Population Genetics of Anopheles pretoriensis in Grande Comore Island
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Mosquito Sampling
2.3. Mitogenome Sequencing and Analysis
2.4. COI Sequencing and Analysis
3. Results
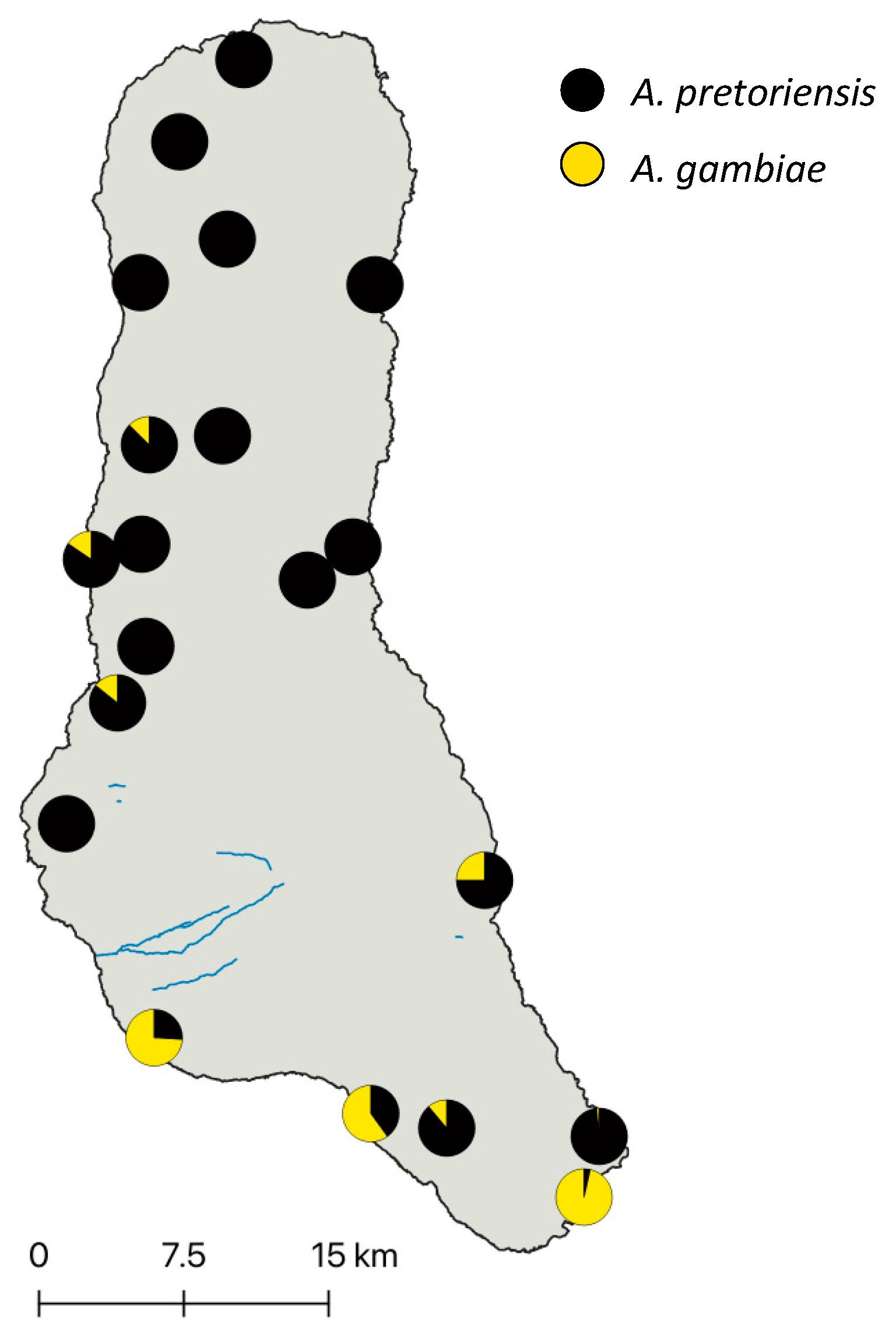
3.1. Mosquito Identification and Abundance
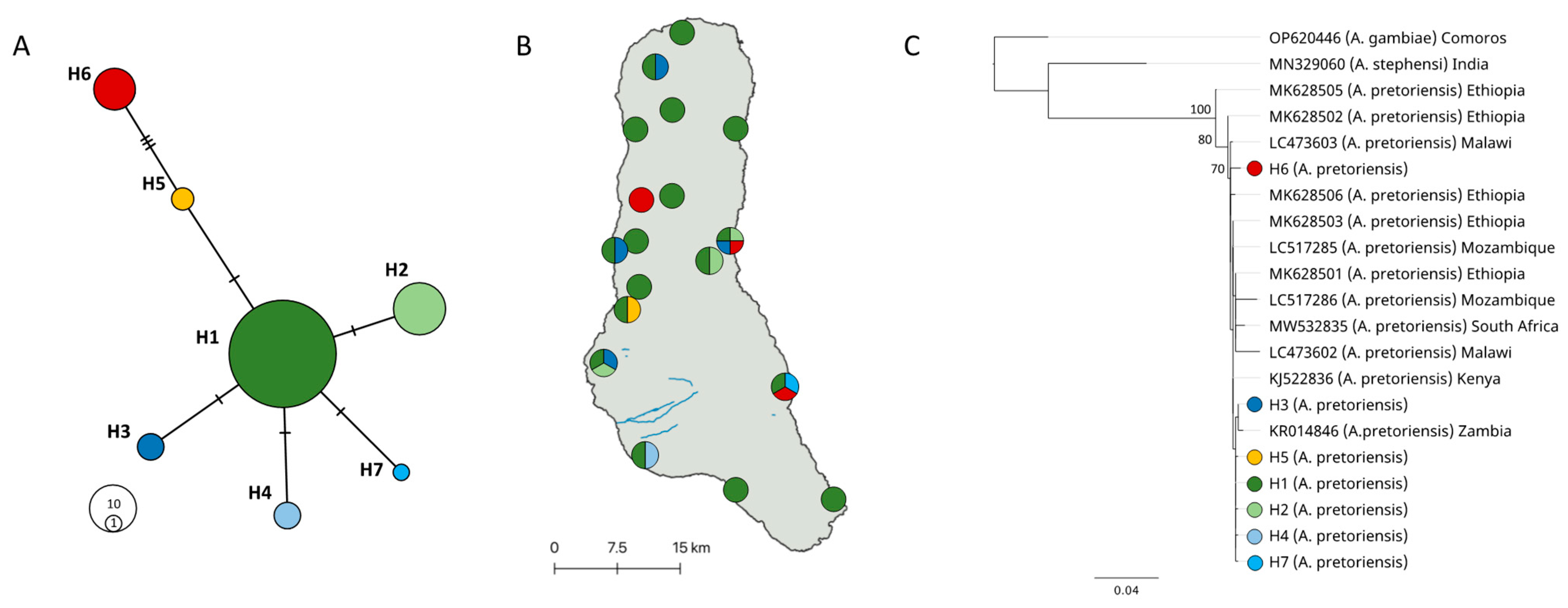
3.2. Anopheles Pretoriensis Mitogenome Phylogeny
3.3. Anopheles pretoriensis Population Genetics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Rubio-Palis, Y.; Chareonviriyaphap, T.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; et al. A global map of dominant malaria vectors. Parasit Vectors 2012, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Lobo, N.F.; St Laurent, B.; Sikaala, C.H.; Hamainza, B.; Chanda, J.; Chinula, D.; Krishnankutty, S.M.; Mueller, J.D.; Deason, N.A.; Hoang, Q.T.; et al. Unexpected diversity of Anopheles species in Eastern Zambia: Implications for evaluating vector behavior and interventions using molecular tools. Sci. Rep. 2015, 5, 17952. [Google Scholar] [CrossRef] [Green Version]
- Antonio-Nkondjio, C.; Kerah, C.H.; Simard, F.; Awono-Ambene, P.; Chouaibou, M.; Tchuinkam, T.; Fontenille, D. Complexity of the malaria vectorial system in Cameroon: Contribution of secondary vectors to malaria transmission. J. Med. Entomol. 2006, 43, 1215–1221. [Google Scholar] [CrossRef]
- Braack, L.; Bornman, R.; Kruger, T.; Dahan-Moss, Y.; Gilbert, A.; Kaiser, M.; Oliver, S.V.; Cornel, A.J.; Lee, Y.; Norris, D.E.; et al. Malaria Vectors and Vector Surveillance in Limpopo Province (South Africa): 1927 to 2018. Int. J. Environ. Res. Public Health 2020, 17, 4125. [Google Scholar] [CrossRef]
- Chakir, I.; Said, A.I.; Affane, B.; Jambou, R. Control of malaria in the Comoro Islands over the past century. Malar. J. 2017, 16, 387. [Google Scholar] [CrossRef] [Green Version]
- Kassim, S.; James, P.; Alolga, R.; Assanhou, A.; Kassim, S.; Bacar, A.; Silai, R.; Tian, L.; Li, H.; Ma, A. Major decline in malaria morbidity and mortality in the Union of Comoros between 2010 and 2014: The effect of a combination of prevention and control measures. S. Afr. Med. J. 2016, 106, 709–714. [Google Scholar] [CrossRef] [Green Version]
- Attoumane, A.; Silai, R.; Bacar, A.; Cardinale, E.; Pennober, G.; Herbreteau, V. Changing Patterns of Malaria in Grande Comore after a Drastic Decline: Importance of Fine-Scale Spatial Analysis to Inform Future Control Actions. Remote Sens. 2020, 12, 4082. [Google Scholar] [CrossRef]
- Marsden, C.D.; Cornel, A.; Lee, Y.; Sanford, M.R.; Norris, L.C.; Goodell, P.B.; Nieman, C.C.; Han, S.; Rodrigues, A.; Denis, J.; et al. An analysis of two island groups as potential sites for trials of transgenic mosquitoes for malaria control. Evol. Appl. 2013, 6, 706–720. [Google Scholar] [CrossRef]
- Lanzaro, G.C.; Campos, M.; Crepeau, M.; Cornel, A.; Estrada, A.; Gripkey, H.; Haddad, Z.; Kormos, A.; Palomares, S. Selection of sites for field trials of genetically engineered mosquitoes with gene drive. Evol. Appl. 2021, 14, 2147–2161. [Google Scholar] [CrossRef]
- Ciubotariu, I.I.; Jones, C.M.; Kobayashi, T.; Bobanga, T.; Muleba, M.; Pringle, J.C.; Stevenson, J.C.; Carpi, G.; Norris, D.E. Genetic Diversity of Anopheles coustani (Diptera: Culicidae) in Malaria Transmission Foci in Southern and Central Africa. J. Med. Entomol. 2020, 57, 1782–1792. [Google Scholar] [CrossRef]
- Nieman, C.C.; Yamasaki, Y.; Collier, T.C.; Lee, Y. A DNA extraction protocol for improved DNA yield from individual mosquitoes. F1000Research 2015, 4, 1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamasaki, Y.K.; Nieman, C.C.; Chang, A.N.; Collier, T.C.; Main, B.J.; Lee, Y. Improved tools for genomic DNA library construction of small insects. F1000Research 2016, 5, 211. [Google Scholar] [CrossRef]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017, 45, e18. [Google Scholar] [CrossRef] [Green Version]
- Bernt, M.; Donath, A.; Juhling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Putz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [Green Version]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Leigh, J.W.; Bryant, D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Guo, J.; Yan, Z.T.; Fu, W.B.; Yuan, H.; Li, X.D.; Chen, B. Complete mitogenomes of Anopheles peditaeniatus and Anopheles nitidus and phylogenetic relationships within the genus Anopheles inferred from mitogenomes. Parasit Vectors 2021, 14, 452. [Google Scholar] [CrossRef]
- Theobald, F.V. A Monograph of the Culicidae of the World; British Museum (Natural History): London, UK, 1903; Volume 3. [Google Scholar]
- Gillies, M.T.; De Meillon, B.A. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region). S. Afr. Inst. Med. Res. Johannesbg. 1968, 54, 3–343. [Google Scholar]
- Kyalo, D.; Amratia, P.; Mundia, C.W.; Mbogo, C.M.; Coetzee, M.; Snow, R.W. A geo-coded inventory of anophelines in the Afrotropical Region south of the Sahara: 1898–2016. Wellcome Open Res. 2017, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.; Alves, J.; Dia, I.; Gómez, L.F. Susceptibility of mosquito vectors of the city of Praia, Cabo Verde, to Temephos and Bacillus thuringiensis var israelensis. PLoS ONE 2020, 15, e0234242. [Google Scholar] [CrossRef] [PubMed]
- Tedrow, R.E.; Rakotomanga, T.; Nepomichene, T.; Howes, R.E.; Ratovonjato, J.; Ratsimbasoa, A.C.; Svenson, G.J.; Zimmerman, P.A. Anopheles mosquito surveillance in Madagascar reveals multiple blood feeding behavior and Plasmodium infection. PLoS Negl. Trop. Dis. 2019, 13, e0007176. [Google Scholar] [CrossRef]
- Le Goff, G.; Goodman, S.M.; Elguero, E.; Robert, V. Survey of the mosquitoes (Diptera: Culicidae) of Mayotte. PLoS ONE 2014, 9, e100696. [Google Scholar] [CrossRef] [Green Version]
- Al-Sheik, A.A. Larval habitat, ecology, seasonal abundance and vectorial role in malaria transmission of Anopheles arabiensis in Jazan Region of Saudi Arabia. J. Egypt Soc. Parasitol. 2011, 41, 615–634. [Google Scholar]
- Al-Eryani, S.M.A.; Kelly-Hope, L.; Harbach, R.E.; Briscoe, A.G.; Barnish, G.; Azazy, A.; McCall, P.J. Entomological aspects and the role of human behaviour in malaria transmission in a highland region of the Republic of Yemen. Malar. J. 2016, 15, 130. [Google Scholar] [CrossRef] [Green Version]
- Jacques, B. Les moustiques de l’archipel Comores. Sér. Entomol. Médicale Parasitol. 1977, 15, 153–170. [Google Scholar]
- Gutiérrez, L.A.; González, J.J.; Gómez, G.F.; Castro, M.I.; Rosero, D.A.; Luckhart, S.; Conn, J.E.; Correa, M.M. Species composition and natural infectivity of anthropophilic Anopheles (Diptera: Culicidae) in the states of Córdoba and Antioquia, Northwestern Colombia. Memórias Inst. Oswaldo Cruz 2009, 104, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Naranjo-Díaz, N.; Conn, J.E.; Correa, M.M. Behavior and population structure of Anopheles darlingi in Colombia. Infect. Genet. Evol. 2016, 39, 64–73. [Google Scholar] [CrossRef]
- Frankham, R. Do island populations have less genetic variation than mainland populations? Heredity 1997, 78, 311–327. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, M.; Patel, N.; Marshall, C.; Gripkey, H.; Ditter, R.E.; Crepeau, M.W.; Toilibou, A.; Amina, Y.; Cornel, A.J.; Lee, Y.; et al. Population Genetics of Anopheles pretoriensis in Grande Comore Island. Insects 2023, 14, 14. https://doi.org/10.3390/insects14010014
Campos M, Patel N, Marshall C, Gripkey H, Ditter RE, Crepeau MW, Toilibou A, Amina Y, Cornel AJ, Lee Y, et al. Population Genetics of Anopheles pretoriensis in Grande Comore Island. Insects. 2023; 14(1):14. https://doi.org/10.3390/insects14010014
Chicago/Turabian StyleCampos, Melina, Nikita Patel, Carly Marshall, Hans Gripkey, Robert E. Ditter, Marc W. Crepeau, Ali Toilibou, Yssouf Amina, Anthony J. Cornel, Yoosook Lee, and et al. 2023. "Population Genetics of Anopheles pretoriensis in Grande Comore Island" Insects 14, no. 1: 14. https://doi.org/10.3390/insects14010014
APA StyleCampos, M., Patel, N., Marshall, C., Gripkey, H., Ditter, R. E., Crepeau, M. W., Toilibou, A., Amina, Y., Cornel, A. J., Lee, Y., & Lanzaro, G. C. (2023). Population Genetics of Anopheles pretoriensis in Grande Comore Island. Insects, 14(1), 14. https://doi.org/10.3390/insects14010014






