Knockdown of the Sodium/Potassium ATPase Subunit Beta 2 Reduces Egg Production in the Dengue Vector, Aedes aegypti
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Rearing
2.2. Total RNA Isolation and cDNA Synthesis
2.3. qRT-PCR
2.4. Na+/K+ ATPase Subunit β2 (nkaβ2) Annotation
2.5. RNAi Knockdown of nkaβ2
2.6. Mortality Assay
2.7. Ovary Morphology Assay
2.8. Clutch Size and Hatch Rate Assay
2.9. Statistical Analysis
3. Results
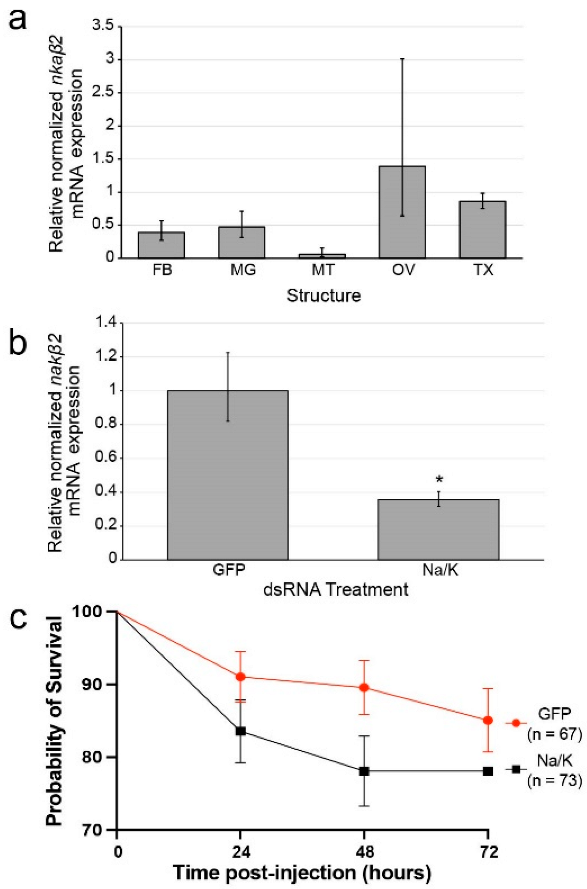
3.1. nkaβ2 Expression Is High in Ovaries of Female Ae. aegypti
3.2. nkaβ2 Is an Ortholog of Drosophila Melanogaster Nervana 3
3.3. dsRNA Reduces nkaβ2 Transcript Levels in Mosquitoes
3.4. Na/K ATPase Subunit Beta Knockdown Does/Does Not Cause Significantly Increased Mortality Relative to Control
3.5. Na/K ATPase Subunit Beta Knockdown Has No Significant Effect on Ovary or Follicle Size
3.6. Na/K ATPase Subunit Beta Knockdown Significantly Reduces Egg Numbers but Not Hatch Rates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rose, A.M.; Valdes, R., Jr. Understanding the sodium pump and its relevance to disease. Clin. Chem. 1994, 40, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, K.; Drengstig, T.; Ruoff, P. Transepithelial glucose transport and Na+/K+ homeostasis in enterocytes: An integrative model. Am. J. Physiol.-Cell Physiol. 2014, 307, C320–C337. [Google Scholar] [CrossRef] [PubMed]
- Rossier, B.C.; Geering, K.; Kraehenbuhl, J.P. Regulation of the sodium pump: How and why? Trends Biochem. Sci. 1987, 12, 483–487. [Google Scholar] [CrossRef]
- Tosteson, D.C.; Hoffman, J.F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J. Gen. Physiol. 1960, 44, 169–194. [Google Scholar] [CrossRef]
- Armstrong, C.M. The Na/K pump, Cl ion, and osmotic stabilization of cells. Proc. Natl. Acad. Sci. USA 2003, 100, 6257–6262. [Google Scholar] [CrossRef]
- Vagin, O.; Tokhtaeva, E.; Yakubov, I.; Shevchenko, E.; Sachs, G. Inverse correlation between the extent of N-glycan branching and intercellular adhesion in epithelia: Contribution of the Na, K-ATPase β1 subunit. J. Biol. Chem. 2008, 283, 2192–2202. [Google Scholar] [CrossRef]
- Gloor, S.; Antonicek, H.; Sweadner, K.J.; Pagliusi, S.; Frank, R.; Moos, M.; Schachner, M. The adhesion molecule on glia (AMOG) is a homologue of the beta subunit of the Na, K-ATPase. J. Cell Biol. 1990, 110, 165–174. [Google Scholar] [CrossRef]
- Nie, Y.; Bai, F.; Chaudhry, M.A.; Pratt, R.; Shapiro, J.I.; Liu, J. The Na/K-ATPase α1 and c-Src form signaling complex under native condition: A crosslinking approach. Sci. Rep. 2020, 10, 6006. [Google Scholar] [CrossRef]
- Tian, J.; Cai, T.; Yuan, Z.; Wang, H.; Liu, L.; Haas, M.; Maksimova, E.; Huang, X.Y.; Xie, Z.J. Binding of Src to Na+/K+-ATPase Forms a Functional Signaling Complex. Mol. Biol. Cell. 2006, 17, 317–326. [Google Scholar] [CrossRef]
- Lingrel, J.B.; Williams, M.T.; Vorhees, C.V.; Moseley, A.E. Na,K-ATPase and the role of α isoforms in behavior. J. Bioenerg. Biomembr. 2007, 39, 385–389. [Google Scholar] [CrossRef]
- Jorgensen, P.L. Mechanism of the Na+ K+ Pump, Protein, Structure and Conformations of the Pure, Na++K+-ATPase. Biochim. Biophys. Acta (BBA)—Rev. Biomembr. 1982, 694, 27–68. [Google Scholar] [CrossRef]
- Horisberger, J.D. Recent insights into the structure and mechanism of the sodium pump. Physiology 2004, 19, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The structure and function of the Na, K-ATPase isoforms in health and disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef]
- Orlowski, J.; Lingrel, J.B. Tissue-specific and developmental regulation of rat NaK-ATPase catalytic alpha isoform and beta subunit mRNAs. J. Biol. Chem. 1988, 263, 10436–10442. [Google Scholar] [CrossRef] [PubMed]
- Hundal, H.S.; Marette, A.; Mitsumoto, Y.; Ramlal, T.; Blostein, R.; Klip, A. Insulin induces translocation of the alpha 2 and beta 1 subunits of the Na+/K(+)-ATPase from intracellular compartments to the plasma membrane in mammalian skeletal muscle. J Biol. Chem. 1992, 267, 5040–5043. [Google Scholar] [CrossRef]
- McGrail, K.M.; Phillips, J.M.; Sweadner, K.J. Immunofluorescent localization of three, Na,K-ATPase isozymes in the rat central nervous system: Both neurons and glia can express more than one, NaK-ATPase. J. Neurosci. 1991, 11, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Zahler, R.; Brines, M.; Kashgarian, M.; Benz, E.J., Jr.; Gilmore-Hebert, M. The cardiac conduction system in the rat expresses the alpha 2 and alpha 3 isoforms of the Na+K(+)-ATPase. Proc. Natl. Acad. Sci. USA 1992, 89, 99–103. [Google Scholar] [CrossRef]
- Jiao, S.; Johnson, K.; Moreno, C.; Yano, S.; Holmgren, M. Comparative description of the mRNA expression profile of Na(+) /K(+) -ATPase isoforms in adult mouse nervous system. J. Comp. Neurol. 2022, 530, 627–647. [Google Scholar] [CrossRef]
- Hlivko, J.T.; Chakraborty, S.; Hlivko, T.J.; Sengupta, A.; James, P.F. The human, Na,K-ATPase alpha4 isoform is a ouabain-sensitive alpha isoform that is expressed in sperm. Mol. Reprod. Dev. 2006, 73, 101–115. [Google Scholar] [CrossRef]
- Beggah, A.T.; Jaunin, P.; Geering, K. Role of glycosylation and disulfide bond formation in the beta subunit in the folding and functional expression of NaK-ATPase. J. Biol. Chem. 1997, 272, 10318–10326. [Google Scholar] [CrossRef]
- Hasler, U.; Wang, X.; Crambert, G.; Beguin, P.; Jaisser, F.; Horisberger, J.D.; Geering, K. Role of beta-subunit domains in the assembly, stable expression, intracellular routing, and functional properties of Na,K-ATPase. J. Biol. Chem. 1998, 273, 30826–30835. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.A.; Gopal, J.; Willis, D.; Espineda, C.; Twiss, J.L.; Rajasekaran, A.K. Na, K-ATPase β1-subunit increases the translation efficiency of the α1-subunit in MSV-MDCK cells. Mol. Biol. Cell 2004, 15, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Tokhtaeva, E.; Sachs, G.; Souda, P.; Bassilian, S.; Whitelegge, J.P.; Shoshani, L.; Vagin, O. Epithelial junctions depend on intercellular trans-interactions between the Na,K-ATPase β₁ subunits. J. Biol. Chem. 2011, 286, 25801–25812. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.H.; Hamilton, E.J.; Liu, C.C.; Figtree, G.A. Reversible oxidative modification: Implications for cardiovascular physiology and pathophysiology. Trends. Cardiovasc. Med. 2010, 20, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.R.; Assentoft, M.; Cotrina, M.L.; Hua, S.Z.; Nedergaard, M.; Kaila, K.; Voipio, J.; MacAulay, N. Contributions of the Na⁺/K⁺-ATPase, NKCC1 and Kir4.1 to hippocampal K.⁺ clearance and volume responses. Glia 2014, 62, 608–622. [Google Scholar] [CrossRef]
- Therien, A.G.; Pu, H.X.; Karlish, S.J.D.; Blostein, R.E. Molecular and functional studies of the gamma subunit of the sodium pump. J. Bioenerg. Biomembr. 2001, 33, 407–414. [Google Scholar] [CrossRef]
- Therien, A.G.; Goldshleger, R.; Karlish, S.J.; Blostein, R. Tissue-specific distribution and modulatory role of the γ subunit of the Na, K-ATPase. J. Biol. Chem. 1997, 272, 32628–32634. [Google Scholar] [CrossRef]
- Scaraffia, P.Y.; Zhang, Q.; Thorson, K.; Wysocki, V.H.; Miesfeld, R.L. Differential ammonia metabolism in Aedes aegypti fat body and midgut tissues. J. Insect. Physiol. 2010, 56, 1040–1049. [Google Scholar] [CrossRef]
- Hansen, I.A.; Attardo, G.M.; Rodriguez, S.D.; Drake, L.L. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front. Physiol. 2014, 5, 103. [Google Scholar] [CrossRef]
- Lorenz, M.C.; Heitman, J. The M.EP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Embo. J. 1998, 17, 1236–1247. [Google Scholar] [CrossRef]
- Iraqui, I.; Vissers, S.; Bernard, F.; de Craene, J.O.; Boles, E.; Urrestarazu, A.; André, B. Amino acid signaling in Saccharomyces cerevisiae: A permease-like sensor of external amino acids and F-Box protein Grr1p are required for transcriptional induction of the AGP1 gene, which encodes a broad-specificity amino acid permease. Mol. Cell Biol. 1999, 19, 989–1001. [Google Scholar] [CrossRef]
- Hansen, I.A.; Attardo, G.M.; Park, J.H.; Peng, Q.; Raikhel, A.S. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proc. Natl. Acad. Sci. USA 2004, 101, 10626–10631. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.A.; Attardo, G.M.; Roy, S.G.; Raikhel, A.S. Target of rapamycin-dependent activation of S.6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. J. Biol. Chem. 2005, 280, 20565–20572. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, V.K.; Drake, L.L.; Aguirre, S.E.; Price, D.P.; Rodriguez, S.D.; Hansen, I.A. SLC7 amino acid transporters of the yellow fever mosquito, Aedes aegypti and their role in fat body, TOR signaling and reproduction. J. Insect. Physiol. 2012, 58, 513–522. [Google Scholar] [CrossRef]
- Closs, E.I.; Boissel, J.P.; Habermeier, A.; Rotmann, A. Structure and function of cationic amino acid transporters (CATs). J Membr, Biol. 2006, 213, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Verrey, F.; Closs, E.I.; Wagner, C.A.; Palacin, M.; Endou, H.; Kanai, Y. CATs and H.ATs: The S.LC7 family of amino acid transporters. Pflugers. Arch. 2004, 447, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Attardo, G.M.; Hansen, I.A.; Shiao, S.H.; Raikhel, A.S. Identification of two cationic amino acid transporters required for nutritional signaling during mosquito reproduction. J. Exp. Biol. 2006, 209, 3071–3078. [Google Scholar] [CrossRef] [PubMed]
- Pinch, M.; Muka, T.; Kandel, Y.; Lamsal, M.; Martinez, N.; Teixeira, M.; Boudko, D.Y.; Hansen, I.A. General, Control, Nonderepressible 1 interacts with, Cationic, Amino, Acid, Transporter 1 and affects, Aedes aegypti fecundity. Parasit. Vectors 2022, 15, 383. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Mitra, S.; Pinch, M.; Kandel, Y.; Li, Y.; Rodriguez, S.D.; Hansen, I.A. Olfaction-Related Gene Expression in the Antennae of Female Mosquitoes From Common Aedes aegypti Laboratory Strains. Front. Physiol. 2021, 12, 668236. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular, Evolutionary, Genetics, Analysis, Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.L.; Price, D.P.; Aguirre, S.E.; Hansen, I.A. RNAi-mediated gene knockdown and in vivo diuresis assay in adult female, Aedes aegypti mosquitoes. J. Vis. Exp. 2012, 65, e3479. [Google Scholar] [CrossRef]
- Miller, S.C.; Miyata, K.; Brown, S.J.; Tomoyasu, Y. Dissecting systemic, R.NA interference in the red flour beetle, Tribolium castaneum: Parameters affecting the efficiency of R.NAi. PLoS ONE 2012, 7, e47431. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to, ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Benelli, G.; Mehlhorn, H. Declining malaria, rising of dengue and Zika virus: Insights for mosquito vector control. Parasitol. Res. 2016, 115, 1747–1754. [Google Scholar] [CrossRef]
- Clements, A.N. The Biology of Mosquitoes; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Dengue and, Severe, Dengue 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 22 November 2022).
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors, Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Amelia-Yap, Z.H.; Chen, C.D.; Sofian-Azirun, M.; Low, V.L. Pyrethroid resistance in the dengue vector, Aedes aegypti in Southeast Asia: Present situation and prospects for management. Parasites Vectors 2018, 11, 332. [Google Scholar] [CrossRef]
- Badolo, A.; Sombie, A.; Pignatelli, P.M.; Sanon, A.; Yameogo, F.; Wangrawa, D.W.; Sanon, A.; Kanuka, H.; McCall, P.J.; Weetman, D. Insecticide resistance levels and mechanisms in Aedes aegypti populations in and around Ouagadougou, Burkina Faso. PLoS Negl. Trop. Dis. 2019, 13, e0007439. [Google Scholar] [CrossRef]
- Chareonviriyaphap, T.; Bangs, M.J.; Suwonkerd, W.; Kongmee, M.; Corbel, V.; Ngoen-Klan, R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasit. Vectors 2013, 6, 280. [Google Scholar] [CrossRef]
- Demok, S.; Endersby-Harshman, N.; Vinit, R.; Timinao, L.; Robinson, L.J.; Susapu, M.; Makita, L.; Laman, M.; Hoffmann, A.; Karl, S. Insecticide resistance status of Aedes aegypti and Aedes albopictus mosquitoes in Papua New Guinea. Parasites Vectors 2019, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Goindin, D.; Delannay, C.; Gelasse, A.; Ramdini, C.; Gaude, T.; Faucon, F.; David, J.P.; Gustave, J.; Vega-Rua, A.; Fouque, F. Levels of insecticide resistance to deltamethrin, malathion, and temephos, and associated mechanisms in Aedes aegypti mosquitoes from the Guadeloupe and Saint Martin islands (French West Indies). Infect. Dis. Poverty 2017, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Kandel, Y.; Vulcan, J.; Rodriguez, S.D.; Moore, E.; Chung, H.N.; Mitra, S.; Cordova, J.J.; Martinez, K.J.; Moon, A.S.; Kulkarni, A.; et al. Widespread insecticide resistance in Aedes aegypti L. from New Mexico, U.S.A. PLoS ONE 2019, 14, e0212693. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Caputo, B.; Tsunoda, T.; Cuong, T.C.; Maekawa, Y.; Lam-Phua, S.G.; Pichler, V.; Itokawa, K.; Murota, K.; Komagata, O.; et al. First detection of a Vssc allele V.1016G conferring a high level of insecticide resistance in Aedes albopictus collected from, Europe (Italy) and Asia (Vietnam), 2016: A new emerging threat to controlling arboviral diseases. Euro. Surveill. 2019, 24, 1700847. [Google Scholar] [CrossRef] [PubMed]
- Macoris, M.L.; Martins, A.J.; Andrighetti, M.T.M.; Lima, J.B.P.; Valle, D. Pyrethroid resistance persists after ten years without usage against, Aedes aegypti in governmental campaigns: Lessons from, Sao, Paulo, State, Brazil. PLoS Negl. Trop. Dis. 2018, 12, e0006390. [Google Scholar] [CrossRef]
- Marcombe, S.; Fustec, B.; Cattel, J.; Chonephetsarath, S.; Thammavong, P.; Phommavanh, N.; David, J.P.; Corbel, V.; Sutherland, I.W.; Hertz, J.C.; et al. Distribution of insecticide resistance and mechanisms involved in the arbovirus vector, Aedes aegypti in Laos and implication for vector control. PLoS Negl. Trop. Dis. 2019, 13, e0007852. [Google Scholar] [CrossRef]
- Raikhel, A.S.; Kokoza, V.A.; Zhu, J.; Martin, D.; Wang, S.-F.; Li, C.; Sun, G.; Ahmed, A.; Dittmer, N.; Attardo, G. Molecular biology of mosquito vitellogenesis: From basic studies to genetic engineering of antipathogen immunity. Insect. Biochem. Mol. Biol. 2002, 32, 1275–1286. [Google Scholar] [CrossRef]
- Barnett, M.W.; Larkman, P.M. The action potential. Pract. Neurol. 2007, 7, 192–197. [Google Scholar]
- Lang, F.; Busch, G.L.; Ritter, M.; Volkl, H.; Waldegger, S.; Gulbins, E.; Haussinger, D. Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 1998, 78, 247–306. [Google Scholar] [CrossRef]
- Rose, E.M.; Koo, J.C.; Antflick, J.E.; Ahmed, S.M.; Angers, S.; Hampson, D.R. Glutamate transporter coupling to, Na,K-ATPase. J. Neurosci. 2009, 29, 8143–8155. [Google Scholar] [CrossRef]
- Manoharan, P.; Gayam, S.; Arthur, S.; Palaniappan, B.; Singh, S.; Dick, G.M.; Sundaram, U. Chronic and selective inhibition of basolateral membrane, Na-K-ATPase uniquely regulates brush border membrane, Na absorption in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2015, 308, C650–C656. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.; Reddig, K.; Li, H.S. Loss of Na(+)/K(+)-ATPase in Drosophila photoreceptors leads to blindness and age-dependent neurodegeneration. Exp. Neurol. 2014, 261, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, G.R.; Davey, K.G. Cellular and molecular actions of juvenile hormone. II. Roles of juvenile hormone in adult insects. Adv. Insect. Phys. 1996, 26, 1–155. [Google Scholar]
- Song, J.; Zhou, S. Post-transcriptional regulation of insect metamorphosis and oogenesis. Cell. Mol. Life Sci. 2020, 77, 1893–1909. [Google Scholar] [CrossRef] [PubMed]
- Sevala, V.; Davey, K. Juvenile hormone dependent phosphorylation of a 100 kDa polypeptide is mediated by protein kinase C in the follicle cells of Rhodnius prolixus. Invertebr. Reprod. Dev. 1993, 23, 189–193. [Google Scholar] [CrossRef]
- Jing, Y.-P.; An, H.; Zhang, S.; Wang, N.; Zhou, S. Protein kinase, C. mediates juvenile hormone–dependent phosphorylation of Na+/K+-ATPase to induce ovarian follicular patency for yolk protein uptake. J. Biol. Chem. 2018, 293, 20112–20122. [Google Scholar] [CrossRef]
- Sevala, V.; Davey, K. Action of juvenile hormone on the follicle cells ofRhodnius prolixus: Evidence for a novel regulatory mechanism involving protein kinase C. Experientia 1989, 45, 355–356. [Google Scholar] [CrossRef]
- Anderson, W.A.; Spielman, A. Permeability of the ovarian follicle of Aedes aegypti mosquitoes. J. Cell Biol. 1971, 50, 201–221. [Google Scholar] [CrossRef]
- Roth, T.F.; Porter, K.R. Yolk protein uptake in the oocyte of the mosquito Aedes aegypti L. J. Cell Biol. 1964, 20, 313–332. [Google Scholar] [CrossRef]
- Yonge, C.; Hagedorn, H.H. Dynamics of vitellogenin uptake in Aedes aegypti as demonstrated by trypan blue. J. Insect. Physiol. 1977, 23, 1199–1203. [Google Scholar] [CrossRef]
- Fausto, A.M.; Fava, E.; Mazzini, M.; Cecchettini, A.; Giorgi, F. Confocal scanning laser microscopy of the follicular epithelium in ovarioles of the stick insect, Carausius morosus. Cell Tissue Res. 1998, 293, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Koller, C.N.; Raikhel, A.S. Initiation of vitellogenin uptake and protein synthesis in the mosquito (Aedes aegypti) ovary in response to a blood meal. J. Insect. Physiol. 1991, 37, 703–711. [Google Scholar] [CrossRef]
- Valzania, L.; Mattee, M.T.; Strand, M.R.; Brown, M.R. Blood feeding activates the vitellogenic stage of oogenesis in the mosquito, Aedes aegypti through inhibition of glycogen synthase kinase 3 by the insulin and TOR pathways. Dev. Biol. 2019, 454, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Dick, K.B.; Ross, C.R.; Yampolsky, L.Y. Genetic variation of dietary restriction and the effects of nutrient-free water and amino acid supplements on lifespan and fecundity of Drosophila. Genet. Res. 2011, 93, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.N.; Rodriguez, S.D.; Carpenter, V.K.; Vulcan, J.; Bailey, C.D.; Nageswara-Rao, M.; Li, Y.; Attardo, G.M.; Hansen, I.A. Fat body organ culture system in Aedes aegypti, a vector of zika virus. J. Vis. Exp. 2017, 126, e55508. [Google Scholar] [CrossRef]
- Hung, R.J.; Hu, Y.; Kirchner, R.; Liu, Y.; Xu, C.; Comjean, A.; Tattikota, S.G.; Li, F.; Song, W.; Ho Sui, S.; et al. A cell atlas of the adult, Drosophila midgut. Proc. Natl. Acad. Sci. USA 2020, 117, 1514–1523. [Google Scholar] [CrossRef]
- Paul, S.M.; Palladino, M.J.; Beitel, G.J. A pump-independent function of the Na, K-ATPase is required for epithelial junction function and tracheal tube-size control. Development 2007, 134, 147–155. [Google Scholar] [CrossRef]
- Roy, M.; Sivan-Loukianova, E.; Eberl, D.F. Cell-type-specific roles of Na+/K+ ATPase subunits in Drosophila auditory mechanosensation. Proc. Natl. Acad. Sci. USA 2013, 110, 181–186. [Google Scholar] [CrossRef]
- Field, L.H.; Matheson, T. Chordotonal organs of insects. In Advances in Insect Physiology; Evans, P.D., Ed.; Academic Press: San Diego, CA, USA, 1998; Volume 27, pp. 1–228. [Google Scholar]

| Primer | Sequence | Tm (°C) |
|---|---|---|
| sodium/potassium ATPase subunit beta 2 (nkaβ2) forward | TCCCACTGAGGAGCAGAAATACC | 60 |
| nkaβ2 reverse | TGCTGCGGGCAAACTCTACC | |
| ribosomal protein s7 (rps7) forward * | TCAGTGTACAAGAAGCTGACCGGA | 60 |
| rps7 reverse * | TTCCGCGCGCGCTCACTTATTAGATT |
| Primer | Sequence | Size (bp) |
|---|---|---|
| NKAβ2 forward | TAATACGACTCACTATAGGGAGAAATCGACTTCCTTCCTTTGGGG | 618 |
| NKAβ2 reverse | TAATACGACTCACTATAGGGAGATTCTTCTTGGTGGTATGGCTCC | |
| GFP forward | TAATACGACTCACTATAGGGCGATGCCACCT | 518 |
| GFP reverse | TAATACGACTCACTATAGGGCGGACTGGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, N.P.; Pinch, M.; Kandel, Y.; Hansen, I.A. Knockdown of the Sodium/Potassium ATPase Subunit Beta 2 Reduces Egg Production in the Dengue Vector, Aedes aegypti. Insects 2023, 14, 50. https://doi.org/10.3390/insects14010050
Martinez NP, Pinch M, Kandel Y, Hansen IA. Knockdown of the Sodium/Potassium ATPase Subunit Beta 2 Reduces Egg Production in the Dengue Vector, Aedes aegypti. Insects. 2023; 14(1):50. https://doi.org/10.3390/insects14010050
Chicago/Turabian StyleMartinez, Nathan P., Matthew Pinch, Yashoda Kandel, and Immo A. Hansen. 2023. "Knockdown of the Sodium/Potassium ATPase Subunit Beta 2 Reduces Egg Production in the Dengue Vector, Aedes aegypti" Insects 14, no. 1: 50. https://doi.org/10.3390/insects14010050
APA StyleMartinez, N. P., Pinch, M., Kandel, Y., & Hansen, I. A. (2023). Knockdown of the Sodium/Potassium ATPase Subunit Beta 2 Reduces Egg Production in the Dengue Vector, Aedes aegypti. Insects, 14(1), 50. https://doi.org/10.3390/insects14010050





