Nishimura, G.; Hoshizaki, S.; Ishikawa, Y. Two kinds of sex ratio distorters in a moth, Ostrinia scapulalis. Genome 2003, 46, 974–982. [Google Scholar] [CrossRef]
- Paini, D.R.; Bailey, W.J. Seasonal sex ratio and unbalanced investment sex ratio in the Banksia bee Hylaeus alcyoneus. Ecol. Entomol. 2002, 27, 713–719. [Google Scholar] [CrossRef]
- Perez-Mendoza, J.; Campbell, J.F.; Throne, J.E. Influence of age, mating status, sex, quantity of food, and long-term food deprivation on red flour beetle (Coleoptera: Tenebrionidae) flight initiation. J. Econ. Entomol. 2011, 104, 2078–2086. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, L.; Jiang, X.; Sappington, T.W. Synchronized oviposition triggered by migratory flight intensifies larval outbreaks of beet webworm. PLoS ONE 2012, 7, e31562. [Google Scholar] [CrossRef]
- Moiroux, J.; Brodeur, J.; Boivin, G. Sex ratio variations with temperature in an egg parasitoid: Behavioural adjustment and physiological constraint. Anim. Behav. 2014, 91, 61–66. [Google Scholar] [CrossRef]
- Wehi, P.M.; Nakagawa, S.; Trewick, S.A.; Morgan-Richards, M. Does predation result in adult sex ratio skew in a sexually dimorphic insect genus? J. Evol. Biol. 2011, 24, 2321–2328. [Google Scholar] [CrossRef]
- Mopper, S.; Whitham, T.G. The plant stress paradox: Effects on pinyon sawfly sex ratios and fecundity. Ecology 1992, 73, 515–525. [Google Scholar] [CrossRef]
- Schenkel, M.A.; Billeter, J.C.; Beukeboom, L.W.; Pen, I.D. Divergent evolution of genetic sex determination mechanisms along environmental gradients. Evol. Lett. 2023, 7, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.M. Sex determination in invertebrates. In Sex Ratios Concepts and Research Methods; Hardy, I.C.W., Ed.; Cambridge University Press: New York, NY, USA, 2002; pp. 178–194. [Google Scholar]
- Jiggins, F.M.; Hurst, G.D.D.; Majerus, M.E.N. Sex ratio distortion in Acraea encedon (Lepidoptera: Nymphalidae) is caused by a male-killing bacterium. Heredity 1998, 81, 87–91. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, M. A preliminary study on sex ratios of insects. Acta Agr. Univ. Jiangxiensis 1986, 8–13. [Google Scholar]
- Beranek, C.T.; Maynard, C.; McHenry, C.; Clulow, J.; Mahony, M. Identifying a limiting factor in the population dynamics of a threatened amphibian: The influence of extended female maturation on operational sex ratio. Austral. Ecol. 2022, 47, 239–250. [Google Scholar] [CrossRef]
- Kvarnemo, C.; Ahnesjo, I. The dynamics of operational sex ratios and competition for mates. Trends Ecol. Evol. 1996, 11, 404–408. [Google Scholar] [CrossRef]
- Rodríguez-Muñoz, R.; Boonekamp, J.J.; Fisher, D.; Hopwood, P.; Tregenza, T. Slower senescence in a wild insect population in years with a more female-biased sex ratio. Proc. R. Soc. B-Biol. Sci. 2019, 286, 20190286. [Google Scholar] [CrossRef]
- Radhakrishnan, P.; Fedorka, K.M. Influence of female age, sperm senescence and multiple mating on sperm viability in female Drosophila melanogaster. J. Insect Physiol. 2011, 57, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Mellström, H.L.; Wiklund, C. What affects mating rate? Polyandry is higher in the directly developing generation of the butterfly Pieris napi. Anim. Behav. 2010, 80, 413–418. [Google Scholar] [CrossRef]
- Hayashi, F. Multiple mating and lifetime reproductive output in female dobsonflies that receive nuptial gifts. Ecol. Res. 2010, 13, 283–289. [Google Scholar] [CrossRef]
- Pai, A.; Bennett, L.; Yan, G. Female multiple mating for fertility assurance in red flour beetles (Tribolium castaneum). Can. J. Zool. 2005, 83, 913–919. [Google Scholar] [CrossRef]
- Dunn, D.W.; Sumner, J.P.; Goulson, D. The benefits of multiple mating to female seaweed flies, Coelopa frigida (Diptera: Coelpidae). Behav. Ecol. Sociobiol. 2005, 58, 128–135. [Google Scholar] [CrossRef]
- de Guzman, L.I.; Rinderer, T.E.; Frake, A.M. The effects of diet, mating duration, female to male ratios, and temperature on ovary activation, mating success, and fecundity of Aethina tumida. Apidologie 2015, 46, 326–336. [Google Scholar] [CrossRef]
- Jiménez-Pérez, A.; Wang, Q. Male remating behavior and its effect on female reproductive fitness in Cnephasia jactatana Walker (Lepidoptera: Tortricidae). J. Insect Behav. 2004, 17, 685–694. [Google Scholar] [CrossRef]
- Fitz-Earle, M.; Barclay, H.J. Is there an optimal sex ratio for insect mass rearing? Ecol. Model. 1989, 45, 205–220. [Google Scholar] [CrossRef]
- Knor, I.B.; Bashev, A.N.; Alekseev, A.A.; Kirov, E.I. Effect of population density on the dynamics of the beet webworm Loxostege sticticalis L. (Lepidoptera: Pyralidae). Entomol. Rev. 1993, 72, 117–124. [Google Scholar]
- Luo, L.; Li, G. The threshold temperature, thermal constant and division of generation regions of meadow moth (Loxostege sticticalis L.) in China. Acta Entomol. Sin. 1993, 36, 332–339. [Google Scholar]
- Pepper, J.H. The effect of certain climate factors on the distribution of the beet webworm (Loxostege sticticalis L.) in North American. Ecology 1938, 19, 565–571. [Google Scholar] [CrossRef]
- Chen, X.; Hao, L.; Jiang, Y.; Zhai, B. Spatio-temporal dynamics of meadow moth outbreaks in Eurasia over the past 100 years. Chin. J. Appl. Entomol. 2022, 59, 375–385. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, H.; Kang, A. The causes for the outbreak of the first generation of larval beet webworm, Loxostege sticticalis in Zhangjiakou during 1997. J. Nat. Disast. 1998, 7, 158–164. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Hayat, M.; George, S. Factors influencing mating success, mating frequency, and fecundity in Phthorimaea operculella (Lepidoptera: Gelechiidae). Environ. Entomol. 2001, 30, 31–36. [Google Scholar]
- Azrag, A.G.A.; Ndlela, S.; Mkiga, A.M.; Mohamed, S.A. Mating frequency of female false codling moth, Thaumatotibia leucotreta (Lepidoptera: Tortricidae): Trade-off between fecundity and longevity. J. Insect Behav. 2021, 34, 319–333. [Google Scholar] [CrossRef]
- Liu, X.P.; He, H.M.; Xue, F.S. The effect of mating frequency and mating pattern on female reproductive fitness in cabbage beetle, Colaphellus bowringi. Entomol. Exp. Appl. 2013, 146, 379–385. [Google Scholar] [CrossRef]
- Kolodziejczyk, M.; Radwan, J. The effect of mating frequency on female lifetime fecundity in the bulb mite, Rhizoglyphus robini (Acari: Acaridae). Behav. Ecol.y Sociobiol. 2003, 53, 110–115. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, K.; Sappington, T.W.; Luo, L.; Jiang, X. Response of reproductive traits and longevity of beet webworm to temperature, and implications for migration. J. Insect Sci. 2015, 15, 154. [Google Scholar] [CrossRef]
- Price, T.A.R.; Bretman, A.J.; Avent, T.D.; Snook, R.R.; Hurst, G.D.D.; Wedell, N. Sex ratio distorter reduces sperm competitive ability in an insect. Evolution 2008, 62, 1644–1652. [Google Scholar] [CrossRef]
- Royer, L.; McNeil, J. Male investment in the european corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae): Impact on female longevity and reproductive performance. Funct. Ecol. 1993, 7, 209–215. [Google Scholar] [CrossRef]
- Outram, I. Aspects of mating in the spruce budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae). Can. Entomol. 1971, 103, 1121–1128. [Google Scholar] [CrossRef]
- Kong, H.; Cheng, Y.; Luo, L.; Sappington, T.W.; Jiang, X.; Zhang, L. Density-dependent prophylaxis in crowded beet webworm, Loxostege sticticalis (Lepidoptera: Pyralidae) larvae to a parasitoid and a fungal pathogen. Int. J. Pest Manag. 2013, 59, 174–179. [Google Scholar] [CrossRef]
- Li, M. The Influences of Larval Rearing Density, Temperature, and Sex Ratio on the Development and Reproduction of Beet Webworm, Loxostege sticticalis L. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2014. [Google Scholar]
- Parkash, R.; Lambhod, C. Plastic changes in cold and drought tolerance of Drosophila nepalensis correlate with sex-specific differences in body melanization, cuticular lipid mass, proline accumulation, and seasonal abundance. Comp. Biochem. Phys. A. 2021, 258, 110985. [Google Scholar] [CrossRef]
- Niveditha, S.; Deepashree, S.; Ramesh, S.R.; Shivanandappa, T. Sex differences in oxidative stress resistance in relation to longevity in Drosophila melanogaster. J. Comp. Physiol. B. 2017, 187, 899–909. [Google Scholar] [CrossRef]
- Parkash, R.; Ranga, P. Sex-specific divergence for adaptations to dehydration stress in Drosophila kikkawai. J. Exp. Biol. 2013, 216, 3301–3313. [Google Scholar] [CrossRef][Green Version]
- Wang, X.H.; Kang, L. Molecular mechanisms of phase change in locusts. Annu. Rev. Entomol. 2014, 59, 225–244. [Google Scholar] [CrossRef]
- Narasimhan, A.; Jigisha, N.G.; Kapila, R.; Meena, A.; Santhosh; Prasad, N.G. Consequences of adaptation to larval crowding on sexual and fecundity selection in Drosophila melanogaster. J. Evol. Biol. 2023, 36, 730–737. [Google Scholar] [CrossRef]


| Sex | Maximum Mating Frequency | Number of Progenies | Net Reproductive Rate |
|---|---|---|---|
| Female | 1.70 ± 0.15 * | 154.96 ± 22.57 * | 56.18 ± 7.49 |
| Male | 4.95 ± 0.49 | 461.05 ± 79.05 | 73.59 ± 9.92 |
| Sex Ratio (Female vs. Male) | Progeny per Female | Progeny per Male | Hatching Rate (%) | Female Mating Frequency | Male Mating Frequency |
|---|---|---|---|---|---|
| 3:1 | 171.62 ± 22.81 a | 514.86 ± 68.44 a | 83.40 ± 1.17 a | 1.00 ± 0.10 c | 3.00 ± 0.30 a |
| 2:1 | 246.65 ± 12.00 a | 493.30 ± 24.00 a | 84.80 ± 1.93 a | 1.23 ± 0.05 bc | 2.45 ± 0.09 a |
| 1:1 | 270.29 ± 27.12 a | 270.29 ± 27.12 b | 85.00 ± 1.78 a | 1.29 ± 0.14 b | 1.29 ± 0.14 b |
| 1:2 | 315.85 ± 37.16 a | 157.92 ± 18.73 bc | 86.60 ± 1.03 a | 1.3 ± 0.05 b | 0.65 ± 0.03 bc |
| 1:3 | 183.36 ± 71.42 a | 61.12 ± 23.80 c | 88.00 ± 2.68 a | 1.58 ± 0.08 a | 0.56 ± 0.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Hu, M.; Kang, A.; Xiao, Y.; Luo, L.; Jiang, X. The Sex Ratio Indicates the Conclusion and Onset of Population Cycles in the Beet Webworm Loxostege sticticalis L. (Lepidoptera: Pyralidae). Insects 2023, 14, 781. https://doi.org/10.3390/insects14100781
Cheng Y, Hu M, Kang A, Xiao Y, Luo L, Jiang X. The Sex Ratio Indicates the Conclusion and Onset of Population Cycles in the Beet Webworm Loxostege sticticalis L. (Lepidoptera: Pyralidae). Insects. 2023; 14(10):781. https://doi.org/10.3390/insects14100781
Chicago/Turabian StyleCheng, Yunxia, Min Hu, Aiguo Kang, Yonghong Xiao, Lizhi Luo, and Xingfu Jiang. 2023. "The Sex Ratio Indicates the Conclusion and Onset of Population Cycles in the Beet Webworm Loxostege sticticalis L. (Lepidoptera: Pyralidae)" Insects 14, no. 10: 781. https://doi.org/10.3390/insects14100781
APA StyleCheng, Y., Hu, M., Kang, A., Xiao, Y., Luo, L., & Jiang, X. (2023). The Sex Ratio Indicates the Conclusion and Onset of Population Cycles in the Beet Webworm Loxostege sticticalis L. (Lepidoptera: Pyralidae). Insects, 14(10), 781. https://doi.org/10.3390/insects14100781







