Cold Acclimation and Supercooling Capacity of Agasicles hygrophila Adults
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Host Plants and Insects
2.2. The SCP and FP of Different A. hygrophila Life History Stages
2.3. Determination of the Identification Temperature of A. hygrophila
2.4. Effects of Rapid Cold Hardening on A. hygrophila Viability
2.5. Persistence of Rapid Cold Resistance
2.6. Effects of Different Acclimation Methods on the Cold Tolerance of A. hygrophila
2.7. Effects of Cold Acclimation on A. hygrophila Longevity
2.8. Data Analysis
3. Results
3.1. The SCP and FP of Different A. hygrophila Life History Stages
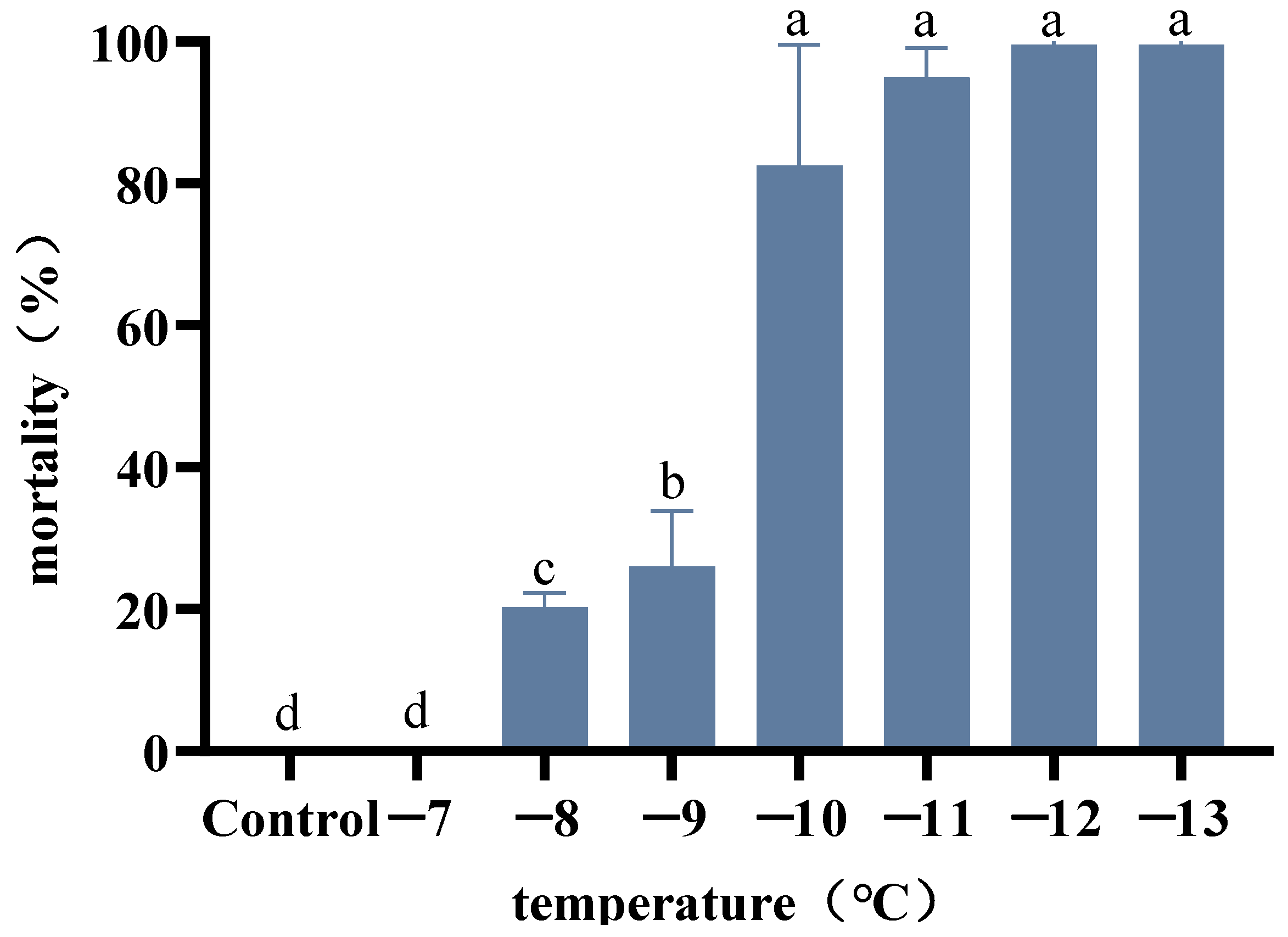
3.2. Determination of the Identification Temperature of A. hygrophila
3.3. Effects of Rapid Cold Hardening on A. hygrophila Viability
3.4. Persistence of Rapid Cold Resistance
3.5. Effects of Different Acclimation Methods on the Cold Tolerance of A. hygrophila
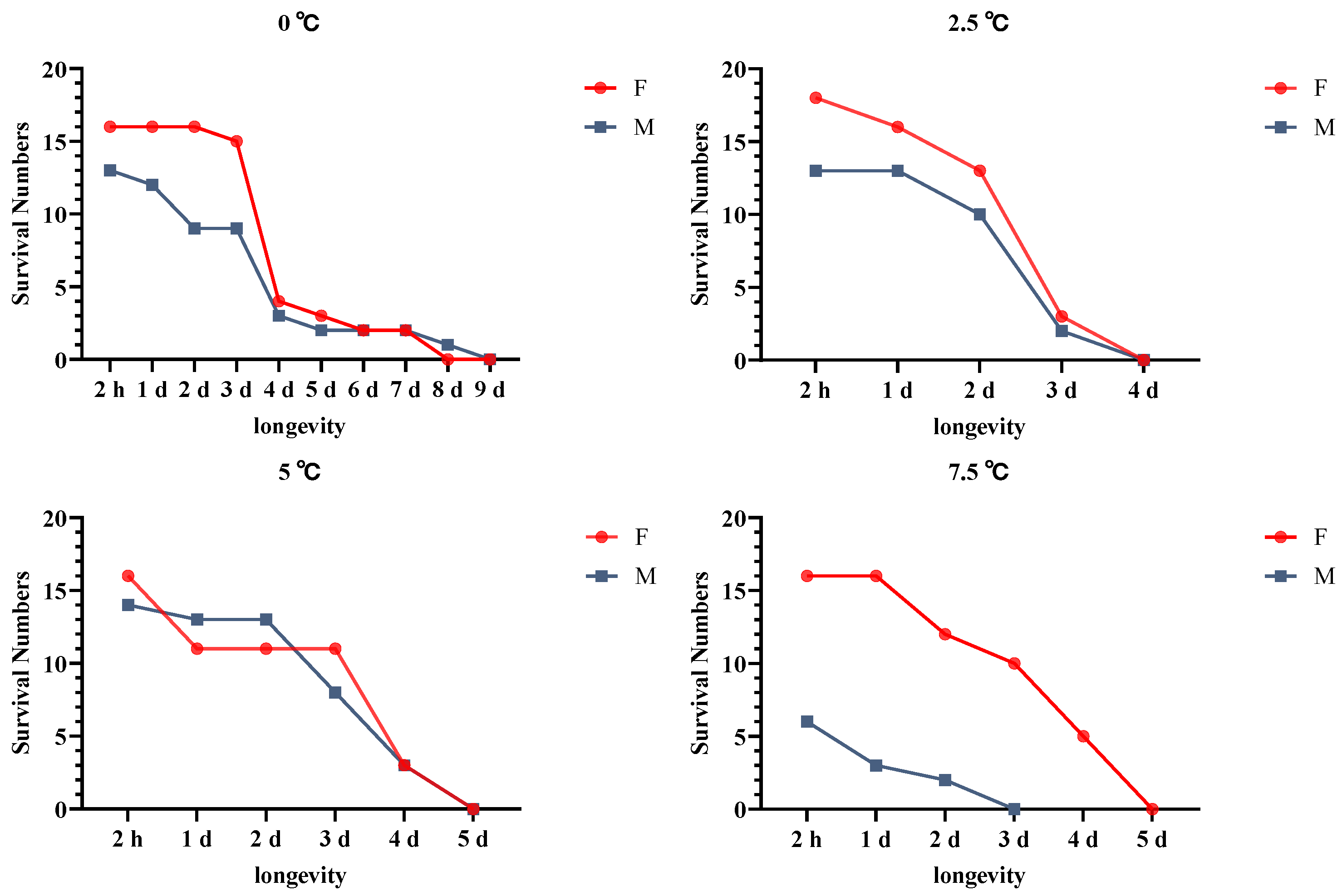
3.6. Effects of Cold Acclimation on A. hygrophila Longevity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bowler, K. Acclimation, heat shock and hardening. J. Therm. Biol. 2005, 30, 125–130. [Google Scholar] [CrossRef]
- Shintani, Y.; Ishikawa, Y. Relationship between rapid cold-hardening and cold acclimation in the eggs of the yellow-spotted longicorn beetle, Psacothea hilaris. J. Insect Physiol. 2007, 53, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E., Jr.; Chen, C.P.; Denlinger, D.L. A rapid cold-hardening process in insects. Science 1987, 238, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Bale, J.S.; Hayward, S.A. Insect overwintering in a changing climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef]
- Coulson, S.J.; Bale, J.S. Characterisation and limitation of the rapid cold hardening response in the house fly Musca domestica. J. Insect Physiol. 1990, 36, 207–211. [Google Scholar] [CrossRef]
- Bai, Y.; Dong, J.J.; Guan, D.L.; Xie, J.Y.; Xu, S.Q. Geographic variation in wing size and shape of the grasshopper Trilophidia annulata (Orthoptera: Oedipodidae): Morphological trait variations follow an ecogeographical rule. Sci. Rep. 2016, 6, 32680. [Google Scholar] [CrossRef] [PubMed]
- Ayrinhac, A.; Debat, V.; Gibert, P.; Gkister, A.; Legout, H.; Moreteau, B.; Vergilino, R.; David, J.R. Cold adaptation in geographical populations of Drosophila melanogaster: Phenotypic plasticity is more important than genetic variability. Funct. Ecol. 2004, 18, 700–706. [Google Scholar] [CrossRef]
- Broufas, G.D.; Koveos, D.S. Rapid cold hardening in the predatory mite Euseius (Amblyseius) finlandicus (Acari: Phytoseiidae). J Insect Physiol. 2001, 47, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Ju, R.T.; Xiao, Y.Y.; Li, B. Rapid cold hardening increases cold and chilling tolerances more than acclimation in the adults of the sycamore lace bug, Corythucha ciliata (Say) (Hemiptera: Tingidae). J. Insect. Physiol. 2011, 57, 1577–1582. [Google Scholar] [CrossRef]
- Burks, C.S.; Hagstrum, D.W. Rapid cold hardening capacity in five species of coleopteran pests of stored grain. J. Stored Prod. Res. 1999, 35, 65–75. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Flinn, P.W. Survival of Rhyzopertha dominica (Coleoptera: Bostrichidae) in stored wheat under fall and winter conditions. Environ. Entomol. 1994, 23, 390–395. [Google Scholar] [CrossRef]
- Smith, L.B. Effects of cold-acclimation on supercooling and survival of the rusty grain beetle, Cryptolestes ferrugineus (Stephens) (Coleoptera: Cucujidae) at sub-zero temperatures. Can. J. Zool. 1970, 48, 853–858. [Google Scholar] [CrossRef]
- Abdelghany, A.; Suthisut, D.; Fields, P. The effect of diapause and cold acclimation on the cold-hardiness of the warehouse beetle, Trogoderma variabile (Coleoptera: Dermestidae). Can. Entomol. 2015, 147, 158–168. [Google Scholar] [CrossRef]
- Lee, R.E.; Denlinger, D.L. Cold tolerance in diapausing and non-diapausing stages of the flesh fly, Sarcophaga crassipalpis. Physiol. Entomol. 1985, 10, 309–315. [Google Scholar] [CrossRef]
- Lee, R.E.; Elnitsky, M.A.; Rinehart, J.P.; Hayward, S.A.L.; Sandro, L.H.; Denlinger, D.L. Rapid cold-hardening increases the freezing tolerance of the Antarctic midge Belgica antarctica. J. Exp. Biol. 2006, 209, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kawarasaki, Y.; Teets, N.M.; Denlinger, D.L.; Lee, R.E. The protective effect of rapid cold-hardening develops more quickly in frozen versus supercooled larvae of the Antarctic midge, Belgica antarctica. J. Exp. Biol. 2013, 216, 3937–3945. [Google Scholar] [CrossRef] [PubMed]
- Kawarasaki, Y.; Teets, N.M.; Denlinger, D.L.; Lee, R.E. Alternative overwintering strategies in an Antarctic midge: Freezing vs. cryoprotective dehydration. Funct. Ecol. 2014, 28, 933–943. [Google Scholar] [CrossRef]
- Julien, M.H.; Skarratt, B.; Maywald, G.F. Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. J. Aquat. Plant Manag. 1995, 33, 55–60. [Google Scholar]
- Ma, R.Y.; Wang, R. Invasive mechanism and biological control of alligator weed, Alternanthera philoxeroides (Amaranthaceae), China. Chin. J. Appl. Environ. Biol. 2005, 11, 246–250. [Google Scholar]
- Buckingham, G.R.; Doucias, D.; Theriot, R.F. Reintroduction of the alligatorweed flea beetle (Agasicles hygrophila Selman and Vogt) into the United States from Argentina. J. Aquat. Plant Manag. 1983, 21, 101–102. [Google Scholar]
- Stewart, C.A.; Emberson, R.M.; Syrett, P. Temperature effects on the alligator weed flea-beetle, Agasicles hygrophila (Coleoptera: Chrysomelidae): Implications for biological control in New Zealand. In Proceedings of the IX International Symposium on Biological Control of Weeds, Stellenbosch, South Africa, 19–26 January 1996; pp. 393–398. [Google Scholar]
- Zhao, L.L.; Jia, D.; Yuan, X.S.; Guo, Y.Q.; Zhou, W.W.; Ma, R.Y. Cold hardiness of the biological control agent, Agasicles hygrophila, and implications for its potential distribution. Biol. Contr. 2015, 87, 1–5. [Google Scholar] [CrossRef]
- Bonnot, G.; Peypelut, L.; Febvay, G.; Lavenseau, L.; Fleurat-Lessard, F.; Fields, P.G. The effect of cold acclimation and deacclimation on cold tolerance, trehalose and free amino acid levels in Sitophilus granarius and Cryptolestes ferrugineus (Coleoptera). J. Insect Physiol. 1998, 44, 955–965. [Google Scholar] [PubMed]
- Scharf, I.; Segal, D.; Bar, A.; Gottlieb, D. Negative effects of fluctuating temperatures around the optimal temperature on reproduction and survival of the red flour beetle. J. Therm. Biol. 2022, 103, 103165. [Google Scholar] [CrossRef] [PubMed]
- Howe, R.W. A summary of estimates of optimal and minimal conditions for population increase of some stored products insects. J. Stored Prod. Res. 1965, 1, 177–184. [Google Scholar] [CrossRef]
- Lutterschmidt, W.I.; Hutchison, V.H. The critical thermal maximum: History and critique. Can. J. Zool. 1997, 75, 1561–1574. [Google Scholar] [CrossRef]
- Toxopeus, J.; Sinclair, B.J. Mechanisms underlying insect freeze tolerance. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1891–1914. [Google Scholar] [CrossRef] [PubMed]
- Bale, J.S. Classes of insect cold hardiness. Funct. Ecol. 1993, 7, 751–753. [Google Scholar]
- Sinclair, B.J. Insect cold tolerance: How many kinds of frozen? Eur. J. Entomol. 1999, 96, 157–164. [Google Scholar]
- Pörtner, H.O.; Farrell, A.P. Physiology and Climate Change. Science. 2008, 322, 690–692. [Google Scholar] [CrossRef]
- Czajka, M.C.; Lee, R.E. A rapid cold-hardening response protecting against cold shock injury in Drosophila melanogaster. J. Exp. Biol. 1990, 148, 245–254. [Google Scholar] [CrossRef]
- McDonald, J.; Bale, J.; Walters, K.J. Rapid cold hardening in the western flower thrips Frankliniella occidentalis. J. Insect Physiol. 1997, 43, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Zhao, F.; Ma, C.S. Effects of temperature fluctuation on life history traits of different developmental stages of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Acta Entomol. Sin. 2015, 58, 160–168. [Google Scholar]
- Gerken, A.R.; Eller, O.C.; Hahn, D.A.; Morgan, T.J. Constraints, independence, and evolution of thermal plasticity: Probing genetic architecture of long-and short-term thermal acclimation. Proc. Natl. Acad. Sci. USA 2015, 112, 4399–4404. [Google Scholar] [CrossRef]
- Gerken, A.R.; Eller-Smith, O.C.; Morgan, T.J. Speed of exposure to rapid cold hardening and genotype drive the level of acclimation response in Drosophila melanogaster. J. Therm. Biol. 2018, 76, 21–28. [Google Scholar] [CrossRef]
- Izadi, H.; Mohammadzadeh, M.; Mehrabian, M. Cold Tolerance of the Tribolium castaneum (Coleoptera: Tenebrionidae), Under Different Thermal Regimes: Impact of Cold Acclimation. J. Econ. Entomol. 2019, 112, 1983–1988. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Arthur, F.H.; Kavallieratos, N.G.; Hartzer, K.L. To Acclimate or Not to Acclimate? Simultaneous Positive and Negative Effects of Acclimation on Susceptibility of Tribolium confusum (Coleoptera: Tenebrionidae) and Oryzaephilus surinamensis (Coleoptera: Silvanidae) to Low Temperatures. J. Econ. Entomol. 2019, 112, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.C.; Jing, X. Observation and Research on the Development and Reproduction of Agasicles Hygrophila; Plant Protection: Wuhan, China, 1999; Volume 6. [Google Scholar]
- Sgro, C.M.; Terblanche, J.S.; Hoffmann, A.A. What can plasticity contribute to insect responses to climate change? Annu. Rev. Entomol. 2016, 61, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Zhang, X.-X.; Chang, Y.-W.; Du, Y.-Z. Differential Response of Leafminer Flies Liriomyza trifolii (Burgess) and Liriomyza sativae (Blanchard) to Rapid Cold Hardening. Insects 2021, 12, 1041. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Jia, D.; Yuan, X.F. Response to Short-Term Cold Storage for Eggs of Agasicles hygrophila (Coleoptera: Chrysomelidae), a Biological Control Agent of Alligator Weed Alternanthera philoxeroides (Caryophyllales: Amaranthaceae). J. Econ. Entomol. 2018, 111, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.M.; Aitkenhead, I.; Janion-Scheepers, C.; King, C.K.; McGeoch, M.A.; Nielsen, U.N.; Terauds, A.; Liu, W.P.A.; Chown, S.L. Basal tolerance but not plasticity gives invasive springtails the advantage in an assemblage setting. Conserv. Physiol. 2020, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, F.; Mikani, A.; Moharramipour, S. Thermal tolerance variations and physiological adjustments in a winter active and a summer active aphid species. J. Therm. Biol. 2021, 98, 102950. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA. 2008, 105, 6668–6672. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, B.J.; Vernon, P.; Klok, C.J.; Chown, S.L. Insects at low temperatures: An ecological perspective. Trends Ecol. Evol. 2003, 18, 257–262. [Google Scholar] [CrossRef]
- Coulson, J.R. Biological Control of Alligator Weed, 1959–1972. A Review and Evaluation; Technical Bulletin, Agricultural Research Service, United States Department of Agriculture: Washinton, DC, USA, 1977. [Google Scholar]
- Sinclair, B.J.; Marshall, K.E.; Sewell, M.A.; Levesque, D.L.; Willett, C.S.; Slotsbo, S.; Dong, Y.; Harley, C.D.G.; Marshall, D.J.; Helmuth, B.S.; et al. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol. Lett. 2016, 19, 1372–1385. [Google Scholar] [CrossRef] [PubMed]



| Developmental Stage | SCP | FP |
|---|---|---|
| 1st instar | –14.54 ± 4.06 | –10.33 ± 5.22 |
| 2nd instar | –11.77 ± 1.96 | –4.69 ± 2.51 |
| 3rd instar | –10.67 ± 2.93 | –2.68 ± 2.19 |
| Pupa | –10.85 ± 3.89 | –1.92 ± 1.41 |
| Adult female | –9.81 ± 2.06 | –4.19 ± 2.61 |
| Adult male | –8.38 ± 2.85 | –3.12 ± 2.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.; Jin, J.; Wu, Q.; Liang, X.; Lv, C.; Guo, J. Cold Acclimation and Supercooling Capacity of Agasicles hygrophila Adults. Insects 2023, 14, 58. https://doi.org/10.3390/insects14010058
Pei Y, Jin J, Wu Q, Liang X, Lv C, Guo J. Cold Acclimation and Supercooling Capacity of Agasicles hygrophila Adults. Insects. 2023; 14(1):58. https://doi.org/10.3390/insects14010058
Chicago/Turabian StylePei, Yiming, Jisu Jin, Qiang Wu, Xiaocui Liang, Chen Lv, and Jianying Guo. 2023. "Cold Acclimation and Supercooling Capacity of Agasicles hygrophila Adults" Insects 14, no. 1: 58. https://doi.org/10.3390/insects14010058
APA StylePei, Y., Jin, J., Wu, Q., Liang, X., Lv, C., & Guo, J. (2023). Cold Acclimation and Supercooling Capacity of Agasicles hygrophila Adults. Insects, 14(1), 58. https://doi.org/10.3390/insects14010058






