Predicting the Weathering Time by the Empty Puparium of Sarcophaga peregrina (Diptera: Sarcophagidae) with the ANN Models
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.3. Regression Algorithms
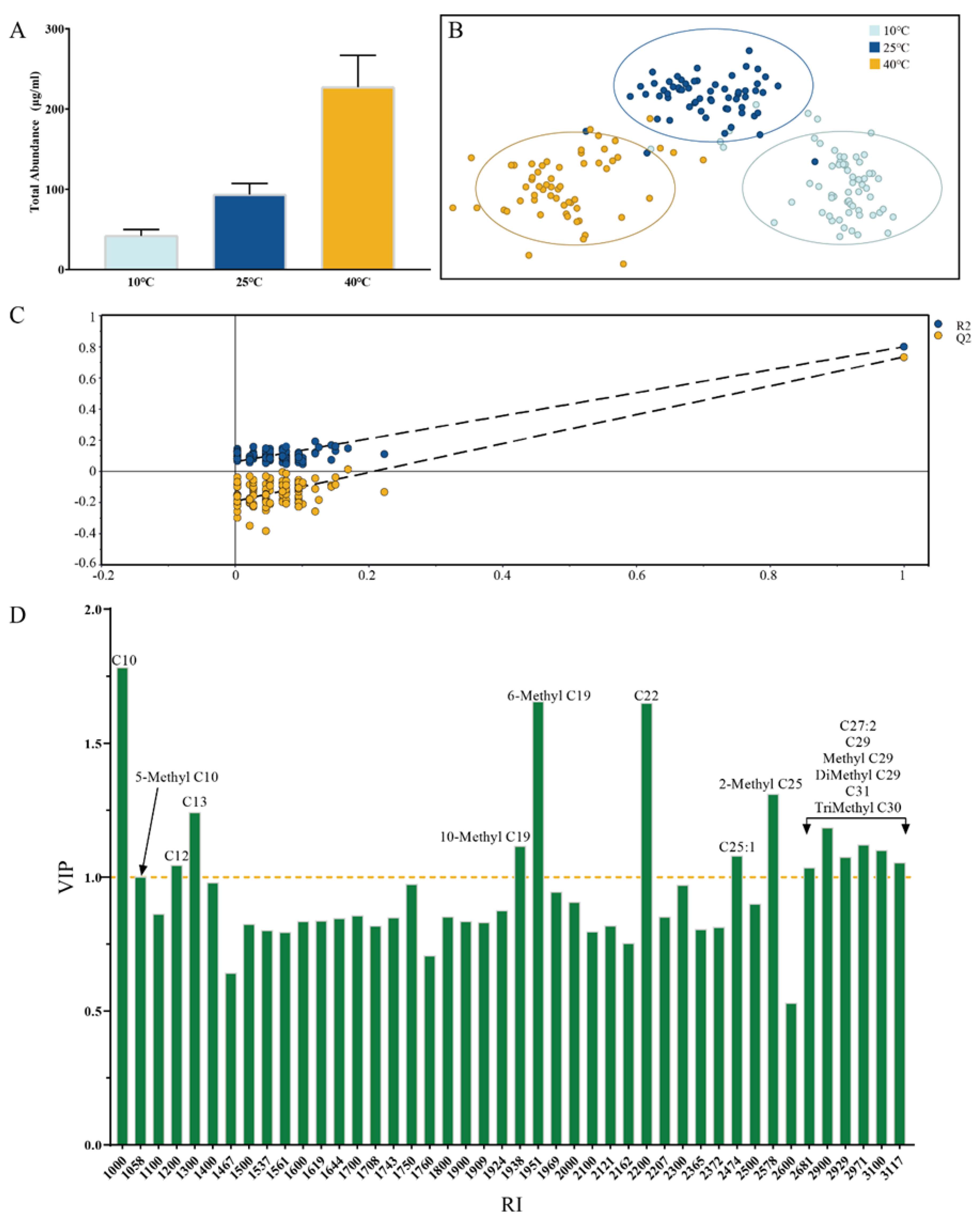
3. Results
3.1. The OPLS-DA Models of S. peregrina Puparium Classified by Temperatures
3.2. The PLS Model of S. peregrina Puparium
3.3. The SVR Model of S. peregrina Puparium
3.4. The ANN Model of S. peregrina Puparium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pape, T. Catalogue of the Sarcophagidae of the world (Insecta: Diptera). Mem. Entomol. Int. 1996, 8, 558. [Google Scholar]
- Abela, G. Benefits of maggot debridement therapy on leg ulcers: A literature review. Br. J. Community Nurs. 2017, 22, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Piwczyński, M.; Szpila, K.; Grzywacz, A.; Pape, T. A large-scale molecular phylogeny of flesh flies (Diptera: Sarcophagidae). Syst. Entomol. 2015, 39, 783–799. [Google Scholar]
- Ren, L.; Shang, Y.; Chen, W.; Meng, F.; Cai, J.; Zhu, G.; Chen, L.; Wang, Y.; Deng, J.; Guo, Y. A brief review of forensically important flesh flies (Diptera: Sarcophagidae). Forensic. Sci. Res. 2018, 3, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Sukontason, K.; Sribanditmongkol, P.; Ngoen-klan, R.; Klong-klaew, T.; Moophayak, K.; Sukontason, K.L. Differentiation between Lucilia cuprina and Hemipyrellia ligurriens (Diptera: Calliphoridae) larvae for use in forensic entomology applications. Parasitol. Res. 2010, 106, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Sukontason, K.; Bunchu, N.; Chaiwong, T.; Moophayak, K.; Sukontason, K.L. Forensically important flesh fly species in Thailand: Morphology and developmental rate. Parasitol. Res. 2010, 106, 1055–1064. [Google Scholar] [CrossRef]
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J. Forensic entomology: Applications and limitations. Forensic. Sci. Med. Pathol. 2011, 7, 379–392. [Google Scholar] [CrossRef]
- Archer, M.S.; Elgar, M.A. Effects of decomposition on carcass attendance in a guild of carrion-breeding flies. Med. Vet. Entomol. 2003, 17, 263–271. [Google Scholar]
- Frere, B.; Suchaud, F.; Bernier, G.; Cottin, F.; Vincent, B.; Dourel, L.; Lelong, A.; Arpino, P. GC-MS analysis of cuticular lipids in recent and older scavenger insect puparia. An approach to estimate the postmortem interval (PMI). Anal. Bioanal. Chem. 2014, 406, 1081–1088. [Google Scholar] [CrossRef]
- Amendt, J.; Krettek, R.; Niess, C.; Zehner, R.; Bratzke, H. Forensic entomology in Germany. Forensic Sci. Int. 2000, 113, 309–314. [Google Scholar] [CrossRef]
- Zhu, G.H.; Xu, X.H.; Yu, X.J.; Zhang, Y.; Wang, J.F. Puparial case hydrocarbons of Chrysomya megacephala as an indicator of the postmortem interval. Forensic. Sci. Int. 2007, 169, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.H.; Yu, X.J.; Xie, L.X.; Luo, H.; Wang, D.; Lv, J.Y.; Xu, X.H. Time of death revealed by hydrocarbons of empty puparia of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae): A field experiment. PLoS ONE 2013, 8, e73043. [Google Scholar] [CrossRef] [PubMed]
- Sukontason, K.L.; Ngern-Klun, R.; Sripakdee, D.; Sukontason, K. Identifying fly puparia by clearing technique: Application to forensic entomology. Parasitol. Res. 2007, 101, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Li, K.; Zhu, J.; Zhu, G.; Hu, C. Cuticular hydrocarbon composition in pupal exuviae for taxonomic differentiation of six necrophagous flies. J. Med. Entomol. 2007, 44, 450–456. [Google Scholar] [CrossRef]
- Braga, M.V.; Pinto, Z.T.; de Carvalho Queiroz, M.M.; Matsumoto, N.; Blomquist, G.J. Cuticular hydrocarbons as a tool for the identification of insect species: Puparial cases from Sarcophagidae. Acta Trop. 2013, 128, 479–485. [Google Scholar] [CrossRef]
- Ginzel, M.D.; Blomquist, G.J. Insect Hydrocarbons: Biochemistry and Chemical Ecology. In Extracellular Composite Matrices in Arthropods; Cohen, E., Moussian, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 221–252. [Google Scholar] [CrossRef]
- Chung, H.; Carroll, S.B. Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. Bioessays 2015, 37, 822–830. [Google Scholar] [CrossRef]
- Botella-Cruz, M.; Velasco, J.; Millán, A.; Hetz, S.; Pallarés, S. Cuticle Hydrocarbons Show Plastic Variation under Desiccation in Saline Aquatic Beetles. Insects 2021, 12, 285. [Google Scholar] [CrossRef]
- Moore, H.E.; Butcher, J.B.; Adam, C.D.; Day, C.R.; Drijfhout, F.P. Age estimation of Calliphora (Diptera: Calliphoridae) larvae using cuticular hydrocarbon analysis and Artificial Neural Networks. Forensic Sci. Int. 2016, 268, 81–91. [Google Scholar] [CrossRef][Green Version]
- Moore, H.E.; Butcher, J.B.; Day, C.R.; Drijfhout, F.P. Adult fly age estimations using cuticular hydrocarbons and Artificial Neural Networks in forensically important Calliphoridae species. Forensic Sci. Int. 2017, 280, 233–244. [Google Scholar] [CrossRef]
- Moore, H.E.; Hall, M.J.R.; Drijfhout, F.P.; Cody, R.B.; Whitmore, D. Cuticular hydrocarbons for identifying Sarcophagidae (Diptera). Sci. Rep. 2021, 11, 7732. [Google Scholar] [CrossRef]
- Carlson, D.A.; Langley, P.A.; Huyton, P. Sex pheromone of the tsetse fly: Isolation, identification, and synthesis of contact aphrodisiacs. Science 1978, 201, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Vaníčková, L.; Virgilio, M.; Tomčala, A.; Břízová, R.; Ekesi, S.; Hoskovec, M.; Kalinová, B.; Do Nascimento, R.R.; De Meyer, M. Resolution of three cryptic agricultural pests (Ceratitis fasciventris, C. anonae, C. rosa, Diptera: Tephritidae) using cuticular hydrocarbon profiling. Bull Entomol. Res. 2014, 104, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Shang, Y.; Yang, L.; Wang, S.; Wang, X.; Chen, S.; Bao, Z.; An, D.; Meng, F.; Cai, J.; et al. Chromosome-level de novo genome assembly of Sarcophaga peregrina provides insights into the evolutionary adaptation of flesh flies. Mol. Ecol. Resour. 2021, 21, 251–262. [Google Scholar] [CrossRef]
- Sukontason, K.L.; Sanit, S.; Klong-Klaew, T.; Tomberlin, J.K.; Sukontason, K. Sarcophaga (Liosarcophaga) dux (Diptera: Sarcophagidae): A flesh fly species of medical importance. Biol. Res. 2014, 47, 14. [Google Scholar] [PubMed]
- Zhang, X.; Shang, Y.; Ren, L.; Qu, H.; Zhu, G.; Guo, Y. A Study of Cuticular Hydrocarbons of All Life Stages in Sarcophaga peregrina (Diptera: Sarcophagidae). J. Med. Entomol. 2021, 59, 108–119. [Google Scholar] [CrossRef]
- Carlson, D.A.; Bernier, U.R.; Sutton, B.D. Elution Patterns from Capillary GC for Methyl-Branched Alkanes. J. Chem. Ecol. 1998, 24, 1845–1865. [Google Scholar] [CrossRef]
- Espelie, K.E.; Bernays, E.A. Diet-related differences in the cuticular lipids ofManduca sexta larvae. J. Chem. Ecol. 1989, 15, 2003–2017. [Google Scholar] [CrossRef]
- Bernier, U.R.; Carlson, D.A.; Geden, C.J. Gas chromatography/mass spectrometry analysis of the cuticular hydrocarbons from parasitic wasps of the genus Muscidifurax. J. Am. Soc. Mass Spectrom. 1998, 9, 320–332. [Google Scholar] [CrossRef]
- Park, S.J.; Pandey, G.; Castro-Vargas, C.; Oakeshott, J.G.; Taylor, P.W.; Mendez, V. Cuticular Chemistry of the Queensland Fruit Fly Bactrocera tryoni (Froggatt). Molecules 2020, 25, 4185. [Google Scholar] [CrossRef]
- Krkosova, Z.; Kubinec, R.; Sojak, L.; Amann, A. Temperature-programmed gas chromatography linear retention indices of all C4-C30 monomethylalkanes on methylsilicone OV-1 stationary phase. Contribution towards a better understanding of volatile organic compounds in exhaled breath. J. Chromatogr. A. 2008, 1179, 59–68. [Google Scholar] [CrossRef]
- Pang, T.; Zhu, S.; Lu, X.; Xu, G. Identification of unknown compounds on the basis of retention index data in comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2007, 30, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Kováts, E.S.; Weisz, P.B. Über den Retentionsindex und seine Verwendung zur Aufstellung einer Polaritätsskala für Lösungsmittel. Berichte der Bunsengesellschaft für physikalische Chemie 1965, 69, 812–820. [Google Scholar] [CrossRef]
- Roux, O.; Gers, C.; Legal, L. Ontogenetic study of three Calliphoridae of forensic importance through cuticular hydrocarbon analysis. Med. Vet. Entomol. 2008, 22, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Haywood, A.L.; Redshaw, J.; Hanson-Heine, M.W.D.; Taylor, A.; Brown, A.; Mason, A.M.; Gärtner, T.; Hirst, J.D. Kernel Methods for Predicting Yields of Chemical Reactions. J. Chem. Inf. Model 2021, 62, 2077–2092. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, F.; Rännar, S. Alternative Partial Least-Squares (PLS) Algorithms. In 3D QSAR in Drug Design: Recent Advances; Kubinyi, H., Folkers, G., Martin, Y.C., Eds.; Springer: Dordrecht, The Netherlands.
- Fong, S.S.; Sági-Kiss, V.; Brereton, R.G. Self-Organizing Maps and Support Vector Regression as aids to coupled chromatography: Illustrated by predicting spoilage in apples using volatile organic compounds. Talanta 2011, 83, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, R.; Vogt, M.; Bajorath, J. Support Vector Machine Classification and Regression Prioritize Different Structural Features for Binary Compound Activity and Potency Value Prediction. ACS Omega 2017, 2, 6371–6379. [Google Scholar] [CrossRef]
- Awad, M.; Khanna, R. Support Vector Regression. In Efficient Learning Machines: Theories, Concepts, and Applications for Engineers and System Designers; Apress: Berkeley, CA, USA, 2015; pp. 67–80. [Google Scholar] [CrossRef]
- Wydra, J.; Matuszewski, S. The optimal post-eclosion interval while estimating the post-mortem interval based on an empty puparium. Forensic Sci. Med. Pathol. 2021, 17, 192–198. [Google Scholar] [CrossRef]
- Zhu, G.H.; Jia, Z.J.; Yu, X.J.; Wu, K.S.; Chen, L.S.; Lv, J.Y.; Eric Benbow, M. Predictable weathering of puparial hydrocarbons of necrophagous flies for determining the postmortem interval: A field experiment using Chrysomya rufifacies. Int. J. Legal Med. 2017, 131, 885–894. [Google Scholar] [CrossRef]
- Kuhn, M.; Johnson, K. Linear Regression and Its Cousins. In Applied Predictive Modeling; Springer: New York, NY, USA, 2013; pp. 101–139. [Google Scholar] [CrossRef]
- Paula, M.C.; Michelutti, K.B.; Eulalio, A.; Piva, R.C.; Cardoso, C.A.L.; Antonialli-Junior, W.F. New method for estimating the post-mortem interval using the chemical composition of different generations of empty puparia: Indoor cases. PLoS ONE 2018, 13, e0209776. [Google Scholar] [CrossRef]
- Sharma, P.; Schiewer, S. Assessment of crude oil biodegradation in arctic seashore sediments: Effects of temperature, salinity, and crude oil concentration. Environ. Sci. Pollut Res. Int. 2016, 23, 14881–14888. [Google Scholar] [CrossRef]
- Ma, M.; Gao, W.; Li, Q.; Han, B.; Zhu, A.; Yang, H.; Zheng, L. Biodiversity and oil degradation capacity of oil-degrading bacteria isolated from deep-sea hydrothermal sediments of the South Mid-Atlantic Ridge. Mar. Pollut. Bull. 2021, 171, 112770. [Google Scholar] [CrossRef] [PubMed]
- Acosta, X.; Corronca, J.A.; González-Reyes, A.X.; Centeno, N.D. Postmortem Interval Estimation and Validation Through a Comparative Study of South American Flies Reared in the Field Versus Laboratory Conditions. J. Med. Entomol. 2022, 59, 147–161. [Google Scholar] [CrossRef] [PubMed]
| Group | Training Set | Validation Set |
|---|---|---|
| 10 °C | (50, 49) | (12, 49) |
| 25 °C | (50, 49) | (12, 49) |
| 40 °C | (46, 49) | (12, 49) |
| Group | Number of Principal Components | Max Iters |
|---|---|---|
| 10 °C | 9 | 10 |
| 25 °C | 2 | 10 |
| 40 °C | 3 | 10 |
| Group | Model | Training Set | Validation Set | Total Set | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | MSE | MAE | R2 | RMSE | MSE | MAE | R2 | RMSE | MSE | MAE | ||
| 10 °C | ANN | 0.96 | 7.6 | 57.78 | 5.39 | 0.81 | 17.9 | 320.36 | 12.44 | 0.94 | 9.5 | 90.32 | 5.76 |
| SVR | 0.98 | 6.22 | 38.73 | 2.08 | 0.57 | 20.94 | 438.61 | 18.92 | 0.92 | 11.07 | 122.58 | 5.61 | |
| PLS | 0.86 | 14.27 | 203.76 | 10.84 | 0.55 | 27.92 | 779.3 | 22.89 | 0.78 | 18.01 | 324.44 | 13.36 | |
| 25 °C | ANN | 0.79 | 17.22 | 296.5 | 11.25 | 0.69 | 23.19 | 537.77 | 15.88 | 0.77 | 18.63 | 347.09 | 12.22 |
| SVR | 0.91 | 11.89 | 141.38 | 3.79 | 0.43 | 24.23 | 587.13 | 19.11 | 0.84 | 15.32 | 234.84 | 7 | |
| PLS | 0.53 | 25.95 | 673.16 | 20.18 | 0.57 | 27.2 | 739.58 | 20.27 | 0.54 | 26.21 | 687.08 | 20.2 | |
| 40 °C | ANN | 0.88 | 13.5 | 182.32 | 10.21 | 0.76 | 15.88 | 252.06 | 12.4 | 0.86 | 14.03 | 196.75 | 10.66 |
| SVR | 1 | 0.66 | 0.44 | 0.19 | 0.66 | 21.38 | 457.18 | 16.21 | 0.93 | 9.74 | 94.94 | 3.51 | |
| PLS | 0.71 | 21.02 | 441.89 | 16.59 | 0.64 | 19.39 | 376.14 | 14.44 | 0.7 | 20.7 | 428.29 | 16.15 | |
| Group | C | γ |
|---|---|---|
| 10 °C | 310 | 0.045 |
| 25 °C | 499 | 0.293 |
| 40 °C | 200 | 0.1 |
| Group | Learning Rate | Epochs | Batch Size |
|---|---|---|---|
| 10 °C | 0.001 | 1000 | 32 |
| 25 °C | 0.01 | 100 | 32 |
| 40 °C | 0.0001 | 1000 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Bai, Y.; Ngando, F.J.; Qu, H.; Shang, Y.; Ren, L.; Guo, Y. Predicting the Weathering Time by the Empty Puparium of Sarcophaga peregrina (Diptera: Sarcophagidae) with the ANN Models. Insects 2022, 13, 808. https://doi.org/10.3390/insects13090808
Zhang X, Bai Y, Ngando FJ, Qu H, Shang Y, Ren L, Guo Y. Predicting the Weathering Time by the Empty Puparium of Sarcophaga peregrina (Diptera: Sarcophagidae) with the ANN Models. Insects. 2022; 13(9):808. https://doi.org/10.3390/insects13090808
Chicago/Turabian StyleZhang, Xiangyan, Yang Bai, Fernand Jocelin Ngando, Hongke Qu, Yanjie Shang, Lipin Ren, and Yadong Guo. 2022. "Predicting the Weathering Time by the Empty Puparium of Sarcophaga peregrina (Diptera: Sarcophagidae) with the ANN Models" Insects 13, no. 9: 808. https://doi.org/10.3390/insects13090808
APA StyleZhang, X., Bai, Y., Ngando, F. J., Qu, H., Shang, Y., Ren, L., & Guo, Y. (2022). Predicting the Weathering Time by the Empty Puparium of Sarcophaga peregrina (Diptera: Sarcophagidae) with the ANN Models. Insects, 13(9), 808. https://doi.org/10.3390/insects13090808







