Survival-Larval Density Relationships in the Field and Their Implications for Control of Container-Dwelling Aedes Mosquitoes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General Approach
2.2. Survey Period
2.3. Density Manipulation
2.4. Data Analysis
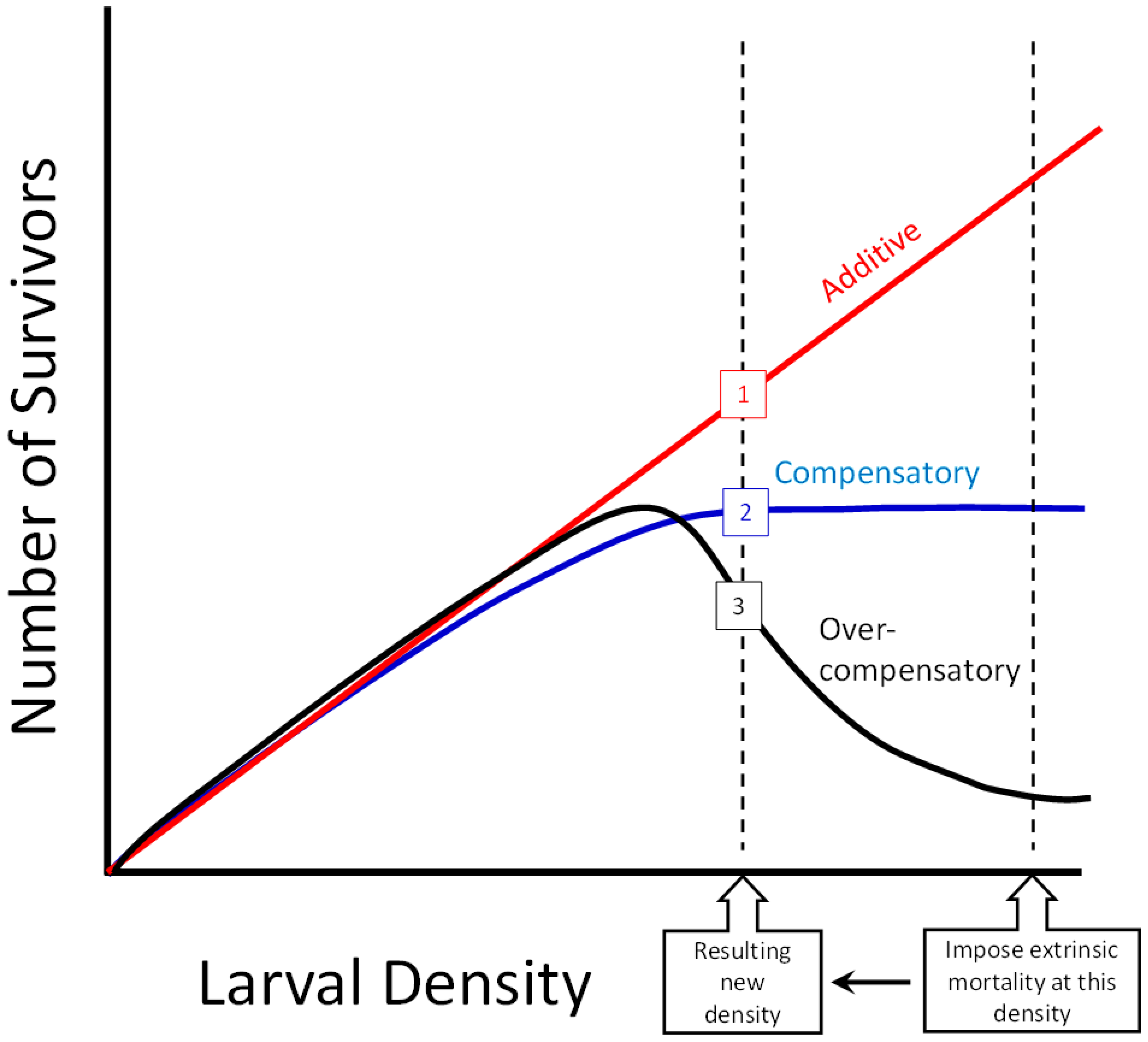
3. Results
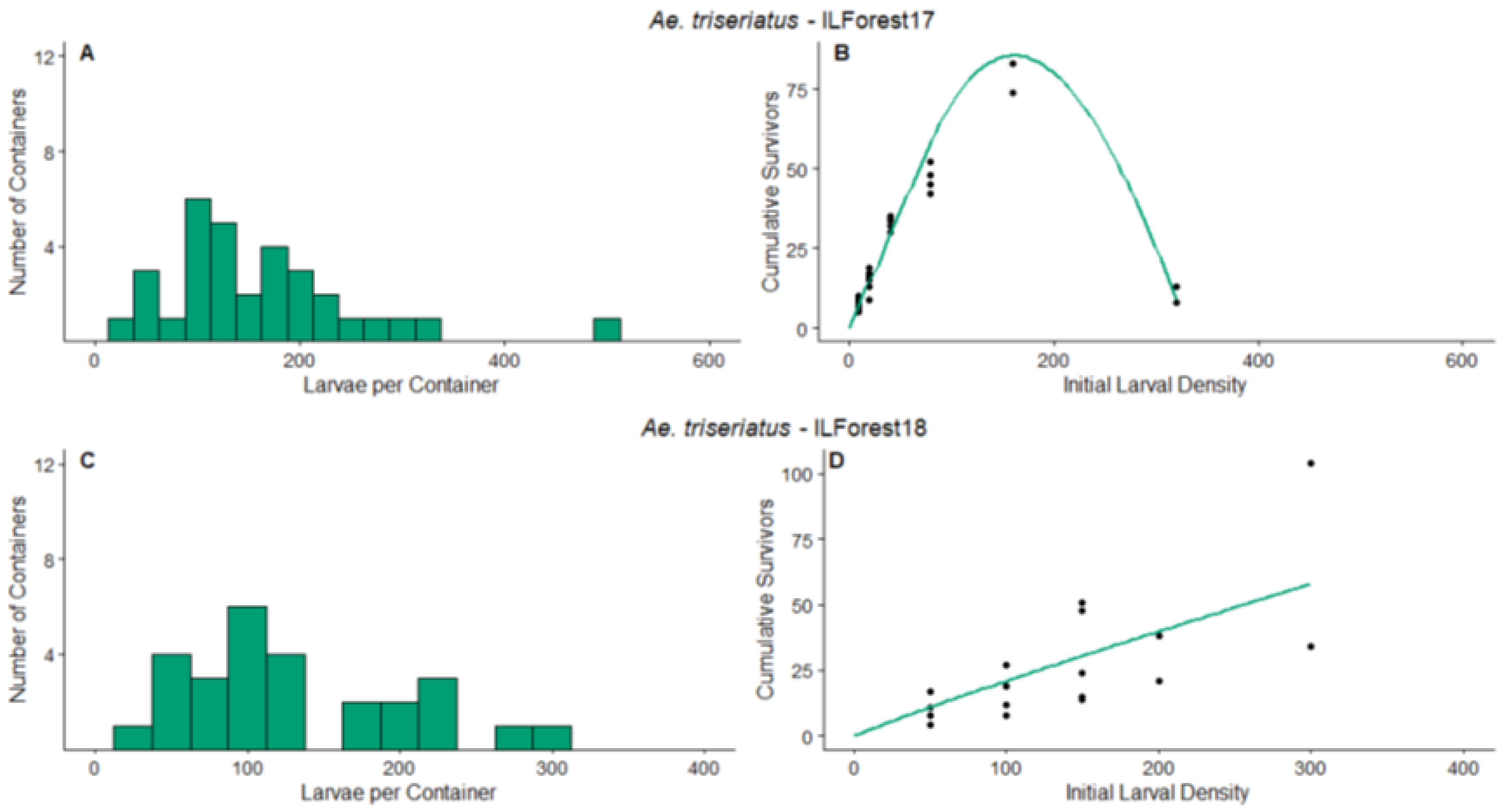
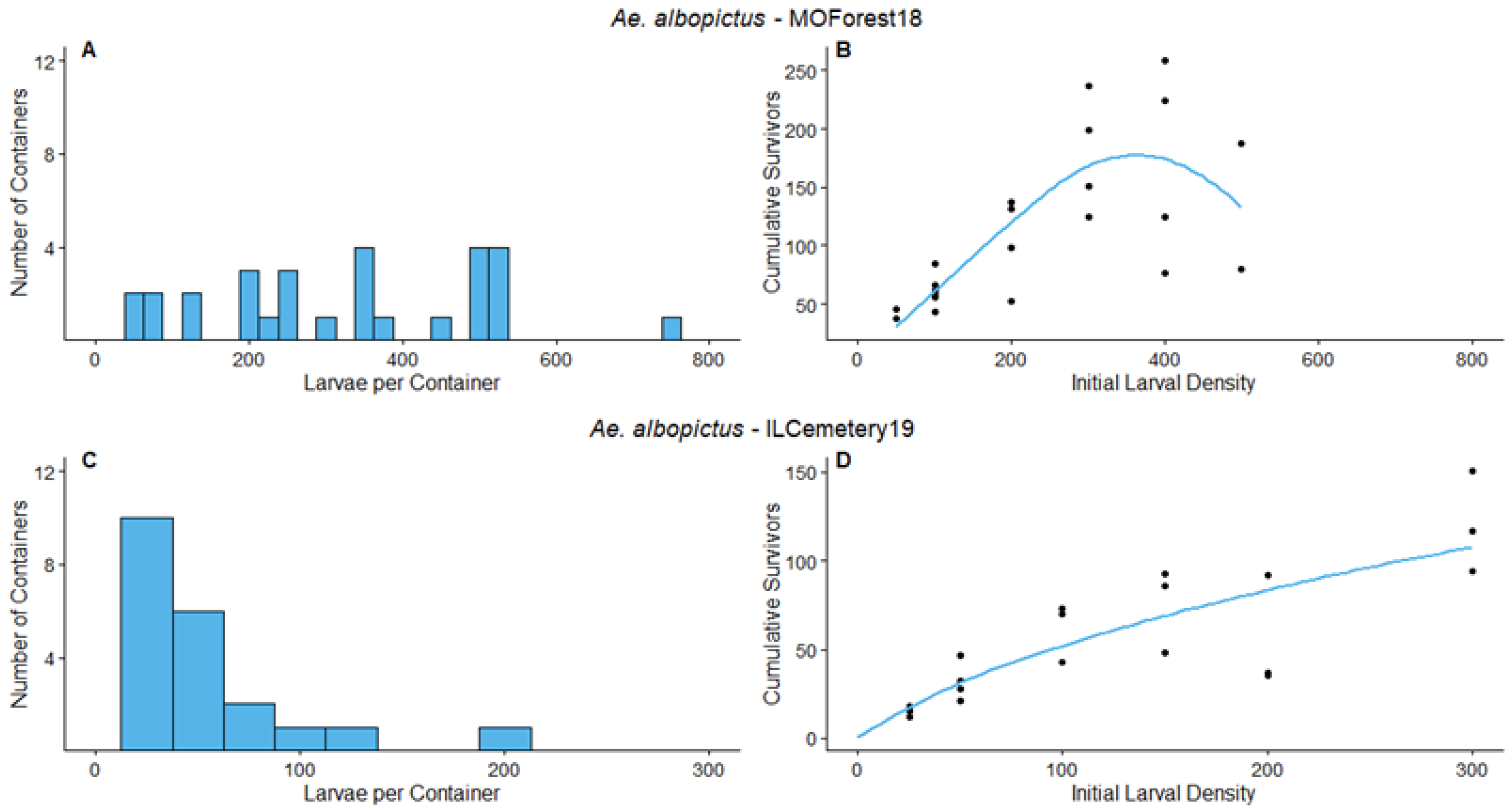
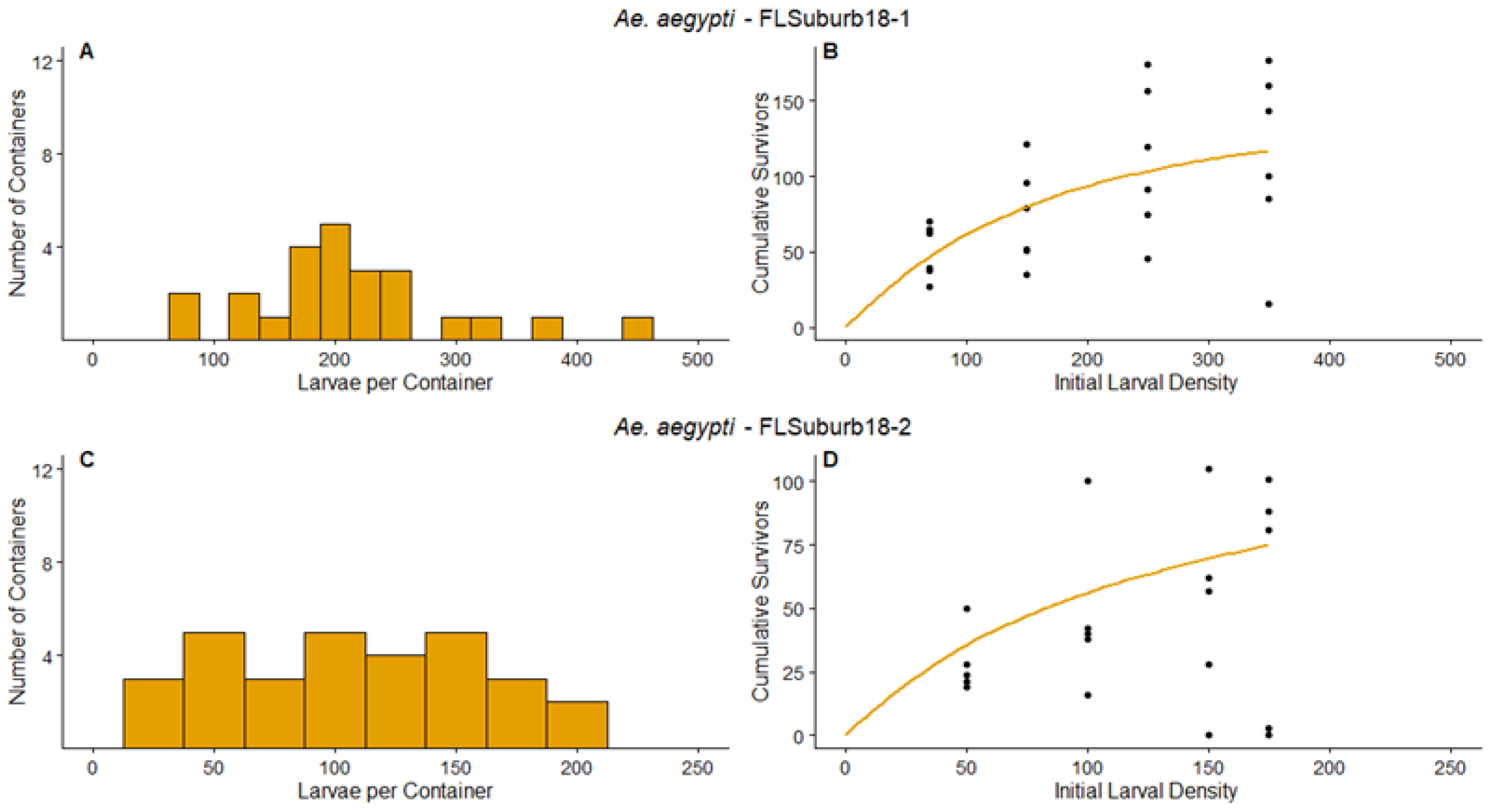
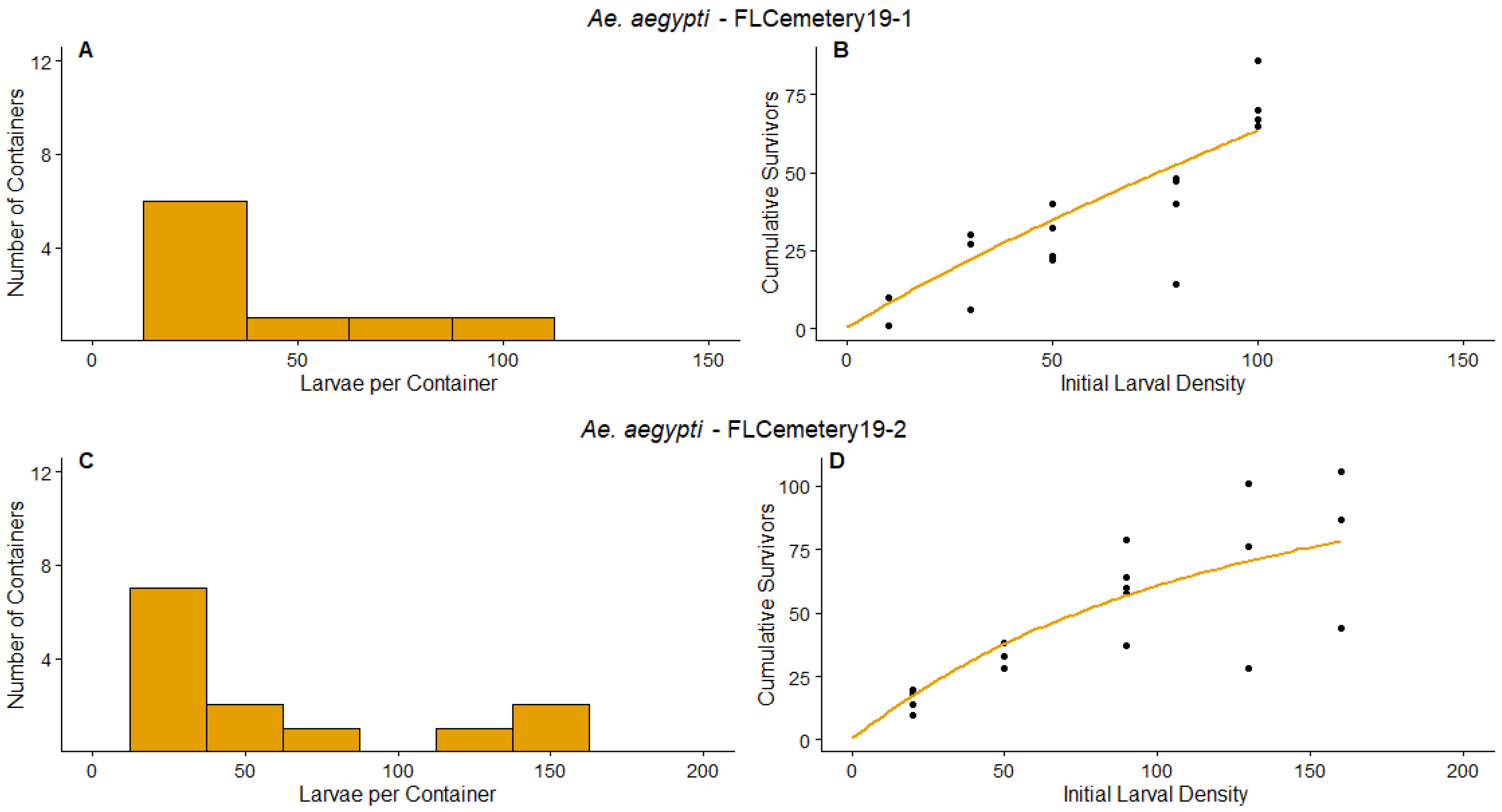
3.1. Survey Results and Density Manipulation
3.2. Detritus Effect
3.3. Regression
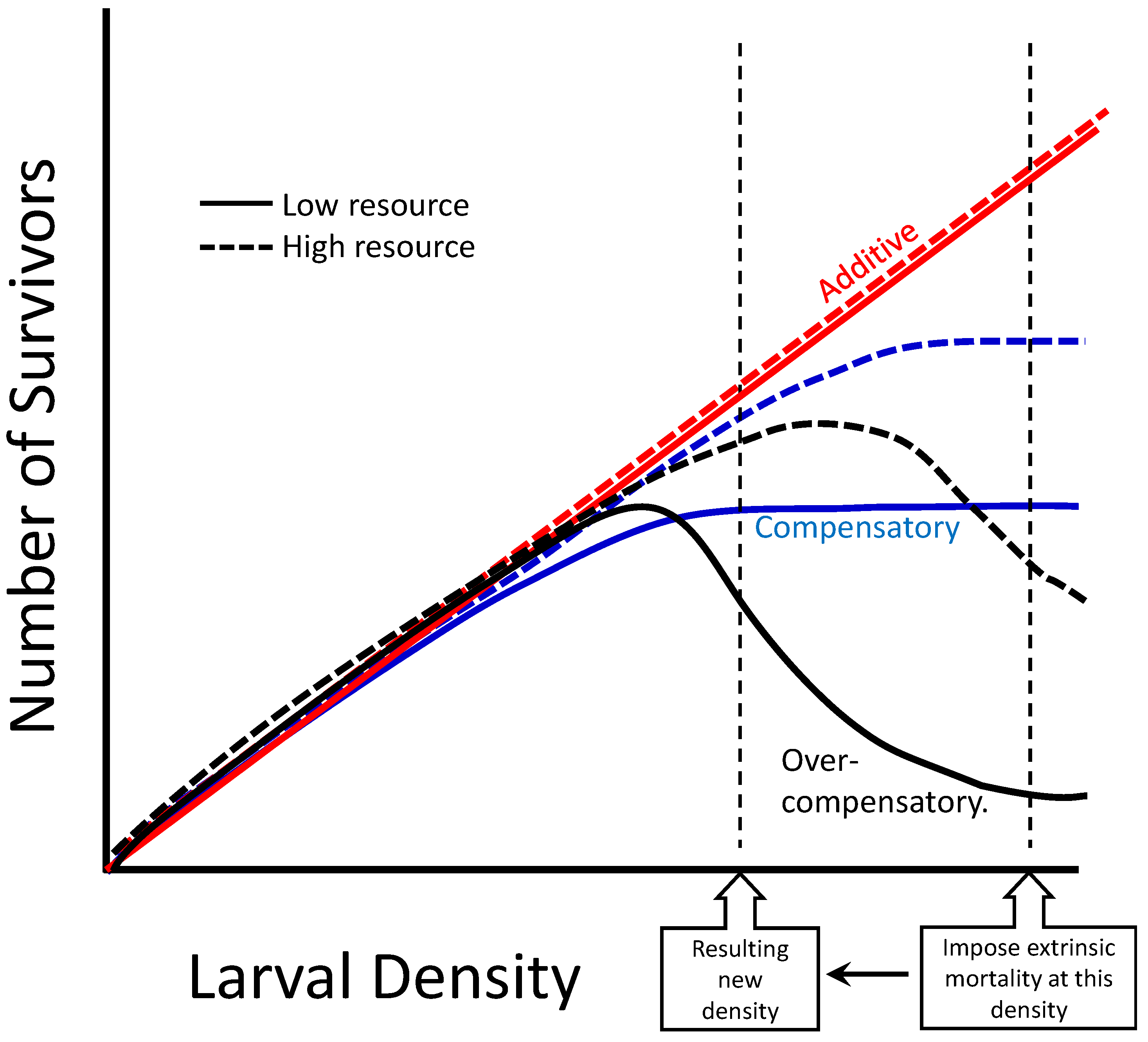
4. Discussion
Author Contributions
Funding
Institutional Animal Care and Use Committee (IACUC) Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ILForest18 | MOForest18 | FLSuburb18 | FLCemetery19-2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | df | F | p | df | F | p | df | F | p | df | F | p |
| Density | 5 | 9.30 | 0.0053 | 5 | 12.86 | 0.0002 | 3 | 4.45 | 0.0200 | 4 | 9.39 | 0.0061 |
| Litter | 1 | 1.28 | 0.2960 | 1 | 1.69 | 0.2184 | 1 | 0.14 | 0.7106 | 1 | 1.93 | 0.2078 |
| Litter × Density | 4 | 4.34 | 0.0445 | 5 | 1.92 | 0.1647 | 3 | 1.41 | 0.2794 | 4 | 0.62 | 0.6615 |
| Error | 7 | 12 | 15 | 7 | ||||||||
References
- Lwande, O.W.; Obanda, V.; Lindström, A.; Ahlm, C.; Evander, M.; Näslund, J.; Bucht, G. Globe-Trotting Aedes aegypti and Aedes albopictus: Risk factors for arbovirus pandemics. Vector Borne Zoonotic Dis. 2020, 20, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Achee, N.L.; Grieco, J.P.; Vatandoost, H.; Seixas, G.; Pinto, J.; Ching-Ng, L.; Martins, A.J.; Juntarajumnong, W.; Corbel, V.; Gouagna, C.; et al. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl. Trop. Dis. 2019, 13, 1–22. [Google Scholar] [CrossRef]
- Lees, R.S.; Gilles, J.R.; Hendrichs, J.; Vreysen, M.J.; Bourtzis, K. Back to the future: The sterile insect technique against mosquito disease vectors. Curr. Opin. Insect Sci. 2015, 10, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.C. Density dependence in larval Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 1998, 35, 825–829. [Google Scholar] [CrossRef]
- Walsh, R.K.; Facchinelli, L.; Ramsey, J.M.; Bond, J.G.; Gould, F. Assessing the impact of density dependence in field populations of Aedes aegypti. J. Vector Ecol. 2011, 36, 300–307. [Google Scholar] [CrossRef]
- Walsh, R.K.; Aguilar, C.L.; Facchinelli, L.; Valerio, L.; Ramsey, J.M.; Scott, T.W.; Lloyd, A.L.; Gould, F. Regulation of Aedes aegypti population dynamics in field systems: Quantifying direct and delayed density dependence. Am. J. Trop. Med. Hyg. 2013, 89, 68–77. [Google Scholar] [CrossRef]
- Walsh, R.K.; Bradley, C.; Apperson, C.S.; Gould, F. An experimental field study of delayed density dependence in natural populations of Aedes albopictus. PLoS ONE 2012, 7, e35959. [Google Scholar] [CrossRef] [PubMed]
- Washburn, J.O. Regulatory factors affecting larval mosquito populations in container and pool habitats: Implications for biological control. J. Am. Mosq. Control Assoc. 1995, 11 Pt 2, 279–283. Available online: http://www.ncbi.nlm.nih.gov/pubmed/7595462 (accessed on 19 January 2022).
- McIntire, K.M.; Juliano, S.A. How can mortality increase population size? A test of two mechanistic hypotheses. Ecology 2018, 99, 1660–1670. [Google Scholar] [CrossRef]
- Neale, Z.R.; Juliano, S.A. Finding the sweet spot: What levels of larval mortality lead to compensation or overcompensation in adult production? Ecosphere 2019, 10, e02855. [Google Scholar] [CrossRef]
- Abrams, P.A. When does greater mortality increase population size? The long history and diverse mechanisms underlying the hydra effect. Ecol. Lett. 2009, 12, 462–474. [Google Scholar] [CrossRef] [PubMed]
- De Roos, A.M.; Schellekens, T.; Van Kooten, T.; Van De Wolfshaar, K.; Claessen, D.; Persson, L. Food-dependent growth leads to overcompensation in stage-specific biomass when mortality increases: The influence of maturation versus reproduction regulation. Am. Nat. 2007, 170, 59–76. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.L.; Connelly, C.R. A review of the control of Aedes aegypti (Diptera: Culicidae) in the continental United States. J. Med. Entomol. 2020, 58, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, J.; Yamada, H.; Pereira, R.; Bourtzis, K.; Vreysen, M.J.B. Phased conditional approach for mosquito management using sterile insect technique. Trends Parasitol. 2020, 36, 325–336. [Google Scholar] [CrossRef]
- Oliva, C.; Benedict, M.; Collins, M.; Baldet, T.; Bellini, R.; Bossin, H.; Bouyer, J.; Corbel, V.; Facchinelli, L.; Fouque, F.; et al. Sterile insect technique (SIT) against Aedes species mosquitoes: A roadmap and good practice framework for designing, implementing and evaluating pilot field trials. Insects 2021, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Dobson, S.L. When more is less: Mosquito population suppression using sterile, incompatible and genetically modified male mosquitoes. J. Med. Entomol. 2021, 58, 1980–1986. [Google Scholar] [CrossRef]
- Gato, R.; Menéndez, Z.; Prieto, E.; Argilés, R.; Rodríguez, M.; Baldoquín, W.; Hernández, Y.; Pérez, D.; Anaya, J.; Fuentes, I.; et al. Sterile insect technique: Successful suppression of an Aedes aegypti field population in Cuba. Insects 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Mains, J.W.; Brelsfoard, C.L.; Rose, R.I.; Dobson, S.L. Female adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef]
- Sauers, L.A.; Hawes, K.E.; Juliano, S.A. Non-linear relationships between density and demographic traits in three Aedes species. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Nannini, M.A.; Juliano, S.A. Effects of the facultative predator Anopheles barberi on population performance of its prey Aedes triseriatus (Diptera Culicidae). Ann. Entomol. Soc. Am. 1998, 91, 33–42. [Google Scholar] [CrossRef]
- Washburn, J.O.; Mercer, D.R.; Anderson, J.R. Regulatory role of parasites: Impact on host population shifts with resource availability. Science 1991, 253, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.G. A family of general production curves for exploited populations. Math. Biosci. 1982, 1982. 59, 77–93. [Google Scholar] [CrossRef]
- Osenberg, C.W.; St Mary, C.M.; Schmitt, R.J.; Holbrook, S.J.; Chesson, P.; Byrne, B. Rethinking ecological inference: Density dependence in reef fishes. Ecol. Lett. 2002, 5, 715–721. [Google Scholar] [CrossRef]
- Schmitt, R.J.; Holbrook, S.J.; Osenberg, C.W. Quantifying the effects of multiple processes on local abundance: A cohort approach for open populations. Ecol. Lett. 1999, 2, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Reiskind, M.H.; Greene, K.L.; Lounibos, L.P. Leaf species identity and combination affect performance and oviposition choice of two container mosquito species. Ecol. Entomol. 2009, 34, 447–456. [Google Scholar] [CrossRef]
- Reiskind, M.H.; Zarrabi, A.A.; Lounibos, L.P. Invasive leaf resources alleviate density dependence in the invasive mosquito, Aedes albopictus. Biol. Invasions 2010, 12, 2319–2328. Available online: https://link.springer.com/article/10.1007/s10530-009-9646-6 (accessed on 18 December 2022). [CrossRef]
- Reiskind, M.H.; Zarrabi, A.A.; Lounibos, L.P. Effects of combination of leaf resources on competition in container mosquito larvae. Bull. Entomol. Res. 2012, 102, 424–434. [Google Scholar] [CrossRef]
- Macchioni, F.; Chiavacci, D.; Biasci, A.; Prati, M.C. Intraspecific competition among larvae of Aedes albopictus in conditions of food abundance and shortage. J. Eur. Mosq. Control Assoc. 2016, 34, 14–16. [Google Scholar]
- Lounibos, L.P.; Suárez, S.; Menéndez, Z.; Nishimura, N.; Escher, R.L.; O’Connell, S.M.; Rey, J.R. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J. Vector Ecol. 2002, 27, 86–95. Available online: https://pubmed.ncbi.nlm.nih.gov/12125878/ (accessed on 18 December 2022).
- Couret, J.; Dotson, E.; Benedict, M.Q. Temperature, larval diet, and density effects on development rate and survival of Aedes aegypti (Diptera: Culicidae). PLoS ONE 2014, 9, e87468. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.V.; Drake, J.M.; Jones, L.; Murdock, C.C. Assessing temperature-dependent competition between two invasive mosquito species. Ecol. Appl. 2021, 31, e02334. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, K.S.; Muturi, E.J.; Alto, B.W. Trait-mediated effects of predation across life-history stages in container mosquitoes. Ecol. Entomol. 2012, 36, 605–615. [Google Scholar] [CrossRef]
- Chandrasegaran, K.; Juliano, S.A. How do trait-mediated non-lethal effects of predation affect population-level performance of mosquitoes? Front. Ecol. Evol. 2019, 7, 25. Available online: https://www.frontiersin.org/articles/10.3389/fevo.2019.00025/full (accessed on 18 December 2022). [CrossRef]
- Aspbury, A.S.; Juliano, S.A. Negative effects of drying and prior exploitation on the detritus resource in an ephemeral aquatic habitat. Oecologia 1998, 115, 137–148. Available online: https://link.springer.com/article/10.1007/s004420050500 (accessed on 18 December 2022). [CrossRef]
- Lounibos, L.P.; Nishimura, N.; Escher, R.L. Seasonality and composition of oak litter fall in southeastern Florida. Fla. Sci. 1992, 55, 92–98. Available online: https://www.jstor.org/stable/24320487 (accessed on 18 December 2022).
- Alto, B.W.; Lampman, R.L.; Kesavaraju, B.; Muturi, E.J. Pesticide-induced release from competition among competing Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 1240–1249. [Google Scholar] [CrossRef]
- Williams, C.R.; Mincham, G.; Ritchie, S.A.; Viennet, E.; Harley, D. Bionomic response of Aedes aegypti to two future climate change scenarios in far north Queensland, Australia: Implications for dengue outbreaks. Parasites Vectors 2014, 7, 447. [Google Scholar] [CrossRef]
- Raharimalala, F.N.; Ravaomanarivo, L.H.; Ravelonandro, P.; Rafarasoa, L.S.; Zouache, K.; Tran-Van, V.; Mousson, L.; Failloux, A.-B.; Hellard, E.; Moro, C.V.; et al. Biogeography of the two major arbovirus mosquito vectors, Aedes aegypti and Aedes albopictus (Diptera, Culicidae), in Madagascar. Parasites Vectors. 2012, 5, 56. [Google Scholar] [CrossRef]
- Norman, B.C.; Walker, E.D. Conditioning of leaf detritus modulates density-dependent growth of Aedes triseriatus larvae (Diptera: Culicidae). J. Med. Entomol. 2018, 55, 342–350. [Google Scholar] [CrossRef]

| Experiment Name | Target Species | Start Date | Experimental Site | Location | Survey Period (Weeks) | Number of Survey Containers | Container Type | Water |
|---|---|---|---|---|---|---|---|---|
| ILForest17 | Ae. triseriatus | June 2017 | Merwin Nature Preserve | McLean County, IL, USA | 6 | 32 | 8 L bucket | Rain barrel, sieved |
| ILForest18 | Ae. triseriatus | June 2018 | Merwin Nature Preserve | McLean County, IL, USA | 6 | 30 | 4 L bucket | Rain barrel, sieved |
| MOForest18 | Ae. albopictus | June 2018 | Tyson Research Center | St. Louis County, MO, USA | 6 | 30 | 4 L bucket | Rain barrel, sieved |
| ILCemetery19 | Ae. albopictus | June 2019 | Graceland/Fairlawn Cemetery | Decatur, IL, USA | 6 | 27 | 1 L vase | Distilled water |
| FLSuburb18-1 | Ae. aegypti | June 2018 | Vero Beach Suburb | Vero Beach, FL, USA | 4 | 26 | 4 L bucket | Distilled water |
| FLSuburb18-2 | Ae. aegypti | July 2018 | Vero Beach Suburb | Vero Beach, FL, USA | 4 | 30 | 4 L bucket | Distilled water |
| FLCemetery19-1 | Ae. aegypti | June 2019 | Memorial Gardens Cemetery | Fort Myers, FL, USA | N/A | 22 | 1 L vase | Rainwater |
| FLCemetery19-2 | Ae. aegypti | June 2019 | Memorial Gardens Cemetery | Fort Myers, FL, USA | 4 | 29 | 1 L vase | Distilled water |
| Experiment Name | Target Species | Start Date (day 0) | Container Type | Initial Larval Densities (Number of Containers) | Census Days | Detritus Addition |
|---|---|---|---|---|---|---|
| ILForest17 | Ae. triseriatus | 21 July 2017 | 8L bucket | 10(6) 20(6) 40(4) 80(4) 160(2) 320(2) | 6, 10, 14, 18 | No |
| ILForest18 | Ae. triseriatus | 11 July 2018 | 4L bucket | 50(4) 100(4) 150(6) 200(2) 300(2) | 6, 10, 14, 18 | Half |
| MOForest18 | Ae. albopictus | 23 June 2018 | 4L bucket | 50(2) 100(8) 200(4) 300(4) 400(4) 500(2) | 6, 10, 14 | Half |
| ILCemetery19 | Ae. albopictus | 28 June 2019 | 1L vase | 25(4) 50(4) 100(4) 150(3) 200(3) 300(3) | 6, 9, 12 | All |
| FLSuburb18-1 | Ae. aegypti | 30 June 2018 | 4L bucket | 70(6) 150(6) 250(6) 350(6) | 6, 9, 12 | Half |
| FLSuburb18-2 | Ae. aegypti | 1 August 2018 | 4L bucket | 50(5) 100(5) 150(5) 175(5) | 6, 9, 13 | All |
| FLCemetery19-1 | Ae. aegypti | 22 July 2019 | 1L vase | 10(4) 30(4) 50(4) 80(4) 100(4) | 6, 9, 12, 15, 17 | All |
| FLCemetery19-2 | Ae. aegypti | 22 July 2019 | 1L vase | 20(4) 50(3) 90(5) 130(3) 160(3) | 6, 9, 12, 15, 17 | Half |
| Experiment Name | Target Species | a | 95% CI | K | 95% CI | d | 95% CI | Interpretation |
|---|---|---|---|---|---|---|---|---|
| ILForest17 | Ae. triseriatus | 0.663 | [0.596, 0.731] | 187.4 | [169.0, 205.9] | 5.9096 | [4.6543, 7.1649] | Overcompensation |
| ILForest18 | Ae. triseriatus | 0.445 | [−0.816, 1.707] | 500 | [−15,885, 16,885] | −0.1693 | [−0.6292, 0.2906] | Additive mortality |
| MOForest18 | Ae. albopictus | 0.605 | [0.5669, 0.6426] | 476.8 | [457.0, 496.7] | 5.4821 | [3.8778, 7.0865] | Overcompensation |
| ILCemetery19 | Ae. albopictus | 0.944 | [0.104, 1.283] | 137.2 | [−272.0, 546.5] | 0.6269 | [−0.0295, 1.2833] | Additive mortality |
| FLSuburb18-1 | Ae. aegypti | 0.826 | [0.466, 1.186] | 250.9 | [48.4, 451.6] | 1.1658 | [0.4167, 1.9150] | Compensation |
| FLSuburb18-2 | Ae. aegypti | 0.635 | [0.379, 0.890] | 167.4 | [95.7, 239.8] | 1.8690 | [0.0219, 3.7161] | Compensation |
| FLCemetery19-1 | Ae. aegypti | 1 | N/A | 400.2 | [−222.5, 1023.0] | 0.3976 | [0.03373, 0.7614] | Additive mortality |
| FLCemetery19-2 | Ae. aegypti | 1 | N/A | 153.8 | [122.8, 184.9] | 1.0134 | [0.5263, 1.5005] | Compensation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, K.G.; Neale, Z.R.; Holly, B.; Canizela, C.C.; Juliano, S.A. Survival-Larval Density Relationships in the Field and Their Implications for Control of Container-Dwelling Aedes Mosquitoes. Insects 2023, 14, 17. https://doi.org/10.3390/insects14010017
Evans KG, Neale ZR, Holly B, Canizela CC, Juliano SA. Survival-Larval Density Relationships in the Field and Their Implications for Control of Container-Dwelling Aedes Mosquitoes. Insects. 2023; 14(1):17. https://doi.org/10.3390/insects14010017
Chicago/Turabian StyleEvans, Katherine G., Zoey R. Neale, Brendan Holly, Cecilia C. Canizela, and Steven A. Juliano. 2023. "Survival-Larval Density Relationships in the Field and Their Implications for Control of Container-Dwelling Aedes Mosquitoes" Insects 14, no. 1: 17. https://doi.org/10.3390/insects14010017
APA StyleEvans, K. G., Neale, Z. R., Holly, B., Canizela, C. C., & Juliano, S. A. (2023). Survival-Larval Density Relationships in the Field and Their Implications for Control of Container-Dwelling Aedes Mosquitoes. Insects, 14(1), 17. https://doi.org/10.3390/insects14010017





