Simple Summary
The name ‘wireworm’ refers to the subterranean larvae of click beetle (Coleoptera: Elateridae) species, of which several are serious pests of a wide range of crops. The limited effectiveness of the available insecticides, their wide host range, their long life cycle, and their cryptic subterranean habitat make wireworms a challenging pest to control. Integrated pest management (IPM) strategies have been recommended to reduce wireworm damage. Although IPM is generally considered to be an approach that is relatively more compatible with the environment and non-target organisms, the implementation of some of the tactics of managing subterranean wireworms is expected to induce stress in the rhizosphere and the established ecological interactions within, some of which could negatively impact various soil health parameters and, subsequently, plant growth. In this paper, we highlight some of the IPM tactics against wireworms and their effects on the rhizosphere and soil microbiome. Awareness of the potential impacts of IPM approaches to the management of subterranean pests will help professionals to develop and implement IPM strategies that minimize disturbance in the rhizosphere and support agroecosystem sustainability.
Abstract
The rhizosphere is where plant roots, physical soil, and subterranean organisms interact to contribute to soil fertility and plant growth. In agroecosystems, the nature of the ecological interactions within the rhizosphere is highly dynamic due to constant disruptions from agricultural practices. The concept of integrated pest management (IPM) was developed in order to promote an approach which is complementary to the environment and non-target organisms, including natural enemies, by reducing the sole reliance on synthetic pesticides to control pests. However, some of the implemented integrated cultural and biological control practices may impact the rhizosphere, especially when targeting subterranean pests. Wireworms, the larval stage of click beetles (Coleoptera: Elateridae), are generalist herbivores and a voracious group of pests that are difficult to control. This paper introduces some existing challenges in wireworm IPM, and discusses the potential impacts of various control methods on the rhizosphere. The awareness of the potential implications of different pest management approaches on the rhizosphere will assist in decision-making and the selection of the control tactics with the least long-term adverse effects on the rhizosphere.
1. Introduction
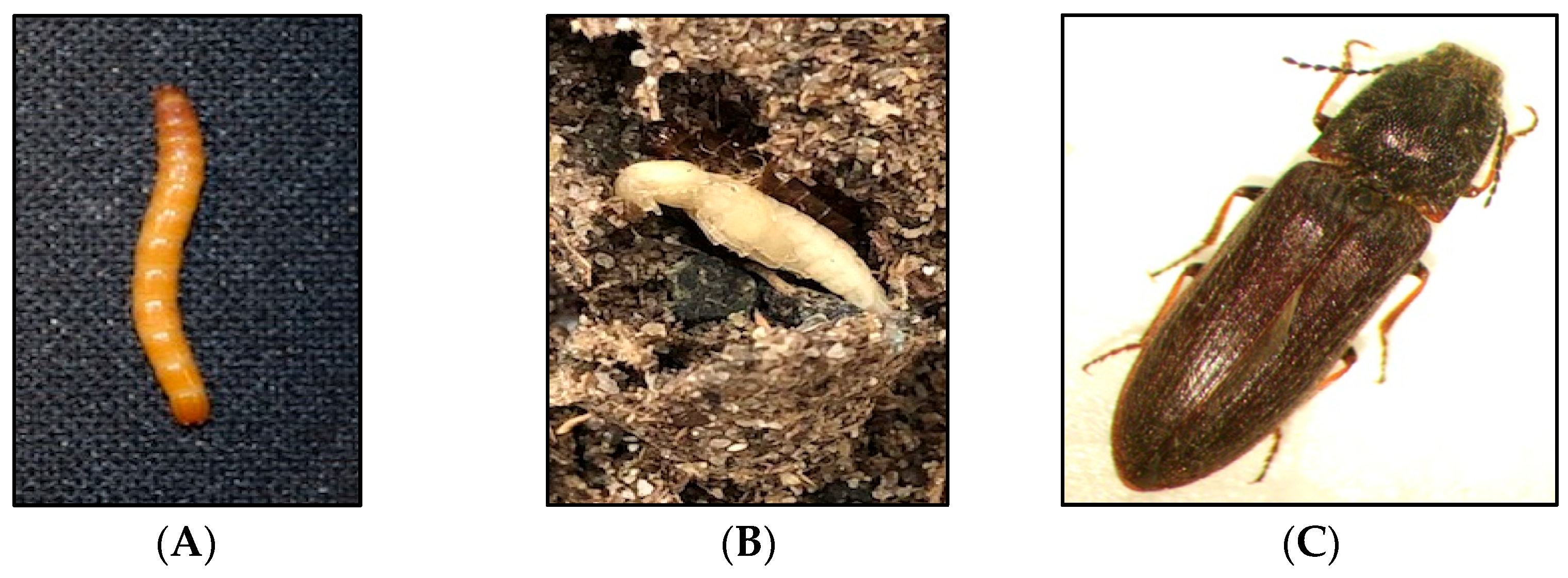
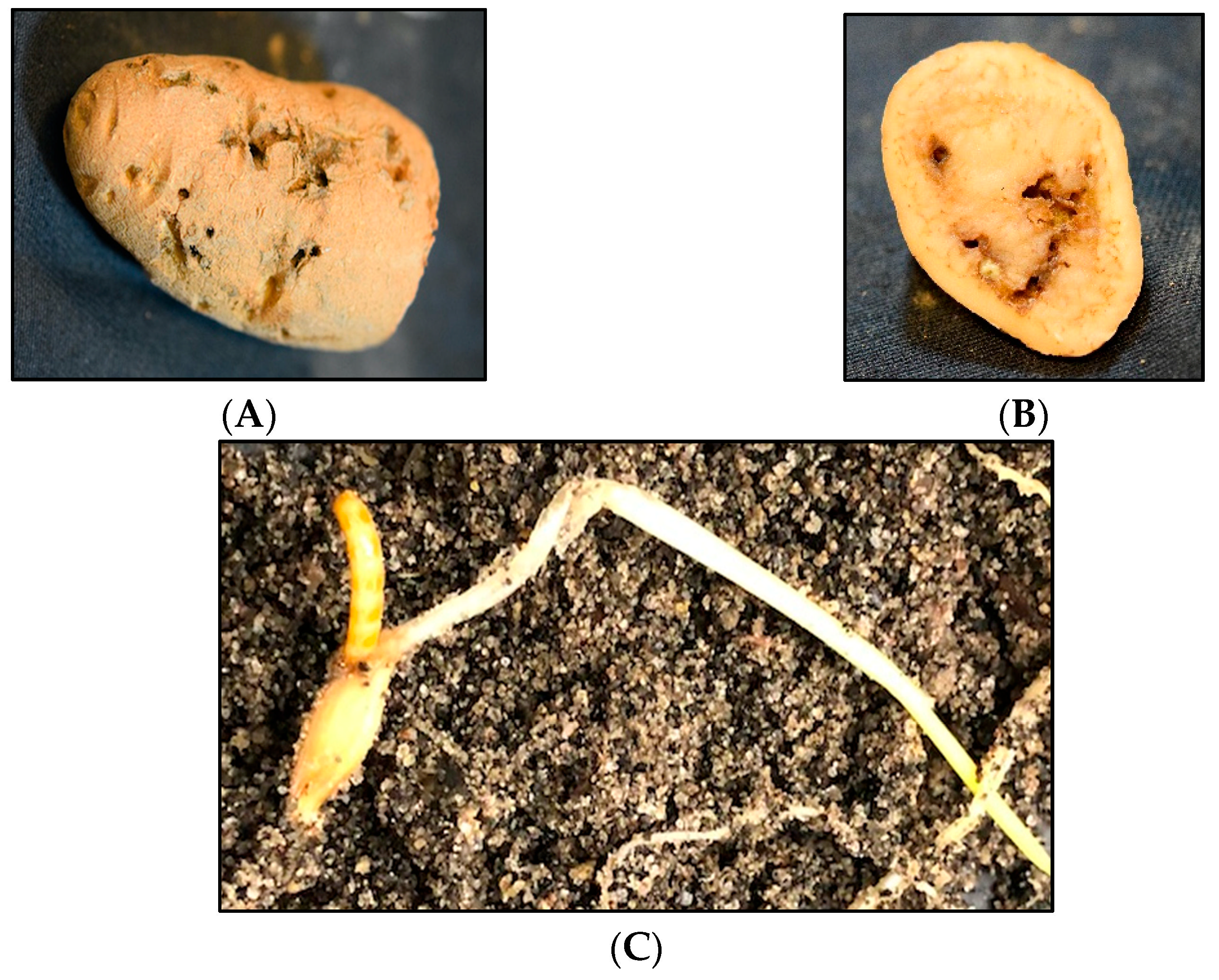
Subterranean wireworms are the larvae of click beetles (Coleoptera: Elateridae; Figure 1A–C), and are one of the most challenging pests to manage in agroecosystems. The adult beetles emerge in spring and early summer, between April and June in the Pacific Northwest (PNW) and the Intermountain West regions of the US, to mate and lay eggs. After egg hatching, the emerged larvae can live in the soil for multiple years, where they complete seven to nine instar larvae within two to four years [1,2]. Wireworms are generalist herbivores [2], and they can cause damage to almost any crop in the rotation because of their multiyear larval stage and broad host range. The damage from wireworms can be reflected in yield reduction and quality loss (Figure 2A–C). The stand thinning resulting from their feeding on seed, emerging sprouts, and young seedlings allows for the establishment of weeds in the wireworm-infested areas [2]. At the field scale, the damage is often patchy, corresponding to the patchiness of wireworm distribution within fields, likely due to the adults’ oviposition site preference [3]. The duration of the larval stage can be influenced by food availability and environmental conditions; wireworms can survive extended periods without live vegetation, and nonliving organic matter constitutes a negligible portion of their diets [4,5,6].
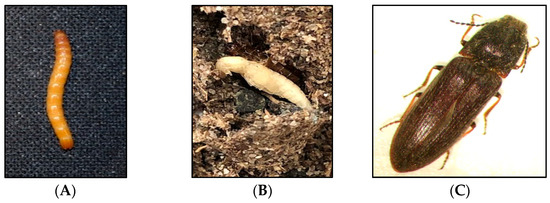
Figure 1.
Different developmental stages of the click beetle, Limonius californicus: (A) late instar larva, (B) pupa, and (C) adult.
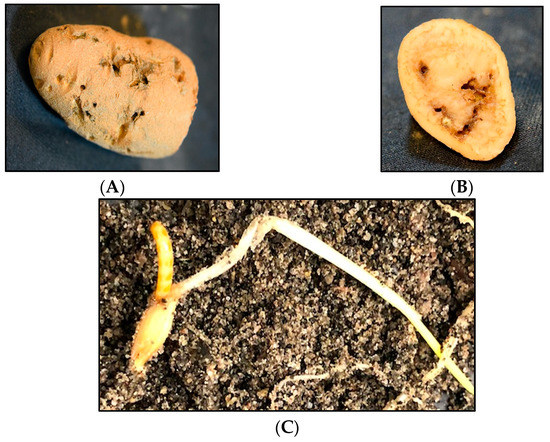
Figure 2.
Feeding damage by the sugar beet wireworm, Limonius californicus. (A,B) Wireworm feeding damage in a potato, and (C) feeding on a barley seed.
Prior to the early 2000s, persistent broad-spectrum organochlorine insecticides were used to keep wireworm populations in check [1,2,7,8]. After the de-registration of organochlorines in the USA, due to their negative impact on non-target organisms and environmental risks, and as the persistent residual activity started to fade away, wireworms re-emerged as a key pest in several regions [9,10]. Despite recent developments in the chemical control of wireworms, insecticides have often failed to adequately control the wireworm population in most crops, especially small grains [11].
Integrated pest management (IPM) is an ecologically based approach that promotes a biorational use of pesticides based on some decision-making guidelines [12,13,14,15]. Although there has been growing interest in the implementation of IPM against wireworms, their subterranean habitat, patchy distribution, vertical movement in the soil, long life-cycle, broad host range, and most importantly, limited location and the species-specific knowledge of their ecology and phenology have made them a difficult pest to control. Despite being a biorational strategy, the implementation of IPM tactics for the management of wireworms can potentially alter the rhizosphere and the ecological interactions within; these effects are often overlooked [16]. The rhizosphere is an interface where plant roots, soil, subterranean pests, and beneficial organisms interact [16]. The agroecosystem’s productivity is dependent on soil fertility and healthy plant development, both of which are influenced by the microbial composition and an array of ecological interactions in the rhizosphere [17,18]. The ecological interactions in the rhizosphere are constantly disrupted by changes in plant species (e.g., crop selection and rotation), the crop developmental stage [19,20,21], pest management [22,23], and other farming practices [16,24,25]. For example, pesticide applications in non-organic production systems are known to alter the chemical and biological soil properties [26,27,28] that are essential for both soil and crop health [27,29,30]. Shifts in the soil microbiome can also, directly and indirectly, influence pest survival and susceptibility to the implemented control practices. For example, some soil microorganisms are known to influence insect feeding [31] or contribute to insecticide detoxification [32].
Therefore, practices that can alter the ecological interactions in the rhizosphere are expected to influence both target (i.e., subterranean pests) and non-target organisms (i.e., beneficial organisms, plants). It is important to note that the ultimate goal of pest management is to maximize the productivity of the agroecosystem. Awareness of the potential impacts of various recommended control tactics on the rhizosphere will assist with the development of IPM strategies that can more effectively contribute to the resilience and sustainability of the agroecosystem. This brief review will first provide an overview of the IPM components associated with the decision-making process which are pertinent to wireworm control. We will then discuss the effectiveness of each recommended control practice in the management of wireworm populations and damage. Under each approach, we will highlight the potential impacts on the rhizosphere and soil microbiome. Finally, we will discuss the implications of those effects on agroecosystem productivity and resilience, and identify the existing gaps needing further investigations.
2. Implementation of IPM Principles to Control Wireworms
2.1. World Fauna and Species Identification
Our current understanding of the global distribution of click beetles is limited, and the existing information is based on studies conducted in specific regions and countries. Moreover, species composition can change over time at both the field and global scales. The appearance of some of the European Agriotes spp. in North America also suggests that human activities can contribute to click beetle dispersal [2]. Overall, there are about 10,000 species (400 genera) of click beetles worldwide [33], of which 921 species (91 genera) are described in North America [34]. Three hundred and sixty-nine species have been reported from Canada and Alaska [35], of which 30 species are of economic importance [36]. In the PNW, Limonius spp. is the most common pest species of click beetles devastating sugar beets, small grains, potatoes, and organic vegetables [37,38,39,40].
In the Holarctic regions of Europe, North Africa, the Middle East, and North Asia, there are about 100 economically important species of click beetles [1,41]. Agriotes spp.—specifically A. lineatus, A. obscurus, and A. sputator—are Europe’s most damaging wireworm species [42]. Agriotes spp. have also been reported in North America [43], damaging various crops such as corn, potato, strawberries, and organic vegetables [44]. Agriotes spp. often is not considered to be a major pest taxon in most of the USA, where Limonius spp., Selatosomus spp., Conoderus spp., Melanotus spp., and Dalopius spp. represent some of the most damaging genera [2,45].
Several identification keys are available to determine adult genera and/or species [46,47,48,49]. However, the identification of the damaging immature wireworms is required in order to inform the selection of adequate and timely control tactics. The limited development of some morphological characteristics in the early larval instars [46], the absence of distinct features to distinguish some species [2], and/or worn-off morphological traits in late instars make larval identification a challenging task. Despite these constraints, the recent advances in molecular techniques have helped clarify several ambiguities in species identification. The Agriotes species complex [3] is one example in which species identification based on the larval morphology is challenging, and the cytochrome oxidase I (COI) and 16 S regions of the mitochondrial DNA helped determine species, and even revealed several haplotypes [50]. Moreover, DNA barcoding was used successfully to identify cryptic species within Hadromorphus glaucus Germer and Hypnoidus bicolor Eschscholtz [51,52]. More recently, COI and 16 S rRNA sequences and nuclear genome-wide single nucleotide polymorphisms generated from restriction site-associated DNA identified cryptic species within the L. californicus and L. infuscatus, where potential genomic responses to broad-spectrum insecticides were also detected [53]. Determining the effects of agricultural practices on population genetic diversity and identifying ecological variables that predict the evolution of adaptations and dispersal patterns [54] based on molecular data are emerging areas of research, and long-term studies with large sample sizes are needed in order to allow for the precise characterization of such effects.
2.2. Monitoring
2.2.1. Adult Monitoring
Sex pheromone lures have been used to estimate the abundance and distribution of the male click beetles [3]. Synthesized female sex pheromones are now available for a few species of click beetles, including several European species [55], and a few pestiferous species in North America, including Cardiophorus tenebrosus [56], Limonius spp. [57,58], and Melanotus communis [59].
In order to estimate the subterranean wireworm populations and species composition, the monitoring of the adult click beetles with the pheromone traps was initially sought [2]. However, establishing the relation between the number of collected adult males and wireworms is complex, and is reflected in inconsistencies across study outcomes. For example, although a field study on Agriotes spp. by Benefer et al. [36] detected no associations between the number of click beetles and wireworms, Furlan et al. [60] reported a significant relationship between male click beetle numbers, wireworm populations, and the extent of damage to maize in Agriotes spp. Furlan et al. [60] also demonstrated that monitoring click beetle populations two years before planting maize could be a practical risk assessment method; in the case of A. ustulatus, capturing > 1000 click beetles/trap two years before planting was associated with a more-than-12-times probability of >15% crop damage. Click beetle behavior and their movement [61,62], the range of pheromone attractiveness [63,64], trapping time and intensity [3], sex ratio, climate, and agricultural practices [65] are examples of the variables that can influence the precision of adult monitoring in the prediction of subterranean wireworm populations. Although the development of pheromone lures provides us with sensitive tools to monitor click beetles [66] and the opportunity to develop distribution maps across landscapes [41], more large-scale species-specific data are needed in order to solidify associations between adult pheromone traps and wireworm bait trap data (Section 2.2.2).
2.2.2. Wireworm Monitoring
Soil core removal [67] and solar bait traps [39] are two of the most frequently used field sampling methods [1]. Soil core sampling can be time-consuming [67,68]. Because the wireworm distribution is often patchy [5], many core samples would be needed in order to accurately estimate the field infestation status. Solar bait traps, on the other hand, are designed to attract wireworms [1], and typically stay in the soil for several days. Germinating cereal seeds are commonly used in solar bait traps as effective baits [69,70,71], attracting wireworms to the CO2 and other volatiles released from the sprouting seed [72]. Covering bait traps with soil, plastic [73,74], and charcoal dust [73] helps to increase the temperature and trap the released CO2 and volatiles in order to maximize wireworm attraction from a distance. Soil temperature [75], moisture [5,76], texture, and other existing sources of CO2 [68] may influence the effectiveness of the bait traps.
The effectiveness of wireworm monitoring also depends on the species-specific phenology and distribution patterns within fields [4,5,40,77]. Wireworm activity is influenced by food availability, molting, and abiotic variables such as moisture and temperature [2,7]. Wireworms dive deeper into the soil profile when the environment is unfavorable and/or become inactive [41,78,79]. Monitoring efforts when the wireworms are inactive or not present in the topsoil may not yield reliable results. Early-season sampling and prophylactic seed treatment application may miss the wireworm species that are active later in the growing season. For example, seed treatment in the PNW is expected to protect plants against L. infuscatus, which is active early in the season, but may not be as effective against L. californicus, which remains active throughout the season [40]. Continuous monitoring during the growing season can help capture an accurate estimate of wireworm populations, species composition, and distribution.
Wireworm distribution within an infested field is often patchy, and multiple species of wireworms can be present within a field [2,36] (AN and AR, unpublished). Because wireworms have limited ability to disperse within the soil [80], their patchy distribution may be explained by the adult female preference for oviposition sites [3]. The adult click beetles are attracted to grasslands for oviposition [2,3,81], and higher wireworm populations can be found in pastures, cereal fields lacking proper rotation, and natural vegetations within and surrounding fields [82,83]. Wireworm distribution and the accuracy of field monitoring can also be driven by other environmental constraints such as soil characteristics and texture [3,84].
2.3. The Risk of Wireworm Damage
2.3.1. Wireworm Economic Threshold
The number of wireworms is not the only variable determining the risk of damage; threshold assessments should also consider wireworm species and the crop of interest [85,86,87]. Cherry et al. [88] estimated that nine larvae of Melanotus communis in 25 soil samples collected from infested sugarcane fields could cause severe economic damage. In another study, Furlan [87] evaluated the damage of Agriotes spp. in corn, and showed that the effect is influenced by both the number and species of the wireworms. The damage by one larva of A. brevis was equal to damage caused by two A. sordidus, or five A. ustulatus. In potatoes, seed treatment is recommended if the number of collected L. californicus or L. canus per trap exceeds two larvae [89]. Moreover, different crops show different levels of susceptibility to wireworm damage; Agriotes spp. can cause 100% and 50% damage in sugar beet and corn, respectively, whereas the damage in soybean remains negligible [77].
2.3.2. Soil Properties and the Risk of Wireworm Damage
Soil temperature, moisture, and texture are among the most important variables associated with wireworm damage [2,68,78,79,90,91,92,93].
In a study conducted in eastern Canada, wireworms started their activity when soil temperatures reached slightly above 1.5 °C, and their movement peaked when soil temperatures reached 12 °C; Dalopius pallidus, Ctenicera lobata, A. mancus, and H. abbreviatus were the five species in the study sites [94]. These reported temperatures are similar to those experienced by wireworms in the early spring when spring-seeded crops are sprouting. Soil temperatures between 8 and 14 °C and soil moistures between 30 and 32% are associated with a high risk of crop damage by Agriotes spp. [79]. The preferred soil moisture of wireworms is also influenced by the soil’s physical structure [79]; the soil texture and compaction influence fluctuations in moisture and temperature, and affect the ability of wireworms to relocate and survive [95,96,97,98]. Soil porosity can also affect the diffusion of carbon dioxide (CO2) and other volatile compounds released from the germinating seeds and roots, which are cues that wireworms use to navigate food sources [41,81,99]. A greenhouse study showed that the frequency of damage by L. californicus to small grains increases in sand-dominated media [100]. Hermann et al. [95] also reported a positive association between sandy soil and potato tuber damage by Agriotes sp. Rapid changes in moisture levels in sandy soil have been proposed as a factor triggering wireworms to burrow into succulent tuber tissue in search of moisture.
Other soil variables that are studied in relation to the risk of wireworm damage are the pH and the organic matter content [101]. However, it is also important to note that the wireworm response to soil pH and soil organic matter can be species-dependent. For example, Agriotes and Melanotus prefer acidic soils [83], whereas Limonius spp. can be active in both alkaline and acidic soils [83]. Soil organic matter is not a major food source for wireworms [6,102], and a long-term survey has suggested an increased risk of Agriotes spp. presence and damage in soils with a high organic matter content [103]. The contribution of soil organic matter to retaining moisture is likely to make conditions favorable for wireworm survival. While the influence of environmental variables on wireworms is often studied (or presented) in isolation, wireworms (and all living organisms) are always exposed to a combination of variables. An understanding of the species-specific wireworm response to combinations of interacting abiotic factors in the rhizosphere is expected to help with the development of relatively more effective control methods (see Section 3.1.4, for an example).
2.3.3. Landscape and Field History and the Risk of Wireworm Damage
The landscape surrounding farmlands and cropping history can also predict the risk of wireworm infestation [95]. Specifically, grassland’s (including small grains with no rotation) presence and duration are strong predictors of the likelihood of wireworm presence [97].
Anecdotal observations are also suggestive of increased wireworm damage on hillsides and slopes (Rashed, personal observations), which supports the trend observed by Parker and Seeney [97], who reported a higher frequency of wireworm infestation in south-facing fields. As mentioned earlier, meadows, natural grass patches (including those dedicated to the ‘Conservation Reserve Program’), and grassy and weedy field margins considerably increase the wireworm damage risk because they attract adult females and provide a favorable place for oviposition and larval development [7,68,95,103,104].
Poggi et al. [105] provided a comprehensive list of factors, including agricultural practices studied for their impacts on the damage risk of wireworms. However, often overlooked are the potential implications of the implemented control practices on the rhizosphere and the ecological interactions involving subterranean micro- and macro-organisms.
3. Wireworm Control Tactics and Impacts on the Rhizosphere
3.1. Cultural Practices
3.1.1. Trap Crop and Intercropping
Because wireworms are limited in their mobility, it may appear that trap cropping would not be a practical approach to reducing crop damage. However, placing attractive trap crops within the main crop has been shown to protect the main crop [43,106]. Intercropping with wheat as the trap crop helped reduce wireworm damage in strawberries [107] and maize [108]. The effectiveness of an attract-and-kill strategy, using insecticide-treated wheat as a trap crop, has also been tested and proven successful in potatoes [109].
Intercropping can negatively impact pests by interfering with their ability to locate host plants and/or by providing an environment which is suitable for the pest’s natural enemies [16,110]. There is also evidence that diversity in trap crops (i.e., using seed mixes) can increase wireworm attraction away from the main crop. Staudacher et al. [111] compared the effectiveness of wheat and a mixture of the wheat, bean, lupine, white mustard, buckwheat, and ryegrass as trap crops in maize; greater protection of maize seedlings was achieved with the mixed-species trap crop.
Intercropping can also positively impact the agroecosystem and soil microbial communities, enhancing plant productivity [112,113,114,115] . However, the microbial community composition and diversity in the rhizosphere can also shift in response to the soil type, plant root exudates, and the interaction between plant species, i.e., nutrient availability and uptake efficiency [112,116,117,118,119,120]. Therefore, selecting suitable plant species for trap cropping is essential in order to sustain soil health, support vigorous plant development, and protect the main crop.
The associations between the nitrogen (N) fixing soil bacteria in legumes make pulse crops suitable candidates for intercropping [115,119,121]. The root tissues of legumes are a source of high organic phosphorus (P) [122], and through the nitrogen-fixing process they increase N in the soil [123]. Intercropping with legumes reduces the need for fertilizers; therefore, it minimizes nitrate and nitrite pollution in the soil [115]. In cereal production, using legumes as intercrops enhances yield and increases microbial diversity and biomass in the rhizosphere [112,113,117,118,119,124,125]. Legumes, such as pea and lentil, are also effective trap crops, attracting wireworms away from the main wheat crop and, subsequently, reducing damage [126,127,128]. Peas planted in potato fields have also been more attractive than wheat and oilseed to wireworms in field trials [126].
Although intercropping with legumes can potentially reduce wireworm damage in some crops, such as small grains and potatoes, the contribution of this approach to N fixation and improved microbial activity could also, in turn, create an environment in the rhizosphere that supports wireworm development and survival through improved N metabolism and availability [129,130] and/or by suppressing the efficacy of entomopathogens [131,132]. When it is abundant, wireworm activity can also increase the microbiome density, functional diversity, and mineralization rate [129]. For example, in higher N and carbon contents, wireworms can shift the soil bacterial composition to increase the abundance of Azotobacter bacteria, which contribute to nitrogen fixation in the soil [129,130].
3.1.2. Crop Rotation
Crop rotation can reduce pest incidence (i.e., pathogens, weeds, and arthropods) [133,134] and contribute to N recovery, nutrient availability, and an increased rate of mineralization [135,136]. However, because wireworms are polyphagous, they can feed on various crops in rotations. Therefore, finding a proper diversified rotation to minimize wireworm damage is particularly challenging. The continuous rotation of small grains with susceptible crops such as potato and sugar beet is not expected to reduce wireworm damage [7,82,93,104,137]. However, rotations with relatively less-susceptible crops such as mustard, soybean, sorghum, cabbage, French marigold, clover, and flax could help to reduce wireworm damage when followed with more susceptible target crops [138]. Long rotations with alfalfa can negatively impact the wireworm population [139]; this effect is attributed to the soil drying and compaction in the rhizosphere due to the high root density developed over the years [140].
A diverse crop rotation plan is expected to enhance the soil microbiome richness [109,136], diversity [24,136,141,142], and crop yield [136,143]. This is because crops differ in the carbon resources that they contribute to the rhizosphere, either through the release of species-specific root exudates or the plant residue that they leave behind in the soil [144,145,146,147], continuously selecting for the microbiome that can utilize the available resources. As discussed earlier, an increase in the diversity and activity of the microbial community in the rhizosphere may not directly negatively impact wireworm populations. Still, it could indirectly reduce damage by supporting vigorous plant growth (i.e., the subsequent crop), which may withstand and overcome feeding by the pest. Moreover, some of the N-fixing crops, such as pea, bean, lentil, and alfalfa, which are relatively more tolerant of wireworm damage, may be potential candidates for rotation with susceptible crops [138]. The selection of a crop rotation plan that fits the regional cropping system and the soil’s physicochemical characteristics is of particular importance. In relation to this, Nettles [148] states: “Several rotations have been suggested by research workers on the wireworm problem, but it should be borne in mind that no one rotation plan will suite all farmers.” In the PNW, peas and lentils are among the most common crops planted in rotation with cereals in rainfed production systems, which are relatively more tolerant of wireworm damage. Promoting diverse long-term rotation with legumes benefits the soil agroecosystem and may help to keep wireworm damage in check.
3.1.3. Tillage
Overall, conventional tillage can negatively impact the wireworm population and reduce the risk of damage to several economically important crops [82,99,149,150]. The effectiveness of tillage as a control method depends on the wireworm’s seasonal activity and life cycle; tillage can be a practical approach when larvae are active in the upper layer of the soil surface [5,149]. Disturbing the topsoil with tillage can expose the click beetle eggs and the newly hatched wireworms to predators, heat, and desiccation [5,77,151]. However, tillage can also stress the rhizosphere by altering the physical structure, aeration, moisture, organic matter decomposition and nutrients, and ecological interactions in the subterranean community that support root development and plant growth [152]. As such, in recent years, conservation agriculture has been promoting minimum soil disturbance (i.e., reduced or no tillage) to improve and conserve soil quality, preserve water, and minimize soil erosion [153,154,155]. The no-tillage practice, with the coverage of plant residue on the soil surface, improves soil microbial diversity and soil organic matter compared to the conventional tillage system [25,141,156], and contributes to the resilience of the agroecosystem [152]. The relatively more stable rhizosphere under no-tillage is expected to provide a suitable environment for wireworms to survive, and has been proposed as an underlying cause for their resurgence as a key pest (e.g., [7]). However, a recent study concluded that in the maize production system, no-tillage neither increased wireworm populations (primarily A. sordidus) nor the rate of damage [157].
While no-tillage and reduced tillage can contribute to soil health and the resilience of the agroecosystem, their impact on wireworm populations and damage require further studies that are species-, crop- and location-specific [41].
3.1.4. Soil Flooding and Drying
Due to the importance of soil moisture in wireworm survival [37,40], water manipulation has been recommended as a possible control approach for wireworms [98,107,158,159]. The effect of soil drying is species-specific; withholding irrigation soon after oviposition to dry topsoil reduced Agriotes spp. [5,68], L. californicus, and L. canus [78] numbers. However, this approach was proven to be ineffective against the dryland species Selatosomus pruininus, known as the Great Basin wireworm [159].
The efficacy of flooding as a control approach against wireworms is both species- and temperature-dependent. Flooding can effectively control L. californicus when the soil temperature exceeds 21 °C [158]. Similarly, in flooded soil, A. obscurus and A. lineatus died more rapidly in 20 °C—an effect which was more pronounced in soils with high salinity [98]. Unlike the Agriotes and Limonius species complexes, the prairie grain wireworm, Selatosomus destructor (AKA. Ctenicera destructor), can feed and molt while submerged in water for up to 6 weeks [160].
Although water manipulation may help to reduce some species of wireworms, both flooding and drought are known to influence biotic and abiotic interactions in the rhizosphere. For example, soil microbial biomass and the bacteria:fungi ratio of the microbiome have been reported to decrease in the flooded, anaerobic environment, especially in 10–20 cm soil depths [161]. A reduction in soil microorganisms may provide the entomopathogenic fungi and nematodes with a competitive edge, improving their efficacy against wireworms. There are studies demonstrating the antagonistic effects of indigenous soil microbes on the applied entomopathogenic agents [162]. The entomopathogenic nematodes Heterorhabditis bacteriophora and Steinernema feltiae have been shown to be more effective against mealworms (Tenebrio molitor) in sterilized compared to non-sterilized soil [131]. In another study, Mazzola [163] suppressed beneficial soil bacteria using soil fumigation in order to apply biological control agents against apple soil-borne pathogens more effectively.
Prolonged periods of drought before planting can also have adverse and long-lasting effects on soil bacterial communities; the addition of N fertilizer failed to improve soil fertility and recover the soil bacterial community [164]. The potential negative impacts of soil drying and flooding as a wireworm control method on soil health parameters need to be further studied and quantified.
3.2. Host Plant Resistance and Tolerance
Because of the wireworms’ subterranean habitat and often-unknown species-specific seasonality, planting resistant and tolerant host plants can be a reliable approach to reducing the damage risk. Potato, corn, and sweet potato are the better-studied crops for variations in susceptibility to wireworm damage. Johnson et al. [165] studied the susceptibility of 12 potato varieties to wireworm feeding. There, the glycoalkaloid concentrations were suggested as one of the mechanisms contributing to the observed differences in susceptibility to Agriotes spp.
Differences in susceptibility to A. sordidus were also reported among corn varieties [166], where the less susceptible corn cultivars released higher concentrations and a more diverse blend of volatile compounds such as hexanal, heptanal, and 2,3-octenanedione than the susceptible genotypes [166]. Hexanal and several other aldehydes have also been detected in barley roots [167], which are a more tolerant crop to wireworm feeding than wheat [100,168]. Oats show resistance to wireworms [168], likely because of their vigorous root development and the presence of defensive steroidal and triterpenoid saponins [169].
Plant roots influence soil microbial composition and activity because they (the roots) shed cells and release various species-specific metabolites into the rhizosphere [170,171]. At the same time, these interactions may develop into a plant-specific microbiome in the rhizosphere (e.g., [116,172,173]). The persistence of such effects needs to be studied in agroecosystems where crop rotations are common. The potential impact of plant defenses on non-pathogenic microorganisms in the rhizosphere is an area that needs further investigation (see [174] for a review).
3.3. Biological Control
3.3.1. Predators
Besides general vertebrate predators such as birds [175], moles and amphibians [2], arthropods including carabid beetles, rove beetles (Coleoptera: Staphylinidae) [176,177] and the stiletto fly Thereva nobilitata (Diptera: Therevidae) larvae [178] have been reported to prey upon wireworms. It is conceivable that, like wireworms, the soil-dwelling predatory beetles and their subterranean larvae are susceptible to some of the agricultural practices that disturb the rhizosphere. Although the impact of these predatory insects on wireworms is more limited than the entomopathogenic organisms, additional studies are needed in order to quantify their impact as biological control agents. Several species of parasitoid wasps from the families Proctotupidae, Bethylidae, and Ichneumonidae, and flies from the family Tachinidae have also been reported to attack several species of wireworms, but again the extent of parasitism in the field appears to be limited [2].
3.3.2. Entomopathogenic Bacteria
Among the resident bacteria in the rhizosphere, Pseudomonas aeruginosa has been reported to infect wireworms during the susceptible molting process [179]. Although the application of entomopathogenic bacteria to control wireworms has not been thoroughly investigated, the modification of the endosymbiotic bacteria associated with wireworms as a biological control method has been the subject of a few studies [180,181,182]. Lacey et al. [183] isolated Rahnella aquatilis, a common bacterium in the rhizosphere in the potato agroecosystem, from L. canus. The genetically modified R. aquatilis expressed wireworm-active toxins and successfully protected seed potatoes from wireworm damage [182]. Rickettsiella agriotidis isolated from Agriotes spp. [181], and Arthrobacter gandavensis, Bacillus thuringiensis, and Pseudomonas plecoglossicida isolated from A. lineatus are a few more examples of bacteria [180] with the potential to be developed into biological control agents against wireworms.
3.3.3. Entomopathogenic Nematodes
Steinernema and Heterorhabditis (Nematoda: Steinernematidae, Heterorhabditidae), along with their endosymbiont bacteria Xenorhabdus spp. and Photorhabdus spp., respectively, are the two main genera of entomopathogenic nematodes (hereafter, EPN) [183]. Although the EPN are expected to perform well in protected and moist habitats, a few challenges exist with regard to wireworms [184]. Their resilience, physical barriers, and behavioral responses make wireworms a difficult host to infect [185]. In the laboratory, Steinernema carpocapsae, S. riobrave and, S. glaseri reduced damage caused by the sugar beet wireworm, L. californicus [186,187]. Heterorhabditis bacteriophora, S. carpocapsae, and S. feltiae were used to manage A. lineatus [188,189], A. obscrus, and A. sputator [189]; the three entomopathogenic nematodes were effective against Agriotes spp. However, S. feltiae has not been effective in controlling S. aeneus [190].
Differences in the efficacy of EPN species may be explained by differences in their foraging behavior [183,191] and/or species-specific differences in wireworm ecology [40]. The efficacy of EPN is also influenced by biotic and abiotic environmental variables [192,193,194]. Therefore, naturally occurring EPN adapted to their soil habitat and endemic hosts might be better than commercial nematode species for the management of local wireworms [184,195,196,197]. More studies are needed in order to evaluate the efficacy of naturally occurring nematodes against wireworms.
3.3.4. Entomopathogenic Fungi (EPF)
Overall, EPF are a promising tool in the management of wireworms. Metarhizium anisopliae (Clavicipitaceae) and Beauveria bassiana (Cordicipitaceae) recovered from the larvae and adults of Hypolithus bicolor caused significant mortality in H. bicolor and S. aeripennis destructor [198]. The B. bassiana isolated from the genera Agriotes, Conoderus, and Hypnoidus [2] successfully reduced Agriotes spp. damage in potatoes [199]. The in-furrow application of B. bassiana also reduced the number of Limonius spp. collected in bait traps in organic fields (unpublished data). However, in a study by Kölliker and colleagues [200], comparing efficacies of M. anisopliae (isolate ART-2825) and the commercial B. bassiana against Agriotes spp., the two EPF species differed significantly in their efficacy against the wireworm, with B. bassiana being ineffective. This finding supported Ansari et al. [189], who determined B. bassiana to be non-pathogenic against A. lineatus. Although the efficacy of M. anisopliae reached as high as 97% against A. obscurus, it was considerably less virulent against A. lineatus and A. sputator [200].
Ansari et al. [189] also demonstrated that M. anisopliae can cause mortality in A. lineatus; however, the mortality rate varied between 10 and 100%, depending on the EPF isolate. Later, Reddy and colleagues [66] reported a reduced number of L. californicus and H. bicolor in experimental plots treated with M. brunneum (F52), M. robertsii (DWR346), and B. bassiana (GHA), with no difference being detected in the efficacy of the EPF species. Field-collected EPF isolates have also shown promise in the reduction of wireworms. For example, M. anisopliae isolated from field-collected A. obscurus was effective against that species in field trials when applied at the high rate of 4 million conidia/cm3 [201].
Application methods that facilitate contact between the biological control agent and wireworms can result in successful infection in the rhizosphere where wireworms are most active; in-furrow and soil drench applications of EPF were more effective than seed coating applications [66,202,203]. However, M. brunneum (1154) still successfully reduced the wireworm population and crop damage when applied as a seed coating [204].
Biotic and abiotic environmental variables in the rhizosphere can determine the efficacy of entomopathogens. The soil temperature [205,206,207], moisture [201,208], and texture [192,209]; the duration of exposure; the pathogen concentrations [206]; the wireworm location at the time of application [202]; and interactions with the soil microbiome [131,132,162,210] are examples of factors that can predict the efficacy of the entomopathogenic organisms against wireworms.
Fluctuations in soil moisture and temperature are affected by the soil porosity and compaction, which are traits that also influence the ability of wireworms and entomopathogens to relocate and survive [95,96,97,98,192]. For example, a porous sandy texture can facilitate EPN locomotion and foraging success [192,193], but it may also increase the rate of damage by wireworms [100]. High soil organic matter is expected to support EPF, likely because soils with high organic matter help with moisture retention [211]. Therefore, selecting entomopathogens which are compatible with environmental and field conditions is critical if the biological control approach is being implemented alone or as a component of IPM against wireworms [66,206].
Soil temperatures above 18 °C are optimal for M. anisopliae to infect wireworms successfully. As wireworms move deeper into the soil in order to evade increasing temperatures and the dry topsoil, fungal conidia may not be able to reach the wireworms [202] effectively; the fatal exposure time for wireworms at 18 °C is estimated to be at least 48 h [206]. Moreover, soil moisture below 6–18% [201] negatively impacts the viability of the fungal conidia, further reducing the efficacy of the EPF.
The optimal temperature to reach higher infectivity differs among EPN species. For example, the pathogenicity of S. carpocapsae [194] and H. bacteriophora [212] at temperatures ranging from 5–25 °C is greater than 35 °C, whereas S. glaseri shows higher pathogenicity at temperatures ranging between 15 and 35 °C [194]. Understanding the species-specific ecology of wireworms and identifying the most effective species and strain of entomopathogens for the environmental conditions are essential variables that can determine the success of the biological control.
The subterranean entomopathogens continuously interact with the microbial organisms in the rhizosphere. These interactions may affect the virulence of the entomopathogens, as well as their ability to survive, move, and locate the host. Susurluk [131] and Shah [132] showed that the efficacy of H. bacteriophora, S. feltiae, and M. anisopliae in sterilized soil is significantly higher than that in non-sterile soil due to microbial suppression in sterilized soil. As mentioned earlier, cultural practices such as trap cropping/intercropping [112,113,114,115] and no-tillage [25,141,156] promote the microbial biomass, diversity, and activity in the rhizosphere, which in turn could interfere with the efficacy of the entomopathogens against subterranean pests. Future studies are warranted to evaluate these potential trade-offs in agroecosystems. Moreover, while wireworms may directly benefit by contributing to N fixation in the soil and enhancing microbial activity and biomass [129], they could also benefit indirectly as the effectiveness of the entomopathogenic organisms is reduced. However, it is also important to note that some entomopathogens, such as Metarhizium, are also known to colonize plant roots, promoting growth, drought resistance, and nitrogen acquisition by the host in the rhizosphere [213,214,215].
One of the less-studied aspects of wireworm management is the role that the associated endosymbionts may play in the determination of wireworm susceptibility to entomopathogenic organisms. Kabaluk et al. [216] reported that the four endosymbiont bacteria Pantoea agglomerans, Pandoraea pnommenusa, Nacardia pseudovaccinii, and Mycobacterium frederiksbergense, associated with wireworm species A. obscurus and A. lineatus, can suppress infection by efficacious M. brunneum.
Although several studies examined the efficacy of various strains of entomopathogenic organisms in isolation, in the rhizosphere wireworms are often exposed to multiple natural enemies simultaneously [217]. A few studies examined the efficacy of a combination of EPF and EPN against wireworms, which yielded inconsistent results [217,218,219,220]. The co-application of EPN and EPF has been reported to result in synergistic [218], additive [219], or even antagonistic [220] outcomes. The time and order of the applications, the developmental stage of the pest [221], the species and strain of the entomopathogens [222], and soil conditions [223] are examples of the variables that may influence the outcome of co-applications of the entomopathogens.
Entomopathogenic organisms have also been used in combination with insecticides. In the laboratory, combining M. anisopliae with the insecticide spinosad, a natural insecticide produced by the soil bacterium Saccharopolyspora spinosa, increased mortality in A. obscurus and A. lineatus [224]. In the field, corn seed coated with M. anisopliae and either spinosad or clothianidin (neonicotinoid) was ineffective against A. obscurus [213]. However, the application of M. anisopliae alone significantly increased the yield [213]. Mixed applications of the bioinsecticides spinosad, azadirachtin, pyrethrin, M. brunneum F52, B. bassiana ANT-03, and B. bassina GHA with each other and in combinations with the neonicotinoid imidacloprid have also been evaluated against the three wireworm species L. californicus, H. bicolor, and Aeolus mellillus in spring wheat [207]. The combined applications of imidacloprid + M. brunneum and M. brunneum + spinosad protected wheat seedlings from the wireworms L. californicus and H. bicolor. However, the wireworm population treated with M. brunneum + spinosad was significantly higher compared to the control [207].
The attract-and-kill approach, based on wireworm responses to attractants such as CO2 and other plant volatiles has also been used to increase contact between biocontrol agents and wireworms [109]. Brandle et al. [209] used M. brunneum in combination with capsules of baker’s yeast as an artificial source of CO2 to attract Agriotes spp. in organic potato fields, and reported a significant reduction in crop damage. Millet grain has also been used as an attractant to draw wireworms to B. bassiana and M. brunneum in spring wheat [208]. Kabaluk et al. [201] used germinating wheat seeds coated with M. anisopliae as a bait trap against A. obscurus in the field. Finally, in the laboratory, alginate beads loaded with a combination of potato extract (as an attractant) and EPN (either S. carpocapsae or H. bacteriophora) were effective in attracting and killing A. obscurus [225].
Using natural enemies to reduce wireworm populations can be costly, especially in large-scale production systems. Therefore, if the natural enemies can persist in the rhizosphere and remain viable for extended periods, their use as a control tactic could be justified from an economic standpoint. In agroecosystems, the rhizosphere is disturbed frequently by various cultural practices, which could influence the populations of natural enemies. For example, entomopathogenic fungi densities are higher in habitats with less soil disturbance, such as grasslands, than cultivated fields [226]. Location-specific studies are needed in order to evaluate further the persistency of the applied biological control agents and the naturally occurring entomopathogens in relation to cultural practices in various agroecosystems.
3.4. Cover Crops and Plant-Derived Biocides as Green Manure
The integration of cover crops into the crop rotation plans can contribute to functional biodiversity, improved pest management [227,228], and nutrient availability [229,230,231] in agroecosystems. There are several species of plants from the family Brassicaceae that contain biocidal glucosinolates, which are used to manage arthropod, pathogen, and weed pests in agricultural systems [110,232,233,234,235]. When hydrolyzed, the biologically inactive glucosinolates produce species-specific and active biocidal compounds [236]. The major glucosinolate in the yellow mustard Sinapis alba is sinalbin, which produces ionic thiocyanate SCN-, a phytotoxic biocidal product [237]. SCN- is ineffective against wireworms [238,239], and is known primarily for its herbicidal effects [240,241]. In the brown mustard Brassica juncea, sinigrin hydrolyzes to produce the volatile 2-propenyl isothiocyanate, a product with proven efficacy against insect pests such as wireworms [242]. The glucosinolates in B. carinata, B. oleracea, and B. nigra are also known for their insecticidal effects on soil-borne insects [243,244,245]. Mustard products with a concentrated glucosinolate content have also been developed and tested against wireworms. For example, defatted B. carinata seed meal caused higher mortality in Agriotes spp. compared to soil-incorporated B. juncea plant tissue [238,245]. Although mustard seed meal can have higher efficacy against wireworms, its application in the field has been challenging due to its bulkiness. In order to address this limitation, a highly concentrated seed meal extract has been developed from B. juncea seed meal [236], which successfully suppressed the reproduction of the plant pathogenic cyst nematode, Globodera spp. [246]. This concentrated seed meal extract also effectively reduced the L. californicus population in the laboratory (unpublished data). Although these newly developed concentrated extracts are relatively easier to apply in the field, their effectiveness and potential impact on the activities of soil microorganisms have yet to be evaluated in the field.
Plant biofumigants are expected to degrade rapidly in the soil [243,245,247]. Hence, green manuring the mustard cover crop is thought to benefit the soil by contributing nutrients and organic matter and, subsequently, improving yield [235]. However, Hansen et al. [248] reported a significant reduction in fungal and mycorrhizal community and microbial biomass in general in the long-term wheat/canola (B. napus L.) rotation compared to the spring wheat/winter wheat rotation. Although the canola contains considerably lower concentrations of glucosinolates than either B. juncea or S. alba, the continuous release of glucosinolates was proposed as a reason for the decline in the microbial community and, subsequently, the wheat yield [248,249]. The effects of glucosinolates and their bioactive derivatives are not limited to soil microorganisms and insect pests; beneficial organisms such as entomopathogenic nematodes and fungi are also negatively impacted [110], which may interfere with the effectiveness of wireworm IPM. However, as discussed earlier, the post-fumigation soil application of entomopathogens has been reported to enhance the efficacy of biological control agents because it reduces the competition with the microbial communities in the rhizosphere [163].
The effects of mustard and its products on soil health in various cropping systems need thorough investigations. The effective dosage based on the infestation rate, the degradation parameters, the homogenous spread of seed meal products across the field, effective incorporation into the soil, the optimal soil temperature and moisture, and most importantly, the timely incorporation of green manure with respect to species-specific wireworm ecology, are a few factors that can predict the probability of success in wireworm management using biofumigants [41,77,238].
3.5. Insecticides
Organochlorines are a group of insecticides with persistent environmental effects [250]; they were used for decades to control agricultural pests, including wireworms [1,3]. This group of insecticides is now banned in the USA and Canada due to environmental and human health risks. Organophosphates (chlorpyrifos, fonofos) and carbamates are two other groups of relatively less persistent insecticides which are used to control soil-dwelling pests such as wireworms, and are again mostly deregistered in the USA [11,251]. Phenylpyrazole, pyrethroids, and neonicotinoids are now the most common groups of insecticides used to control wireworms, offering various degrees of efficacy against this pest [9,11,252,253].
Some pyrethroids, such as tefluthrin [9,11,252], and neonicotinoids, such as thiamethoxam, imidacloprid, and clothianidin [9,10], are known to act as feeding deterrents, and are used as seed treatments to reduce wireworm damage. Although these insecticides are not effective in lowering wireworm populations [252], they are expected to provide crop protection during the vulnerable stages of plant development [254]. The mixed application of insecticides can also improve their efficacy against wireworms [10,252,254]. In the laboratory, combinations of thiamethoxam + fipronil [254,255], thiamethoxam + chlorpyrifos [254], and thiamethoxam + pyrethroid [253] showed higher efficacy against different wireworm species than individual applications of those insecticides.
The soil microbiome plays a critical role in degrading pesticides and removing them from the rhizosphere [251]. Applying insecticides against subterranean pests could alter the chemical and biological properties of the soil. Neonicotinoids, which are one of the most commonly used seed treatments in small grains, are known to alter the structure, genetic diversity, and bioactivity of the soil microbial communities, at least in the short-term [32,256,257,258]. Applying a higher dosage of the neonicotinoid imidacloprid can result in long-term changes in the soil’s microbial composition and metabolic activity [256]. Another neonicotinoid, thiamethoxam, has been reported to temporarily reduce the bacterial abundance of some of plant growth-promoting bacteria, such as Actinobacteria. In contrast, pollutant-degrading bacteria (Firmicutes) are increased in the rhizosphere following insecticide applications [32]. The soil microbiome influences the composition of endosymbionts associated with herbivorous insects [21,23,31]. Therefore, changes in the soil microbial community following insecticide applications could potentially impact the endosymbionts associated with wireworms. Understanding the functional role of various endosymbionts in the life history traits of wireworms is a developing area of research. Symbiotic microorganisms are known to promote insect resistance to biological control agents like B. thuringiensis in gypsy moth [259], M. brunneum in wireworms [216], and parasitoid wasps in aphids [259]. In the context of resistance to insecticides, Burkholderia is an insecticide-degrading group of bacteria present in the soil, and can also be found in the midgut of the stink bug, Riptortus pedestrist [22,260]. There, the soil application of the organophosphate fenitrothion resulted in a shift in the composition of the soil microbiome, increasing insecticide-degrading bacteria, which can be ingested by the herbivores, promoting resistance to insecticides [22].
Despite these reported impacts on the soil microbiome after insecticide applications, the rhizosphere microbiome is expected to reach a stable status within months [257]; soil organic matter, pH, and texture, have direct implications for the leaching, adsorption, and desorption of the applied insecticides [261,262].
The metadiamide broflanilide is the most recent insecticide developed to reduce wireworms in small grains. Although this insecticide has been very effective in knocking down wireworm numbers, little is known about its impact on the rhizosphere and non-target organisms. Nonetheless, our current knowledge of pesticide impacts on the agroecosystem-specific rhizosphere microbiome with respect to soil physiochemical properties is limited [28,263], and additional studies are needed in order to determine whether the adverse effects of pesticides on the non-target organisms in the rhizosphere outweigh the benefits of reduced wireworm damage.
4. Conclusions and Future Directions
The abiotic components of the rhizosphere, e.g., the soil texture, mineral composition, organic matter, and moisture, can be directly affected by the cultural practices recommended for wireworm management, such as tillage, soil flooding and drying, and long-term crop rotations (e.g., alfalfa). However, several soil health parameters—such as microbial biomass, diversity, and activity—can also be negatively impacted by such practices.
Intercropping, planting less susceptible crops, and incorporating biofumigants and cover crops into rotation plans to reduce wireworm populations can cause fluctuations in the microbial community structure and its functional role. The soil microbiome and function can also be impacted by the plant species, variety, and physiological stage of plant development [264,265,266,267]. Moreover, although insecticides may temporarily reduce wireworm losses, they can negatively impact the microorganisms that support healthy plant development. Alternatively, or in addition, the increase in pesticide-degrading bacteria following insecticide applications may increase the rate of their ingestion by herbivores, thereby promoting resistance to the chemistry, which could become heritable in a population [22]. This is a critical area of investigation with regard to the development of sustainable pest management strategies, which to our knowledge have yet to be studied in wireworms.
This brief review was intended to bring attention to the fact that the ultimate goals of IPM are to minimize losses to insect pests and to contribute to the sustainability of the production systems. However, many of the recommended management practices to reduce wireworm populations and damage could potentially negatively impact variables that contribute to soil and crop health. While studies are needed to examine the tradeoffs among various practices, recognizing some of these antagonistic effects may also help to improve the effectiveness of integrated control strategies. For example, the efficacy of entomopathogens against wireworms may be improved if they are applied after biofumigation or soil flooding or drying, which are practices that are expected to temporarily suppress microbial populations in the topsoil.
Our knowledge of the endosymbionts associated with wireworms is limited to a few studies on a very limited number of species [130,180,181,182,216,268]. Future studies are warranted in order to further learn about the species-specific wireworm endosymbionts in relation to the location-specific soil microbiome and understand their functional roles with respect to wireworm development and survival (e.g., resistance to stress).
The understanding of the wider impact of IPM practices on the sustainability of agroecosystems is a developing and continuously evolving area of research, which requires a transdisciplinary framework based on an in-depth interdisciplinary approach to future studies of complex ecological interactions.
Author Contributions
Conceptualization, A.N. and A.R.; writing—original draft preparation, A.N.; writing—review and editing, A.R.; supervision, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank USDA Western SARE (GW20-206)—Sustainable Agricultural Research and Education—for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vernon, R.S.; van Herk, W.G. Wireworms as Pests of Potato. In Insect Pests of Potato: Global Perspectives on Biology and Management; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2013; pp. 103–164. [Google Scholar]
- Traugott, M.; Benefer, C.M.; Blackshaw, R.P.; van Herk, W.G.; Vernon, R.S. Biology, Ecology, and Control of Elaterid Beetles in Agricultural Land. Annu. Rev. Entomol. 2015, 60, 313–334. [Google Scholar] [CrossRef]
- Blackshaw, R.P.; Vernon, R.S. Spatial Relationships between Two Agriotes Click-Beetle Species and Wireworms in Agricultural Fields. Agric. For. Entomol. 2008, 10, 1–11. [Google Scholar] [CrossRef]
- Gough, H.C.; Evans, A.C. Some Notes on the Biology of the Click Beetles Agriotes obscurus L. and A. sputator L. Ann. Appl. Biol. 1942, 29, 275–279. [Google Scholar] [CrossRef]
- Furlan, L. The Biology of Agriotes ustulatus Schäller (Col., Elateridae). II. Larval Development, Pupation, Whole Cycle Description and Practical Implications. J. Appl. Entomol. 1998, 122, 71–78. [Google Scholar] [CrossRef]
- Traugott, M.; Schallhart, N.; Kaufmann, R.; Juen, A. The Feeding Ecology of Elaterid Larvae in Central European Arable Land: New Perspectives Based on Naturally Occurring Stable Isotopes. Soil Biol. Biochem. 2008, 40, 342–349. [Google Scholar] [CrossRef]
- Parker, W.E.; Howard, J.J. The Biology and Management of Wireworms (Agriotes spp.) on Potato with Particular Reference to the U.K. Agric. For. Entomol. 2001, 3, 85–98. [Google Scholar] [CrossRef]
- Jansson, R.K.; Seal, D.R. Biology and Management of Wireworm on Potato. In Proceedings of the International Conference on Potato; APS Press: St.Paul, MN, USA; Jakson Hole, WY, USA, 1994; pp. 31–53. [Google Scholar]
- Vernon, R.S.; Van Herk, W.G.; Clodius, M.; Harding, C. Wireworm Management I: Stand Protection versus Wireworm Mortality with Wheat Seed Treatments. J. Econ. Entomol. 2009, 102, 2126–2136. [Google Scholar] [CrossRef]
- Vernon, R.S.; van Herk, W.G.; Clodius, M.; Harding, C. Crop Protection and Mortality of Agriotes obscurus Wireworms with Blended Insecticidal Wheat Seed Treatments. J. Pest Sci. 2013, 86, 137–150. [Google Scholar] [CrossRef]
- Vernon, R.S.; Van Herk, W.; Tolman, J.; Ortiz Saavedra, H.; Clodius, M.; Gage, B. Transitional Sublethal and Lethal Effects of Insecticides after Dermal Exposures to Five Economic Species of Wireworms (Coleoptera: Elateridae). J. Econ. Entomol. 2008, 101, 365–374. [Google Scholar] [CrossRef]
- Stern, V.M.; Smith, R.F.; van den Bosch, R.; Hagen, K.S. The Integrated Control Concept. Hilgardia 1959, 29, 81–101. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Blacquiere, T.; Field, L.M.; Hails, R.S.; Petrokofsky, G.; Potts, S.G.; Raine, N.E.; Vanbergen, A.J.; McLean, A.R. A Restatement of the Natural Science Evidence Base Concerning Neonicotinoid Insecticides and Insect Pollinators. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140558. [Google Scholar] [CrossRef] [PubMed]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight Principles of Integrated Pest Management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Veres, A.; Wyckhuys, K.A.; Kiss, J.; Tóth, F.; Burgio, G.; Pons, X.; Avilla, C.; Vidal, S.; Razinger, J.; Bazok, R. An Update of the Worldwide Integrated Assessment (WIA) on Systemic Pesticides. Part 4: Alternatives in Major Cropping Systems. Environ. Sci. Pollut. Res. 2020, 27, 29867–29899. [Google Scholar] [CrossRef]
- Johnson, S.N.; Benefer, C.M.; Frew, A.; Griffiths, B.S.; Hartley, S.E.; Karley, A.J.; Rasmann, S.; Schumann, M.; Sonnemann, I.; Robert, C.A.M. New Frontiers in Belowground Ecology for Plant Protection from Root-Feeding Insects. Appl. Soil Ecol. 2016, 108, 96–107. [Google Scholar] [CrossRef]
- Lynch, J.M.; Brimecombe, M.J.; De Leij, F.A. Rhizosphere. In eLS; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2001; ISBN 978-0-470-01590-2. [Google Scholar]
- Dessaux, Y.; Grandclément, C.; Faure, D. Engineering the Rhizosphere. Trends Plant Sci. 2016, 21, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.R.; Lundberg, D.S.; del Rio, T.G.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Host Genotype and Age Shape the Leaf and Root Microbiomes of a Wild Perennial Plant. Nat. Commun. 2016, 7, 12151. [Google Scholar] [CrossRef]
- Strano, C.P.; Malacrinò, A.; Campolo, O.; Palmeri, V. Influence of Host Plant on Thaumetopoea pityocampa Gut Bacterial Community. Microb. Ecol. 2018, 75, 487–494. [Google Scholar] [CrossRef]
- Malacrinò, A.; Karley, A.J.; Schena, L.; Bennett, A.E. Soil Microbial Diversity Impacts Plant Microbiota More than Herbivory. Phytobiomes J. 2021, 5, 408–417. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-Mediated Insecticide Resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef]
- Hannula, S.E.; Zhu, F.; Heinen, R.; Bezemer, T.M. Foliar-Feeding Insects Acquire Microbiomes from the Soil Rather than the Host Plant. Nat. Commun. 2019, 10, 1254. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Rice, W.A.; Clayton, G.W. Soil Microbial Diversity and Community Structure under Wheat as Influenced by Tillage and Crop Rotation. Soil Biol. Biochem. 1998, 30, 1733–1741. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, B.; Zhang, X.; Yang, X.; Zhang, X. Effects of Tillage and Crop Rotation on Soil Microbial Residues in a Rainfed Agroecosystem of Northeast China. Soil Tillage Res. 2011, 114, 43–49. [Google Scholar] [CrossRef]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.D.; Morrissey, C.A.; Noome, D.A.; Van der Sluijs, J.P. Risks of Large-Scale Use of Systemic Insecticides to Ecosystem Functioning and Services. Environ. Sci. Pollut. Res. 2015, 22, 119–134. [Google Scholar] [CrossRef]
- Filimon, M.N.; Voia, S.O.; Popescu, R.; Dumitrescu, G.; Ciochina, L.P.; Mituletu, M.; Vlad, D.C. The Effect of Some Insecticides on Soil Microorganisms Based on Enzymatic and Bacteriological Analyses. Romanian Biotechnol. Lett. 2015, 20, 10439. [Google Scholar]
- Pisa, L.; Goulson, D.; Yang, E.-C.; Gibbons, D.; Sánchez-Bayo, F.; Mitchell, E.; Aebi, A.; van der Sluijs, J.; MacQuarrie, C.J.K.; Giorio, C.; et al. An Update of the Worldwide Integrated Assessment (WIA) on Systemic Insecticides. Part 2: Impacts on Organisms and Ecosystems. Environ. Sci. Pollut. Res. 2021, 28, 11749–11797. [Google Scholar] [CrossRef]
- Cycoń, M.; Piotrowska-Seget, Z.; Kaczyńska, A.; Kozdrój, J. Microbiological Characteristics of a Sandy Loam Soil Exposed to Tebuconazole and λ-Cyhalothrin under Laboratory Conditions. Ecotoxicology 2006, 15, 639–646. [Google Scholar] [CrossRef]
- Vig, K.; Singh, D.K.; Agarwal, H.C.; Dhawan, A.K.; Dureja, P. Soil Microorganisms in Cotton Fields Sequentially Treated with Insecticides. Ecotoxicol. Environ. Saf. 2008, 69, 263–276. [Google Scholar] [CrossRef]
- Badri, D.V.; Zolla, G.; Bakker, M.G.; Manter, D.K.; Vivanco, J.M. Potential Impact of Soil Microbiomes on the Leaf Metabolome and on Herbivore Feeding Behavior. New Phytol. 2013, 198, 264–273. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Z.; Ma, Y.; Luo, J.; Gao, X.; Ning, J.; Mei, X.; She, D. Influence of the Neonicotinoid Insecticide Thiamethoxam on Soil Bacterial Community Composition and Metabolic Function. J. Hazard. Mater. 2021, 405, 124275. [Google Scholar] [CrossRef]
- Johnson, P.J.; Arnett, R.J.R.; Thomas, C.M.; Skelley, P.E. Family 58. Elateridae Leach 1815. Am. Beetles 2002, 2, 160–173. [Google Scholar]
- Marske, K.A.; Ivie, M.A. Beetle Fauna of the United States and Canada. Coleopt. Bull. 2003, 57, 495–503. [Google Scholar] [CrossRef]
- Bousquet, Y. Family Elateridae: Click Beetles. In Checklist of Beetles of Canada and Alaska; Agriculture Canada, Ottawa, Ontario, Publication 1861/E; Agriculture Canada, Research Branch: Ottawa, ON, Canada, 1991; pp. 175–185. [Google Scholar]
- Benefer, C.M.; Knight, M.E.; Ellis, J.S.; Hicks, H.; Blackshaw, R.P. Understanding the Relationship between Adult and Larval Agriotes Distributions: The Effect of Sampling Method, Species Identification and Abiotic Variables. Appl. Soil Ecol. 2012, 53, 39–48. [Google Scholar] [CrossRef]
- Andrews, N.; Ambrosino, M.D.; Fisher, G.C.; Rondon, S.I. Wireworm: Biology and Nonchemical Management in Potatoes in the Pacific Northwest; OSU Extension Service: Grants Pass, OR, USA, 2008. [Google Scholar]
- Morales-Rodriguez, A.; O’Neill, R.P.; Wanner, K.W. A Survey of Wireworm (Coleoptera: Elateridae) Species Infesting Cereal Crops in Montana. Pan-Pac. Entomol. 2014, 90, 116–125. [Google Scholar] [CrossRef]
- Rashed, A.; Etzler, F.; Rogers, C.W.; Marshall, J.M. Wireworms in Idaho Cereals: Monitoring and Identification. Univ. Ida. Ext. Bull. 2015, 898. [Google Scholar]
- Milosavljević, I.; Esser, A.D.; Crowder, D.W. Seasonal Population Dynamics of Wireworms in Wheat Crops in the Pacific Northwestern United States. J. Pest Sci. 2017, 90, 77–86. [Google Scholar] [CrossRef]
- Barsics, F.; Haubruge, E.; Verheggen, F.J. Wireworms’ Management: An Overview of the Existing Methods, with Particular Regards to Agriotes spp.(Coleoptera: Elateridae). Insects 2013, 4, 117–152. [Google Scholar] [CrossRef]
- Burghause, F.; Schmitt, M. Monitoring of the Clickbeetle Genus Agriotes (Elateridae, Coleoptera) in Rhineland-Palatinate in the Years 2008 to 2010. Gesunde Pflanz. 2011, 63, 27–32. [Google Scholar] [CrossRef]
- Ritter, C.; Richter, E. Control Methods and Monitoring of Agriotes Wireworms (Coleoptera: Elateridae). J. Plant Dis. Prot. 2013, 120, 4–15. [Google Scholar] [CrossRef]
- LaGasa, E.; Murray, T.; Mudge, A. Exotic Wireworm Survey and Impact Update 2005-Agriotes lineatus and A. obscurus in Western Washington and Oregon. Wash. State Univ. Ext. Bull. 2006, 44–46. [Google Scholar]
- Toba, H.H.; Campbell, J.D. Wireworm (Coleoptera: Elateridae) Survey in Wheat-Growing Areas of Northcentral and Northeastern Oregon. J. Entomol. Soc. Br. Columbia 1992, 89, 25–30. [Google Scholar]
- Emden, F. van Keys to the Ethiopian Tachinidæ.—I. Phasiinæ. In Proceedings of the Proceedings of the Zoological Society of London; Wiley Online Library: Hoboken, NJ, USA, 1945; Volume 114, pp. 389–436. [Google Scholar]
- Glen, R. Larvae of the Elaterid Beetles of the Tribe Lepturoidini (Coleoptera: Elateridae). Smithson. Misc. Collect. 1950, 111, 1–246. [Google Scholar]
- Wilkinson, A.T. Wireworms of Cultivated Land in British Columbia. J. Entomol. Soc. Br. Columbia 1963, 60, 3–17. [Google Scholar]
- Becker, E.C.; Dogger, J.R.; Stehr, F.W. Elateridae (Elateroidea)(Including Dicronychidae, Lissomidae). Immature Insects 1991, 2, 410–418. [Google Scholar]
- Ellis, J.S.; Blackshaw, R.; Parker, W.; Hicks, H.; Knight, M.E. Genetic Identification of Morphologically Cryptic Agricultural Pests. Agric. For. Entomol. 2009, 11, 115–121. [Google Scholar] [CrossRef]
- Benefer, C.M.; Van Herk, W.G.; Ellis, J.S.; Blackshaw, R.P.; Vernon, R.S.; Knight, M.E. The Molecular Identification and Genetic Diversity of Economically Important Wireworm Species (Coleoptera: Elateridae) in Canada. J. Pest Sci. 2013, 86, 19–27. [Google Scholar] [CrossRef]
- Etzler, F.E.; Wanner, K.W.; Morales-Rodriguez, A.; Ivie, M.A. DNA Barcoding to Improve the Species-Level Management of Wireworms (Coleoptera: Elateridae). J. Econ. Entomol. 2014, 107, 1476–1485. [Google Scholar] [CrossRef]
- Andrews, K.R.; Gerritsen, A.; Rashed, A.; Crowder, D.W.; Rondon, S.I.; van Herk, W.G.; Vernon, R.; Wanner, K.W.; Wilson, C.M.; New, D.D. Wireworm (Coleoptera: Elateridae) Genomic Analysis Reveals Putative Cryptic Species, Population Structure, and Adaptation to Pest Control. Commun. Biol. 2020, 3, 489. [Google Scholar] [CrossRef]
- Balkenhol, N.; Cushman, S.; Storfer, A.; Waits, L. Landscape Genetics: Concepts, Methods, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Furlan, L.; Tóth, M. Occurrence of Click Beetle Pest spp.(Coleoptera, Elateridae) in Europe as Detected by Pheromone Traps: Survey Results of 1998-2006. IOBC WPRS Bull. 2007, 30, 19. [Google Scholar]
- Serrano, J.M.; Collignon, R.M.; Zou, Y.; Millar, J.G. Identification of Sex Pheromones and Sex Pheromone Mimics for Two North American Click Beetle Species (Coleoptera: Elateridae) in the Genus Cardiophorus Esch. J. Chem. Ecol. 2018, 44, 327–338. [Google Scholar] [CrossRef]
- Gries, R.; Alamsetti, S.K.; van Herk, W.G.; Catton, H.A.; Meers, S.; Lemke, E.; Gries, G. Limoniic Acid-Major Component of the Sex Pheromones of the Click Beetles Limonius canus and L. californicus. J. Chem. Ecol. 2021, 47, 123–133. [Google Scholar] [CrossRef]
- Van Herk, W.G.; Lemke, E.; Gries, G.; Gries, R.; Serrano, J.M.; Catton, H.; Wanner, K.; Landolt, P.J.; Cooper, W.R.; Meers, S. Limoniic Acid and Its Analog as Trap Lures for Pest Limonius Species (Coleoptera: Elateridae) in North America. J. Econ. Entomol. 2021, 114, 2108–2120. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Serrano, J.M.; Johnson, P.J.; Millar, J.G. 13-Tetradecenyl Acetate, a Female-Produced Sex Pheromone Component of the Economically Important Click Beetle Melanotus Communis (Gyllenhal)(Coleoptera: Elateridae). Sci. Rep. 2019, 9, 16197. [Google Scholar] [CrossRef] [PubMed]
- Furlan, L.; Contiero, B.; Chiarini, F.; Benvegnù, I.; Tóth, M. The Use of Click Beetle Pheromone Traps to Optimize the Risk Assessment of Wireworm (Coleoptera: Elateridae) Maize Damage. Sci. Rep. 2020, 10, 8780. [Google Scholar] [CrossRef] [PubMed]
- Blackshaw, R.P.; Vernon, R.S. Spatiotemporal Stability of Two Beetle Populations in Non-Farmed Habitats in an Agricultural Landscape. J. Appl. Ecol. 2006, 43, 680–689. [Google Scholar] [CrossRef]
- Schallhart, N.; Wallinger, C.; Juen, A.; Traugott, M. Dispersal Abilities of Adult Click Beetles in Arable Land Revealed by Analysis of Carbon Stable Isotopes. Agric. For. Entomol. 2009, 11, 333–339. [Google Scholar] [CrossRef]
- Sufyan, M.; Neuhoff, D.; Furlan, L. Assessment of the Range of Attraction of Pheromone Traps to Agriotes lineatus and Agriotes obscurus. Agric. For. Entomol. 2011, 13, 313–319. [Google Scholar] [CrossRef]
- Blackshaw, R.P.; van Herk, W.G.; Vernon, R.S. Determination of Agriotes obscurus (C Oleoptera: E Lateridae) Sex Pheromone Attraction Range Using Target Male Behavioural Responses. Agric. For. Entomol. 2018, 20, 228–233. [Google Scholar] [CrossRef]
- Furlan, L.; Tóth, M.; Yatsynin, V.; Ujváry, I. The Project to Implement IPM Strategies against Agriotes Species in Europe: What Has Been Done and What Is Still to Be Done. In Proceedings of the XXI IWGO Conference, Venice, Italy, 27 November 2001; Volume 27, pp. 253–262. [Google Scholar]
- Reddy, G.V.; Tangtrakulwanich, K.; Wu, S.; Miller, J.H.; Ophus, V.L.; Prewett, J.; Jaronski, S.T. Evaluation of the Effectiveness of Entomopathogens for the Management of Wireworms (Coleoptera: Elateridae) on Spring Wheat. J. Invertebr. Pathol. 2014, 120, 43–49. [Google Scholar] [CrossRef]
- Lafrance, J.; Tremblay, R. An Apparatus for Separating Grass and Soil from Turf for Collecting Wireworm Larvae (Coleoptera: Elateridae) in Organic Soils. Can. J. Plant Sci. 1964, 44, 212–213. [Google Scholar] [CrossRef][Green Version]
- Furlan, L. An IPM Approach Targeted against Wireworms: What Has Been Done and What Has to Be Done. IOBCwprs Bull 2005, 28, 91–100. [Google Scholar]
- Horton, D.R.; Landolt, P.J. Orientation Response of Pacific Coast Wireworm (Coleoptera: Elateridae) to Food Baits in Laboratory and Effectiveness of Baits in Field. Can. Entomol. 2002, 134, 357–367. [Google Scholar] [CrossRef]
- Brunner, N.; Kromp, B.; Meindl, P.; Pázmándi, C.; Traugott, M. Evaluation of Different Sampling Techniques for Wireworms (Coleoptera, Elateridae) in Arable Land. IOBCwprs Bull. 2005, 28, 117–122. [Google Scholar]
- Arakaki, N.; Hokama, Y.; Yamamura, K. Efficient Bait for Sampling the Wireworm Melanotus okinawensis (Coleoptera: Elateridae) in a Sugarcane Field. Appl. Entomol. Zool. 2009, 44, 561–568. [Google Scholar] [CrossRef]
- Doane, J.F.; Lee, Y.W.; Klingler, J.; Westcott, N.D. The Orientation Response of Ctencera destructor and Other Wireworms (Coleoptera: Elateridae) to Geminating Grain and to Carbon Dioxide. Can. Entomol. 1975, 107, 1233–1252. [Google Scholar] [CrossRef]
- Ward, R.H.; Keaster, A.J. Wireworm Baiting: Use of Solar Energy to Enhance Early Detection of Melanotus depressus, M. verberans, and Aeolus mellillus in Midwest Cornfields. J. Econ. Entomol. 1977, 70, 403–406. [Google Scholar] [CrossRef]
- Simmons, C.L.; Pedigo, L.P.; Rice, M.E. Evaluation of Seven Sampling Techniques for Wireworms (Coleoptera: Elateridae). Environ. Entomol. 1998, 27, 1062–1068. [Google Scholar] [CrossRef]
- Parker, W.E. Practical Implementation of a Wireworm Management Strategy–Lessons from the UK Potato Industry. Int. Organ. Biol. Integr. Control West Palaearct. Reg. Sect. Bull. 2005, 28, 87–90. [Google Scholar]
- Parker, W.E. Evaluation of the Use of Food Baits for Detecting Wireworms (Agriotes spp., Coleoptera: Elateridae) in Fields Intended for Arable Crop Production. Crop Prot. 1994, 13, 271–276. [Google Scholar] [CrossRef]
- Furlan, L. The Biology of Agriotes ustulatus Schäller (Col., Elateridae). I. Adults and Oviposition. J. Appl. Entomol. 1996, 120, 269–274. [Google Scholar] [CrossRef]
- Campbell, R.E.; Stone, M.W. Dichloroethyl Ether for Wireworm Control. Jour Econ Ent 1937, 30, 212–213. [Google Scholar]
- Jung, J.; Racca, P.; Schmitt, J.; Kleinhenz, B. SIMAGRIO-W: Development of a Prediction Model for Wireworms in Relation to Soil Moisture, Temperature and Type. J. Appl. Entomol. 2014, 138, 183–194. [Google Scholar] [CrossRef]
- Schallhart, N.; Tusch, M.J.; Staudacher, K.; Wallinger, C.; Traugott, M. Stable Isotope Analysis Reveals Whether Soil-Living Elaterid Larvae Move between Agricultural Crops. Soil Biol. Biochem. 2011, 43, 1612–1614. [Google Scholar] [CrossRef] [PubMed]
- Cherry, R.; Stansly, P. Abundance and Spatial Distribution of Wireworms (Coleoptera: Elateridae) in Florida Sugarcane Fields on Muck versus Sandy Soils. Fla. Entomol. 2008, 91, 383–387. [Google Scholar] [CrossRef]
- Saussure, S.; Plantegenest, M.; Thibord, J.-B.; Larroudé, P.; Poggi, S. Management of Wireworm Damage in Maize Fields Using New, Landscape-Scale Strategies. Agron. Sustain. Dev. 2015, 35, 793–802. [Google Scholar] [CrossRef]
- Milosavljević, I.; Esser, A.D.; Crowder, D.W. Effects of Environmental and Agronomic Factors on Soil-Dwelling Pest Communities in Cereal Crops. Agric. Ecosyst. Environ. 2016, 225, 192–198. [Google Scholar] [CrossRef]
- Parker, W.E. The Development of Baiting Techniques to Detect Wireworms (Agriotes spp., Coleoptera: Elateridae) in the Field, and the Relationship between Bait-Trap Catches and Wireworm Damage to Potato. Crop Prot. 1996, 15, 521–527. [Google Scholar] [CrossRef]
- Hinkin, S. Determining the Density of Wireworms. Rastit. Zashchita 1976, 24, 22–25. [Google Scholar]
- Chabert, A.; Blot, Y. Estimation Des Populations Larvaires de Taupins Par Un Piège Attractif. Phytoma 1992, 436, 26–30. [Google Scholar]
- Furlan, L. IPM Thresholds for Agriotes Wireworm Species in Maize in Southern Europe. J. Pest Sci. 2014, 87, 609–617. [Google Scholar] [CrossRef]
- Cherry, R.; Grose, P.; Barbieri, E. Validation of a Sequential Sampling Plan for Wireworms (Coleoptera: Elateridae) at Sugarcane Planting. J. Pest Sci. 2013, 86, 29–32. [Google Scholar] [CrossRef]
- Bechinski, E.; Sandvol, L.; Carpenter, G.; Homan, H. Integrated Pest Management Guide to Wireworms in Potatoes. Univ. Ida. Ext. Bull. 1994, 760. [Google Scholar]
- Lange Jr, W.H.; Carlson, E.C.; Leach, L.D. Seed Treatments for Wireworm Control with Particular Reference to the Use of Lindane. J. Econ. Entomol. 1949, 42, 942–955. [Google Scholar] [CrossRef]
- Jones, E.W. Laboratory Studies on the Moisture Relations of Limonius (Coleoptera: Elaterida). Ecology 1951, 32, 284–293. [Google Scholar] [CrossRef]
- Lefko, S.A.; Pedigo, L.P.; Batchelor, W.D.; Rice, M.E. Spatial Modeling of Preferred Wireworm (Coleoptera: Elateridae) Habitat. Environ. Entomol. 1998, 27, 184–190. [Google Scholar] [CrossRef]
- Poggi, S. A Mechanistic Model for the Population Dynamics of Invertebrate Pests with Above-Belowground Life Stages: Case Study of Click Beetles and Wireworms. In Proceedings of the Annual Meeting ElatPro Project, Mumbai, India, 25 June 2018. [Google Scholar]
- Lafrance, J. The Seasonal Movements of Wireworms (Coleoptera: Elateridae) in Relation to Soil Moisture and Temperature in the Organic Soils of Southwestern Quebec. Can. Entomol. 1968, 100, 801–807. [Google Scholar] [CrossRef]
- Hermann, A.; Brunner, N.; Hann, P.; Wrbka, T.; Kromp, B. Correlations between Wireworm Damages in Potato Fields and Landscape Structure at Different Scales. J. Pest Sci. 2013, 86, 41–51. [Google Scholar] [CrossRef]
- Jones, E.W.; Shirck, F.H. The Seasonal Vertical Distribution of Wireworms in the Soil in Relation to Their Con. J. Agric. Res. 1942, 65, 125. [Google Scholar]
- Parker, W.E.; Seeney, F.M. An Investigation Inot the Use of Multiple Site Characteristics to Predict the Presence and Infestation Level of Wireworms (Agriotes Sup., Coleoptera: Elateridae) in Individual Grass Fields. Ann. Appl. Biol. 1997, 130, 409–425. [Google Scholar] [CrossRef]
- Van Herk, W.G.; Vernon, R.S. Effect of Temperature and Soil on the Control of a Wireworm, Agriotes obscurus L.(Coleoptera: Elateridae) by Flooding. Crop Prot. 2006, 25, 1057–1061. [Google Scholar] [CrossRef]
- Gfeller, A.; Laloux, M.; Barsics, F.; Kati, D.E.; Haubruge, E.; Du Jardin, P.; Verheggen, F.J.; Lognay, G.; Wathelet, J.-P.; Fauconnier, M.-L. Characterization of Volatile Organic Compounds Emitted by Barley (Hordeum Vulgare L.) Roots and Their Attractiveness to Wireworms. J. Chem. Ecol. 2013, 39, 1129–1139. [Google Scholar] [CrossRef]
- Rashed, A.; Rogers, C.W.; Rashidi, M.; Marshall, J.M. Sugar Beet Wireworm Limonius californicus Damage to Wheat and Barley: Evaluations of Plant Damage with Respect to Soil Media, Seeding Depth, and Diatomaceous Earth Application. Arthropod-Plant Interact. 2017, 11, 147–154. [Google Scholar] [CrossRef]
- Kozina, A.; Lemic, D.; Bazok, R.; Mikac, K.M.; McLean, C.M.; Ivezić, M.; Igrc Barčić, J. Climatic, Edaphic Factors and Cropping History Help Predict Click Beetle (Coleoptera: Elateridae)(Agriotes spp.) Abundance. J. Insect Sci. 2015, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Traugott, M.; Pázmándi, C.; Kaufmann, R.; Juen, A. Evaluating 15N/14N and 13C/12C Isotope Ratio Analysis to Investigate Trophic Relationships of Elaterid Larvae (Coleoptera: Elateridae). Soil Biol. Biochem. 2007, 39, 1023–1030. [Google Scholar] [CrossRef]
- Furlan, L.; Vasileiadis, V.P.; Chiarini, F.; Huiting, H.; Leskovšek, R.; Razinger, J.; Holb, I.J.; Sartori, E.; Urek, G.; Verschwele, A. Risk Assessment of Soil-Pest Damage to Grain Maize in Europe within the Framework of Integrated Pest Management. Crop Prot. 2017, 97, 52–59. [Google Scholar] [CrossRef]
- Furlan, L.; Contiero, B.; Chiarini, F.; Colauzzi, M.; Sartori, E.; Benvegnù, I.; Fracasso, F.; Giandon, P. Risk Assessment of Maize Damage by Wireworms (Coleoptera: Elateridae) as the First Step in Implementing IPM and in Reducing the Environmental Impact of Soil Insecticides. Environ. Sci. Pollut. Res. 2017, 24, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Poggi, S.; Le Cointe, R.; Lehmhus, J.; Plantegenest, M.; Furlan, L. Alternative Strategies for Controlling Wireworms in Field Crops: A Review. Agriculture 2021, 11, 436. [Google Scholar] [CrossRef]
- Hokkanen, H.M. Trap Cropping in Pest Management. Annu. Rev. Entomol. 1991, 36, 119–138. [Google Scholar] [CrossRef]
- Vernon, R.S. Aggregation and Mortality of Agriotes obscurus (Coleoptera: Elateridae) at Insecticide-Treated Trap Crops of Wheat. J. Econ. Entomol. 2005, 98, 1999–2005. [Google Scholar] [CrossRef]
- Le Cointe, R.; Girault, Y.; Morvan, T.; Thibord, J.-B.; Larroudé, P.; Lecuyer, G.; Plantegenest, M.; Bouille, D.; Poggi, S. Feeding Pests as an IPM Strategy: Wireworms in Conservation Agriculture as a Case Study. In Proceedings of the 3rd Annual International Branch Virtual Symposium of the Entomological Society of America, Virtual Symposium, 27 April 2020. [Google Scholar]
- Vernon, R.S.; van Herk, W.G.; Clodius, M.; Tolman, J. Companion Planting Attract-and-Kill Method for Wireworm Management in Potatoes. J. Pest Sci. 2016, 89, 375–389. [Google Scholar] [CrossRef]
- Björkman, M.; Klingen, I.; Birch, A.N.; Bones, A.M.; Bruce, T.J.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E. Phytochemicals of Brassicaceae in Plant Protection and Human Health–Influences of Climate, Environment and Agronomic Practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Staudacher, K.; Schallhart, N.; Thalinger, B.; Wallinger, C.; Juen, A.; Traugott, M. Plant Diversity Affects Behavior of Generalist Root Herbivores, Reduces Crop Damage, and Enhances Crop Yield. Ecol. Appl. 2013, 23, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Huang, P.; Huang, G. Effect of intercropping on soil microbial and enzyme activity in the rhizospher. Acta Prataculturae Sin. 2005, 14, 105–110. [Google Scholar]
- Inal, A.; Gunes, A.; Zhang, F.; Cakmak, I. Peanut/Maize Intercropping Induced Changes in Rhizosphere and Nutrient Concentrations in Shoots. Plant Physiol. Biochem. 2007, 45, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Long, L. Intercropping enhances agroecosystem services and functioning: Current knowledge and perspectives. Zhongguo Shengtai Nongye Xuebao Chin. J. Eco-Agric. 2016, 24, 403–415. [Google Scholar]
- Yang, H.; Zhang, W.; Li, L. Intercropping: Feed More People and Build More Sustainable Agroecosystems. Front Agric Sci Eng 2021, 8, 373–386. [Google Scholar]
- Marschner, P.; Crowley, D.; Yang, C.H. Development of Specific Rhizosphere Bacterial Communities in Relation to Plant Species, Nutrition and Soil Type. Plant Soil 2004, 261, 199–208. [Google Scholar] [CrossRef]
- Song, Y.N.; Zhang, F.S.; Marschner, P.; Fan, F.L.; Gao, H.M.; Bao, X.G.; Sun, J.H.; Li, L. Effect of Intercropping on Crop Yield and Chemical and Microbiological Properties in Rhizosphere of Wheat (Triticum aestivum L.), Maize (Zea mays L.), and Faba Bean (Vicia faba L.). Biol. Fertil. Soils 2007, 43, 565–574. [Google Scholar] [CrossRef]
- Zhang, N.N.; Sun, Y.M.; Li, L.; Wang, E.T.; Chen, W.X.; Yuan, H.L. Effects of Intercropping and Rhizobium Inoculation on Yield and Rhizosphere Bacterial Community of Faba Bean (Vicia faba L.). Biol. Fertil. Soils 2010, 46, 625–639. [Google Scholar] [CrossRef]
- Duchene, O.; Vian, J.-F.; Celette, F. Intercropping with Legume for Agroecological Cropping Systems: Complementarity and Facilitation Processes and the Importance of Soil Microorganisms. A Review. Agric. Ecosyst. Environ. 2017, 240, 148–161. [Google Scholar] [CrossRef]
- Rodriguez, C.; Carlsson, G.; Englund, J.-E.; Flöhr, A.; Pelzer, E.; Jeuffroy, M.-H.; Makowski, D.; Jensen, E.S. Grain Legume-Cereal Intercropping Enhances the Use of Soil-Derived and Biologically Fixed Nitrogen in Temperate Agroecosystems. A Meta-Analysis. Eur. J. Agron. 2020, 118, 126077. [Google Scholar] [CrossRef]
- Bedoussac, L.; Journet, E.-P.; Hauggaard-Nielsen, H.; Naudin, C.; Corre-Hellou, G.; Jensen, E.S.; Prieur, L.; Justes, E. Ecological Principles Underlying the Increase of Productivity Achieved by Cereal-Grain Legume Intercrops in Organic Farming. A Review. Agron. Sustain. Dev. 2015, 35, 911–935. [Google Scholar] [CrossRef]
- Schneider, K.D.; Thiessen Martens, J.R.; Zvomuya, F.; Reid, D.K.; Fraser, T.D.; Lynch, D.H.; O’Halloran, I.P.; Wilson, H.F. Options for Improved Phosphorus Cycling and Use in Agriculture at the Field and Regional Scales. J. Environ. Qual. 2019, 48, 1247–1264. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Bandara, M.; Hamel, C.; Knight, J.D.; Gan, Y. Intensifying Crop Rotations with Pulse Crops Enhances System Productivity and Soil Organic Carbon in Semi-Arid Environments. Field Crops Res. 2020, 248, 107657. [Google Scholar] [CrossRef]
- Li, X.; Mu, Y.; Cheng, Y.; Liu, X.; Nian, H. Effects of Intercropping Sugarcane and Soybean on Growth, Rhizosphere Soil Microbes, Nitrogen and Phosphorus Availability. Acta Physiol. Plant. 2013, 35, 1113–1119. [Google Scholar] [CrossRef]
- Gogoi, N.; Baruah, K.K.; Meena, R.S. Grain Legumes: Impact on Soil Health and Agroecosystem. In Legumes for Soil Health and Sustainable Management; Meena, R.S., Das, A., Yadav, G.S., Lal, R., Eds.; Springer: Singapore, 2018; pp. 511–539. ISBN 9789811302534. [Google Scholar]
- Landl, M.; Glauninger, J. Preliminary Investigations into the Use of Trap Crops to Control Agriotes spp.(Coleoptera: Elateridae) in Potato Crops. J. Pest Sci. 2013, 86, 85–90. [Google Scholar] [CrossRef]
- Adhikari, A.; Reddy, G.V. Evaluation of Trap Crops for the Management of Wireworms in Spring Wheat in Montana. Arthropod-Plant Interact. 2017, 11, 755–766. [Google Scholar] [CrossRef]
- Sharma, A.; Sandhi, R.K.; Briar, S.S.; Miller, J.H.; Reddy, G.V.P. Assessing the Performance of Pea and Lentil at Different Seeding Densities as Trap Crops for the Management of Wireworms in Spring Wheat. J. Appl. Entomol. 2019, 143, 460–469. [Google Scholar] [CrossRef]
- Samoilova, E.S.; Kostina, N.V.; Striganova, B.R. Effects of the Vital Activity of Soil Insect Larvae on Microbial Processes in the Soil. Biol. Bull. 2015, 42, 563–569. [Google Scholar] [CrossRef]
- Samoylova, E.S.; Kostina, N.V.; Striganova, B.R. Non-Symbiotic Nitrogen Fixation in the Intestine of Click Beetle Larvae (Coleoptera, Elateridae). In Proceedings of the Doklady Biological Sciences; Springer Nature BV: Dordrecht, The Netherlands, 2015; Volume 461, p. 92. [Google Scholar]
- Susurluk, A. Effectiveness of the Entomopathogenic Nematodes Heterorhabditis bacteriophora and Steinernema feltiae against Tenebrio molitor (Yellow Mealworm) Larvae in Different Soil Types at Different Temperatures. Turk. J. Biol. 2006, 30, 199–205. [Google Scholar]
- Shah, S.; Ash, G.J.; Wilson, B.A. Resporulation of Metarhizium anisopliae Granules on Soil and Mortality of Tenebrio Molitor: Implications for Wireworm Management in Sweetpotato. Ann. Appl. Biol. 2022. [Google Scholar] [CrossRef]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.-F.; Ferrer, A.; Peigné, J. Agroecological Practices for Sustainable Agriculture. A Review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef]
- Nichols, V.; Verhulst, N.; Cox, R.; Govaerts, B. Weed Dynamics and Conservation Agriculture Principles: A Review. Field Crops Res. 2015, 183, 56–68. [Google Scholar] [CrossRef]
- Doltra, J.; Olesen, J.E. The Role of Catch Crops in the Ecological Intensification of Spring Cereals in Organic Farming under Nordic Climate. Eur. J. Agron. 2013, 44, 98–108. [Google Scholar] [CrossRef]
- Town, J.R.; Gregorich, E.G.; Drury, C.F.; Lemke, R.; Phillips, L.A.; Helgason, B.L. Diverse Crop Rotations Influence the Bacterial and Fungal Communities in Root, Rhizosphere and Soil and Impact Soil Microbial Processes. Appl. Soil Ecol. 2022, 169, 104241. [Google Scholar] [CrossRef]
- Furlan, L.; Kreutzweiser, D. Alternatives to Neonicotinoid Insecticides for Pest Control: Case Studies in Agriculture and Forestry. Environ. Sci. Pollut. Res. 2015, 22, 135–147. [Google Scholar] [CrossRef]
- Griffiths, D.C. Susceptibility of Plants to Attack by Wireworms (Agriotes spp.). Ann. Appl. Biol. 1974, 78, 7–13. [Google Scholar] [CrossRef]
- Shirck, F.H. Crop Rotations and Cultural Practices as Related to Wireworm Control in Idaho. J. Econ. Entomol. 1945, 38, 627–633. [Google Scholar] [CrossRef]
- Shirck, F.H. Growth of the Sugar-Beet Wireworm on Different Food Plants. J. Econ. Entomol. 1946, 39, 648–651. [Google Scholar] [CrossRef]
- Dorr de Quadros, P.; Zhalnina, K.; Davis-Richardson, A.; Fagen, J.R.; Drew, J.; Bayer, C.; Camargo, F.A.O.; Triplett, E.W. The Effect of Tillage System and Crop Rotation on Soil Microbial Diversity and Composition in a Subtropical Acrisol. Diversity 2012, 4, 375–395. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.-J. The Impact of Crop Rotation on Soil Microbial Diversity: A Meta-Analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Lu, S.; Lepo, J.E.; Song, H.-X.; Guan, C.-Y.; Zhang, Z.-H. Increased Rice Yield in Long-Term Crop Rotation Regimes through Improved Soil Structure, Rhizosphere Microbial Communities, and Nutrient Bioavailability in Paddy Soil. Biol. Fertil. Soils 2018, 54, 909–923. [Google Scholar] [CrossRef]
- Ryan, M.H.; Kirkegaard, J.A.; Angus, J.F. Brassica Crops Stimulate Soil Mineral N Accumulation. Soil Res. 2006, 44, 367–377. [Google Scholar] [CrossRef]
- Dong, W.-Y.; Zhang, X.-Y.; Dai, X.-Q.; Fu, X.-L.; Yang, F.-T.; Liu, X.-Y.; Sun, X.-M.; Wen, X.-F.; Schaeffer, S. Changes in Soil Microbial Community Composition in Response to Fertilization of Paddy Soils in Subtropical China. Appl. Soil Ecol. 2014, 84, 140–147. [Google Scholar] [CrossRef]
- O’sullivan, O.S.; Heskel, M.A.; Reich, P.B.; Tjoelker, M.G.; Weerasinghe, L.K.; Penillard, A.; Zhu, L.; Egerton, J.J.; Bloomfield, K.J.; Creek, D. Thermal Limits of Leaf Metabolism across Biomes. Glob. Change Biol. 2017, 23, 209–223. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P. Dynamic Root Exudate Chemistry and Microbial Substrate Preferences Drive Patterns in Rhizosphere Microbial Community Assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Nettles, W.C. Effects of Substitute Crops and Rotations on Wireworm Control. J. Econ. Entomol. 1940, 33, 644–646. [Google Scholar] [CrossRef]
- Jansson, R.K.; Lecrone, S.H. Effects of Summer Cover Crop Management on Wireworm (Coleoptera: Elateridae) Abundance and Damage to Potato. J. Econ. Entomol. 1991, 84, 581–586. [Google Scholar] [CrossRef]
- Seal, D.R.; Chalfant, R.B.; Hall, M.R. Effects of Cultural Practices and Rotational Crops on Abundance of Wireworms (Coleoptera: Elateridae) Affecting Sweetpotato in Georgia. Environ. Entomol. 1992, 21, 969–974. [Google Scholar] [CrossRef]
- Salt, G.; Hollick, F.S.J. Studies of Wireworm Population III. Some Effects of Cultivation. Ann. Appl. Biol. 1949, 36, 169–186. [Google Scholar] [CrossRef]
- Kumar, D.; Shivay, Y.S.; Dhar, S.; Kumar, C.; Prasad, R. Rhizospheric Flora and the Influence of Agronomic Practices on Them: A Review. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2013, 83, 1–14. [Google Scholar] [CrossRef]
- Palm, C.; Blanco-Canqui, H.; DeClerck, F.; Gatere, L.; Grace, P. Conservation Agriculture and Ecosystem Services: An Overview. Agric. Ecosyst. Environ. 2014, 187, 87–105. [Google Scholar] [CrossRef]
- Schwilch, G.; Lemann, T.; Berglund, Ö.; Camarotto, C.; Cerdà, A.; Daliakopoulos, I.N.; Kohnová, S.; Krzeminska, D.; Marañón, T.; Rietra, R. Assessing Impacts of Soil Management Measures on Ecosystem Services. Sustainability 2018, 10, 4416. [Google Scholar] [CrossRef]
- Rosa-Schleich, J.; Loos, J.; Mußhoff, O.; Tscharntke, T. Ecological-Economic Trade-Offs of Diversified Farming Systems–a Review. Ecol. Econ. 2019, 160, 251–263. [Google Scholar] [CrossRef]
- Balota, E.L.; Colozzi-Filho, A.; Andrade, D.S.; Dick, R.P. Microbial Biomass in Soils under Different Tillage and Crop Rotation Systems. Biol. Fertil. Soils 2003, 38, 15–20. [Google Scholar] [CrossRef]
- Furlan, L.; Milosavljević, I.; Chiarini, F.; Benvegnù, I. Effects of Conventional versus No-Tillage Systems on the Population Dynamics of Elaterid Pests and the Associated Damage at Establishment of Maize Crops. Crop Prot. 2021, 149, 105751. [Google Scholar] [CrossRef]
- Campbell, R.E.; Stone, M.W. Flooding for the Control of Wireworms in California. J. Econ. Entomol. 1938, 31, 286–291. [Google Scholar] [CrossRef]
- Onsager, J.A.; Landis, B.J.; Rusk, H.W. Control of Wireworms on Potatoes in Eastern Washington by Soil Fumigants and Organophosphorous Insecticides. J. Econ. Entomol. 1966, 59, 441–443. [Google Scholar] [CrossRef]
- Strickland, E.H. Wireworms of Alberta, a Preliminary Report. University of Alberta. Univ. Alta. Bull. Edmenton Alta. 1927, 2. [Google Scholar]
- Unger, I.M.; Kennedy, A.C.; Muzika, R.-M. Flooding Effects on Soil Microbial Communities. Appl. Soil Ecol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Lestan, D.; Lamar, R.T. Development of Fungal Inocula for Bioaugmentation of Contaminated Soils. Appl. Environ. Microbiol. 1996, 62, 2045–2052. [Google Scholar] [CrossRef]
- Mazzola, M. Manipulation of Rhizosphere Bacterial Communities to Induce Suppressive Soils. J. Nematol. 2007, 39, 213. [Google Scholar]
- Nguyen, L.T.T.; Osanai, Y.; Lai, K.; Anderson, I.C.; Bange, M.P.; Tissue, D.T.; Singh, B.K. Responses of the Soil Microbial Community to Nitrogen Fertilizer Regimes and Historical Exposure to Extreme Weather Events: Flooding or Prolonged-Drought. Soil Biol. Biochem. 2018, 118, 227–236. [Google Scholar] [CrossRef]
- Johnson, S.N.; Anderson, E.A.; Dawson, G.; Griffiths, D.W. Varietal Susceptibility of Potatoes to Wireworm Herbivory. Agric. For. Entomol. 2008, 10, 167–174. [Google Scholar] [CrossRef]
- La Forgia, D.; Thibord, J.-B.; Larroudé, P.; Francis, F.; Lognay, G.; Verheggen, F. Linking Variety-Dependent Root Volatile Organic Compounds in Maize with Differential Infestation by Wireworms. J. Pest Sci. 2020, 93, 605–614. [Google Scholar] [CrossRef]
- Barsics, F.; Delory, B.M.; Delaplace, P.; Francis, F.; Fauconnier, M.-L.; Haubruge, É.; Verheggen, F.J. Foraging Wireworms Are Attracted to Root-Produced Volatile Aldehydes. J. Pest Sci. 2017, 90, 69–76. [Google Scholar] [CrossRef]
- Milosavljević, I.; Esser, A.D.; Murphy, K.M.; Crowder, D.W. Effects of Imidacloprid Seed Treatments on Crop Yields and Economic Returns of Cereal Crops. Crop Prot. 2019, 119, 166–171. [Google Scholar] [CrossRef]
- Osbourn, A.E. Saponins in Cereals. Phytochemistry 2003, 62, 1–4. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms. Annu Rev Plant Biol 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Bertin, C.; Yang, X.; Weston, L.A. The Role of Root Exudates and Allelochemicals in the Rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Houlden, A.; Timms-Wilson, T.M.; Day, M.J.; Bailey, M.J. Influence of Plant Developmental Stage on Microbial Community Structure and Activity in the Rhizosphere of Three Field Crops. FEMS Microbiol. Ecol. 2008, 65, 193–201. [Google Scholar] [CrossRef]
- Rudrappa, T.; Czymmek, K.J.; Paré, P.W.; Bais, H.P. Root-Secreted Malic Acid Recruits Beneficial Soil Bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Doornbos, R.F.; van Loon, L.C.; Bakker, P.A. Impact of Root Exudates and Plant Defense Signaling on Bacterial Communities in the Rhizosphere. A Review. Agron. Sustain. Dev. 2012, 32, 227–243. [Google Scholar] [CrossRef]
- Hyslop, J.A. Wireworms Attacking Cereal and Forage Crops; US Department of Agriculture: Washington, DC, USA, 1915. [Google Scholar]
- Fox, C.J.S.; MacLellan, C.R. Some Carabidae and Staphylinidae Shown to Feed on a Wireworm, Agriotes Sputator (L.), by the Precipitin Test1. Can. Entomol. 1956, 88, 228–231. [Google Scholar] [CrossRef]
- Frank, J.H. Carabidae (Coleoptera) as Predators of the Red-Backed Cutworm (Lepidoptera: Noctuidae) in Central Alberta. Can. Entomol. 1971, 103, 1039–1044. [Google Scholar] [CrossRef]
- Van Herk, W.G.; Vernon, R.S.; Cronin, E.M.L.; Gaimari, S.D. Predation of Thereva nobilitata (Fabricius) (Diptera: Therevidae) on Agriotes obscurus L. (Coleoptera: Elateridae). J. Appl. Entomol. 2015, 139, 154–157. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Penetration of the Cuticular Layers of Elaterid Larvae (Coleoptera) by the Fungus Metarrhizium anisopliae, and Notes on a Bacterial Invasion. J. Invertebr. Pathol. 1973, 21, 101–106. [Google Scholar] [CrossRef]
- Danismazoglu, M.; Demir, İ.; Sevim, A.; Demirbag, Z.; Nalcacioglu, R. An Investigation on the Bacterial Flora of Agriotes Lineatus (Coleoptera: Elateridae) and Pathogenicity of the Flora Members. Crop Prot. 2012, 40, 1–7. [Google Scholar] [CrossRef]
- Kleespies, R.G.; Ritter, C.; Zimmermann, G.; Burghause, F.; Feiertag, S.; Leclerque, A. A Survey of Microbial Antagonists of Agriotes Wireworms from Germany and Italy. J. Pest Sci. 2013, 86, 99–106. [Google Scholar] [CrossRef]
- Lacey, L.A.; Unruh, T.R.; Simkins, H.; Thomsen-Archer, K. Gut Bacteria Associated with the Pacific Coast Wireworm, Limonius canus, Inferred from 16s RDNA Sequences and Their Implications for Control. Phytoparasitica 2007, 35, 479–489. [Google Scholar] [CrossRef]
- Lewis, E.E.; Campbell, J.; Griffin, C.; Kaya, H.; Peters, A. Behavioral Ecology of Entomopathogenic Nematodes. Biol. Control 2006, 38, 66–79. [Google Scholar] [CrossRef]
- Campos-Herrera, R.; Gutiérrez, C. A Laboratory Study on the Activity of Steinernema feltiae (Rhabditida: Steinernematidae) Rioja Strain against Horticultural Insect Pests. J. Pest Sci. 2009, 82, 305–309. [Google Scholar] [CrossRef]
- Eidt, D.C.; Thurston, G.S. Physical Deterrents to Infection by Entomopathogenic Nematodes in Wireworms (Coleoptera: Elateridae) and Other Soil Insects. Can. Entomol. 1995, 127, 423–429. [Google Scholar] [CrossRef]
- Sandhi, R.K.; Shapiro-Ilan, D.; Sharma, A.; Reddy, G.V. Efficacy of Entomopathogenic Nematodes against the Sugarbeet Wireworm, Limonius californicus (Mannerheim)(Coleoptera: Elateridae). Biol. Control 2020, 143, 104190. [Google Scholar] [CrossRef]
- Toba, H.H.; Lindegren, J.E.; Turner, J.E.; Vail, P.V. Susceptibility of the Colorado Potato Beetle and the Sugarbeet Wireworm to Steinernema feltiae and S. Glaseri. J. Entomol. 1983, 15, 597–601. [Google Scholar]
- Ester, A.; Huiting, H. Controlling Wireworms (Agriotes Spp.) in a Potato Crop with Biologicals. IOBCwprs Bull. 2007, 30, 189–196. [Google Scholar]
- Ansari, M.A.; Evans, M.; Butt, T.M. Identification of Pathogenic Strains of Entomopathogenic Nematodes and Fungi for Wireworm Control. Crop Prot. 2009, 28, 269–272. [Google Scholar] [CrossRef]
- Lehmhus, J. Wireworm Biology in Middle Europe—What Are We Facing? Microbial and Nematode Control of Invertebrate Pests. IOBCwprs Bull. 2020, 150, 96–99. [Google Scholar]
- Griffin, C.T.; Boemare, N.E.; Lewis, E.E. Biology and Behaviour. In Nematode as Biocontrol Agents; CABI: Wallingford, UK, 2005. [Google Scholar]
- Ensafi, P.; Crowder, D.W.; Esser, A.D.; Zhao, Z.; Marshall, J.M.; Rashed, A. Soil Type Mediates the Effectiveness of Biological Control Against Limonius californicus (Coleoptera: Elateridae). J. Econ. Entomol. 2018, 111, 2058–2253. [Google Scholar] [CrossRef]
- Kaspi, R.; Ross, A.; Hodson, A.K.; Stevens, G.N.; Kaya, H.; Lewis, E.E. Foraging Efficacy of the Entomopathogenic Nematode Steinernema riobrave in Different Soil Types from California Citrus Groves. Appl. Soil Ecol. 2010, 45, 243–253. [Google Scholar] [CrossRef]
- Kung, S.; Gaugler, R.; Kaya, H.K. Effects of Soil Temperature, Moisture, and Relative Humidity on Entomopathogenic Nematode Persistence. J. Invertebr. Pathol. 1991, 57, 242–249. [Google Scholar] [CrossRef]
- Nikoukar, A.; Ensafi, P.; Lewis, E.E.; Crowder, D.W.; Rashed, A. Efficacy of Naturally Occurring and Commercial Entomopathogenic Nematodes Against Sugar Beet Wireworm (Coleoptera: Elateridae). J. Econ. Entomol. 2021, 114, 2241–2244. [Google Scholar] [CrossRef] [PubMed]
- Rojht, H.; Kač, M.; Trdan, S. Nontarget Effect of Entomopathogenic Nematodes on Larvae of Twospotted Lady Beetle (Coleoptera: Coccinellidae) and Green Lacewing (Neuroptera: Chrysopidae) under Laboratory Conditions. J. Econ. Entomol. 2009, 102, 1440–1443. [Google Scholar] [CrossRef]
- Sandhi, R.K.; Shapiro-Ilan, D.; Reddy, G.V. Montana Native Entomopathogenic Nematode Species against Limonius californicus (Coleoptera: Elateridae). J. Econ. Entomol. 2020, 113, 2104–2111. [Google Scholar] [CrossRef]
- Tinline, R.D.; Zacharuk, R.Y. Pathogenicity of Metarrhizium anisopliae (Metch.) Sor. and Beauveria bassiana (Bals.) Vuill. to Two Species of Elateridae. Nature 1960, 187, 794–795. [Google Scholar] [CrossRef]
- Ladurner, E.; Quentin, U.; Franceschini, S.; Benuzzi, M.; Ehlers, R. Efficacy Evaluation of the Entomopathogenic Fungus Beauveria bassiana Strain ATCC 74040 against Wireworms (Agriotes spp.) on Potato. IOBCwprs Bull 2009, 45, 445–448. [Google Scholar]
- Kölliker, U.; Biasio, L.; Jossi, W. Potential Control of Swiss Wireworms with Entomopathogenic Fungi. IOBCwprs Bull 2011, 66, 517–520. [Google Scholar]
- Kabaluk, T.; Vernon, R.; Goettel, M.S. Mortality and Infection of Wireworm, Agriotes obscurus [Coleoptera: Elateridae], with Inundative Field Applications of Metarhizium anisopliae. Phytoprotection 2007, 88, 51–56. [Google Scholar] [CrossRef]
- Sufyan, M.; Abbasi, A.; Gogi, M.D.; Arshad, M.; Nawaz, A.; Neuhoff, D. Efficacy of Beauveria bassiana for the Management of Economically Important Wireworm Species (Coleoptera: Elateridae) in Organic Farming. Gesunde Pflanz. 2017, 69, 197–202. [Google Scholar] [CrossRef]
- Tharp, C.I.; Blodgett, S.L.; Jaronski, S. Control of Wireworm (Elateridae) in potato with Microbial Metarhizium. Arthropod Manag. Tests 2007, 32, E43. [Google Scholar] [CrossRef]
- Razinger, J.; Praprotnik, E.; Schroers, H.-J. Bioaugmentation of Entomopathogenic Fungi for Sustainable Agriotes Larvae (Wireworms) Management in Maize. Front. Plant Sci. 2020, 11, 535005. [Google Scholar] [CrossRef]
- Luz, C.; Fargues, J. Temperature and Moisture Requirements for Conidial Germination of an Isolate of Beauveria bassiana, Pathogenic to Rhodnius prolixus. Mycopathologia 1997, 138, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Kabaluk, T.; Ericsson, J. Environmental and Behavioral Constraints on the Infection of Wireworms by Metarhizium anisopliae. Environ. Entomol. 2014, 36, 1415–1420. [Google Scholar] [CrossRef]
- Antwi, F.B.; Shrestha, G.; Reddy, G.V.; Jaronski, S.T. Entomopathogens in Conjunction with Imidacloprid Could Be Used to Manage Wireworms (Coleoptera: Elateridae) on Spring Wheat. Can. Entomol. 2018, 150, 124–139. [Google Scholar] [CrossRef]
- Sharma, A.; Jaronski, S.; Reddy, G.V. Impact of Granular Carriers to Improve the Efficacy of Entomopathogenic Fungi against Wireworms in Spring Wheat. J. Pest Sci. 2020, 93, 275–290. [Google Scholar] [CrossRef]
- Brandl, M.A.; Schumann, M.; Przyklenk, M.; Patel, A.; Vidal, S. Wireworm Damage Reduction in Potatoes with an Attract-and-Kill Strategy Using Metarhizium brunneum. J. Pest Sci. 2017, 90, 479–793. [Google Scholar] [CrossRef]
- Helmberger, M.; Shields, E.J.; Wicking, K.G. Ecology of Belowground Biological Control: Entomopathogenic Nematode Interactions with Soil Biota. Appl. Soil Ecol. 2017, 121, 201–213. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Navas-Cortés, J.A.; Maranhao, E.A.A.; Ortiz-Urquiza, A.; Santiago-Álvarez, C. Factors Affecting the Occurrence and Distribution of Entomopathogenic Fungi in Natural and Cultivated Soils. Mycol. Res. 2007, 111, 947–966. [Google Scholar] [CrossRef]
- Rohde, C.; Moino Jr, A.; da Silva, M.A.; Carvalho, F.D.; Ferreira, C.S. Influence of Soil Temperature and Moisture on the Infectivity of Entomopathogenic Nematodes (Rhabditida: Heterorhabditidae, Steinernematidae) against Larvae of Ceratitis capitata (Wiedemann)(Diptera: Tephritidae). Neotrop. Entomol. 2010, 39, 608–611. [Google Scholar] [CrossRef]
- Kabaluk, T.; Ericsson, J. Metarhizium anisopliae Seed Treatment Increases Yield of Field Corn When Applied for Wireworm Control. Agron. J. 2007, 99, 1377–1381. [Google Scholar] [CrossRef]
- Barelli, L.; Waller, A.S.; Behie, S.W.; Bidochka, M.J. Plant Microbiome Analysis after Metarhizium Amendment Reveals Increases in Abundance of Plant Growth-Promoting Organisms and Maintenance of Disease-Suppressive Soil. PLoS ONE 2020, 15, e0231150. [Google Scholar] [CrossRef]
- Reinbacher, L.; Bacher, S.; Knecht, F.; Schweizer, C.; Sostizzo, T.; Grabenweger, G. Preventive Field Application of Metarhizium brunneum in Cover Crops for Wireworm Control. Crop Prot. 2021, 150, 105811. [Google Scholar] [CrossRef]
- Kabaluk, T.; Li-Leger, E.; Nam, S. Metarhizium brunneum–an Enzootic Wireworm Disease and Evidence for Its Suppression by Bacterial Symbionts. J. Invertebr. Pathol. 2017, 150, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Milosavljević, I.; Esser, A.D.; Rashed, A.; Crowder, D.W. The Composition of Soil-Dwelling Pathogen Communities Mediates Effects on Wireworm Herbivores and Wheat Productivity. Biol. Control 2020, 149, 104317. [Google Scholar] [CrossRef]
- Ansari, M.A.; Shah, F.A.; Tirry, L.; Moens, M. Field Trials against Hoplia philanthus (Coleoptera: Scarabaeidae) with a Combination of an Entomopathogenic Nematode and the Fungus Metarhizium anisopliae CLO 53. Biol. Control 2006, 39, 453–459. [Google Scholar] [CrossRef]
- Jabbour, R.; Crowder, D.W.; Aultman, E.A.; Snyder, W.E. Entomopathogen Biodiversity Increases Host Mortality. Biol. Control 2011, 59, 277–283. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.; Jackson, M.; Reilly, C.C.; Hotchkiss, M.W. Effects of Combining an Entomopathogenic Fungi or Bacterium with Entomopathogenic Nematodes on Mortality of Curculio caryae (Coleoptera: Curculionidae). Biol. Control 2004, 30, 19–126. [Google Scholar] [CrossRef]
- Wakil, W.; Yasin, M.; Shapiro-Ilan, D. Effects of Single and Combined Applications of Entomopathogenic Fungi and Nematodes against Rhynchophorus ferrugineus (Olivier). Sci. Rep. 2017, 7, 5971. [Google Scholar] [CrossRef]
- Acevedo, J.P.M.; Samuels, R.I.; Machado, I.R.; Dolinski, C. Interactions between Isolates of the Entomopathogenic Fungus Metarhizium anisopliae and the Entomopathogenic Nematode Heterorhabditis bacteriophora JPM4 during Infection of the Sugar Cane Borer Diatraea saccharalis (Lepidoptera: Pyralidae). J. Invertebr. Pathol. 2007, 96, 187–192. [Google Scholar] [CrossRef]
- Barberchek, M.E.; Kaya, H.K. Interactions between Beauveria bassiana and the Entomogenous Nematodes, Steinernema feltiae and Heterorhabditis heliothidis. J. Invertebr. Pathol. 1990, 55, 225–234. [Google Scholar] [CrossRef]
- Ericsson, J.; Kabaluk, T.; Goettel, M.S.; Myers, J.H. Spinosad Interacts Synergistically with the Insect Pathogen Metarhizium anisopliae against the Exotic Wireworms Agriotes lineatus and Agriotes obscurus (Coleoptera: Elateridae). J. Econ. Entomol. 2007, 100, 31–38. [Google Scholar] [CrossRef]
- La Forgia, D.; Bruno, P.; Campos-Herrera, R.; Turlings, T.; Verheggen, F. The Lure of Hidden Death: Development of an Attract-and-Kill Strategy against Wireworms Combining Semiochemical and Entomopathogenic Nematodes. Turk. J. Zool. 2021, 45, 347–355. [Google Scholar] [CrossRef]
- Botelho, A.B.; Alves-Pereira, A.; Prado, R.; Zucchi, M.; Júnior, I. Metarhizium Species in Soil from Brazilian Biomes: A Study of Diversity, Distribution, and Association with Natural and Agricultural Environments.". Fungal Ecol. 2019, 41, 289–300. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Fergen, J.K. Enhancing Predation of a Subterranean Insect Pest: A Conservation Benefit of Winter Vegetation in Agroecosystems. Appl. Soil Ecol. 2011, 51, 9–16. [Google Scholar] [CrossRef]
- Ryan, M.R.; Mirsky, S.B.; Mortensen, D.A.; Teasdale, J.R.; Curran, W.S. Potential Synergistic Effects of Cereal Rye Biomass and Soybean Planting Density on Weed Suppression. Weed Sci. 2011, 59, 238–246. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Barbercheck, M.; Douglas, M.R.; Finney, D.M.; Haider, K.; Kaye, J.P.; Kemanian, A.R.; Mortensen, D.A.; Ryan, M.R.; Tooker, J. A Framework for Evaluating Ecosystem Services Provided by Cover Crops in Agroecosystems. Agric. Syst. 2014, 125, 12–22. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Drinkwater, L.E. Nitrogen Fixation of Red Clover Interseeded with Winter Cereals across a Management-Induced Fertility Gradient. Nutr. Cycl. Agroecosystems 2011, 90, 105–119. [Google Scholar] [CrossRef]
- Tonitto, C.; David, M.B.; Drinkwater, L.E. Replacing Bare Fallows with Cover Crops in Fertilizer-Intensive Cropping Systems: A Meta-Analysis of Crop Yield and N Dynamics. Agric. Ecosyst. Environ. 2006, 112, 58–72. [Google Scholar] [CrossRef]
- Lazzeri, L.; Curto, G.; Leoni, O.; Dallavalle, E. Effects of Glucosinolates and Their Enzymatic Hydrolysis Products via Myrosinase on the Root-Knot Nematode Meloidogyne Incognita (Kofoid et White) Chitw. J. Agric. Food Chem. 2004, 52, 6703–6707. [Google Scholar] [CrossRef]
- Lichtenstein, E.P.; Morgan, D.G.; Mueller, C.H. Insecticides in Nature, Naturally Occurring Insecticides in Cruciferous Crops. J. Agric. Food Chem. 1964, 12, 158–161. [Google Scholar] [CrossRef]
- Manici, L.M.; Lazzeri, L.; Palmieri, S. In Vitro Fungitoxic Activity of Some Glucosinolates and Their Enzyme-Derived Products toward Plant Pathogenic Fungi. J. Agric. Food Chem. 1997, 45, 2768–2773. [Google Scholar] [CrossRef]
- Matthiessen, J.N.; Kirkegaard, J.A. Biofumigation and Enhanced Biodegradation: Opportunity and Challenge in Soilborne Pest and Disease Management. Crit. Rev. Plant Sci. 2006, 25, 235–265. [Google Scholar] [CrossRef]
- Popova, I.; Dubie, J.S.; Morra, M.J. Optimization of Hydrolysis Conditions for Release of Biopesticides from Glucosinolates in Brassica juncea and Sinapis alba Seed Meal Extract. Ind. Crops Prod. 2017, 97, 354–359. [Google Scholar] [CrossRef]
- Borek, V.; Morra, M.J. Ionic Thiocyanate (SCN-) Production from 4-Hydroxybenzyl Glucosinolate Contained in Sinapis alba Seed Meal. J. Agric. Food Chem. 2005, 53, 8650–8654. [Google Scholar] [CrossRef] [PubMed]
- Furlan, L.; Bonetto, C.; Patalano, G.; Lazzeri, L. Potential of Biocidal Meals to Control Wireworm Populations. In Proceedings of the Proceedings of the first International Symposium on “Biofumigation: A possible alternative to methyl bromide; Firenze, Italy, 31 April 2004, Volume 31, pp. 313–316.
- McCaffrey, J.P.; Williams III, L.; Borek, V.; Brown, P.D.; Morra, M.J. Toxicity of Ionic Thiocyanate-Amended Soil to the Wireworm Limonius californicus (Coleoptera: Elateridae). J. Econ. Entomol. 1995, 88, 793–797. [Google Scholar] [CrossRef]
- Brown, J.; Brown, A.P.; Davis, J.B.; Erickson, D. Intergeneric Hybridization between Sinapis alba and Brassica napus. Euphytica 1997, 93, 163–168. [Google Scholar] [CrossRef]
- Morra, M.J.; Popova, I.; Boydston, R.A. Bioherbicidal Activity of Sinapis alba Seed Meal Extracts. Ind. Crops Prod. 2018, 115, 174–181. [Google Scholar] [CrossRef]
- Williams, L.; Morra, M.J.; Brown, P.D.; McCaffrey, J.P. Toxicity of Allyl Isothiocyanate-Amended Soil To Limonius californicus (Mann.)(Coleoptera: Elateridae) Wireworms. J. Chem. Ecol. 1993, 19, 1033–1046. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J.; McCaffrey, J.P.; Auld, D.L.; Williams, L. Allelochemicals Produced during Glucosinolate Degradation in Soi. J. Chem. Ecol. 1991, 17, 2021–2034. [Google Scholar] [CrossRef]
- Elberson, L.R.; Borek, V.; McCaffrey, J.P.; Morra, M.J. Toxicity of Rapeseed Meal-Amended Soil to Wireworms, Limonius californicus (Coleoptera: Elateridae). J. Agric. Entomol. 1996, 13, 232–330. [Google Scholar]
- Furlan, L.; Bonetto, C.; Finotto, A.; Lazzeri, L.; Malaguti, L.; Patalano, G.; Parker, W. The Efficacy of Biofumigant Meals and Plants to Control Wireworm Populations. Ind. Crops Prod. 2010, 31, 245–254. [Google Scholar] [CrossRef]
- Dandurand, L.-M.; Morra, M.J.; Zasada, I.A.; Phillips, W.S.; Popova, I.; Harder, C. Control of Globodera spp. Using Brassica Juncea Seed Meal and Seed Meal Extract. J. Nematol. 2017, 49, 437. [Google Scholar] [CrossRef]
- Sarwar, M.; Kirkegaard, J.A.; Wong, P.T.W.; Desmarchelier, J. Biofumigation Potential of Brassicas. Plant Soil 1998, 201, 103–112. [Google Scholar] [CrossRef]
- Hansen, J.C.; Schillinger, W.F.; Sullivan, T.S.; Paulitz, T.C. Soil Microbial Biomass and Fungi Reduced with Canola Introduced into Long-Term Monoculture Wheat Rotations. Front. Microbiol. 2019, 1488. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.C.; Schillinger, W.F.; Sullivan, T.S.; Paulitz, T.C. Rhizosphere Microbial Communities of Canola and Wheat at Six Paired Field Sites. Appl. Soil Ecol. 2018, 130, 185–193. [Google Scholar] [CrossRef]
- Trudgill, P.W.; Widdus, R.; Rees, J.S. Effects of Organochlorine Insecticides on Bacterial Growth, Respiration and Viability. Microbiology 1971, 69, 1–13. [Google Scholar] [CrossRef][Green Version]
- Digrak, M.; Kazanici, F. Effect of Some Organophosphorus Insecticides on Soil Microorganisms. Turk. J. Biol. 2001, 25, 51–58. [Google Scholar]
- Van Herk, W.G.; Vernon, R.S. Soil Bioassay for Studying Behavioral Responses of Wireworms (Coleoptera: Elateridae) to Insecticide-Treated Wheat Seed. Environ. Entomol. 2007, 36, 1441–1449. [Google Scholar] [CrossRef]
- Van Herk, W.G.; Vernon, R.S. Categorization and Numerical Assessment of Wireworm Mobility over Time Following Exposure to Bifenthrin. J. Pest Sci. 2013, 86, 115–123. [Google Scholar] [CrossRef]
- Van Herk, W.; Vernon, R.S.; Vojtko, B.; Snow, S.; Fortier, J.; Fortin, C. Contact Behaviour and Mortality of Wireworms Exposed to Six Classes of Insecticide Applied to Wheat Seed. J. Pest Sci. 2015, 88, 717–739. [Google Scholar] [CrossRef]
- Morales-Rodriguez, A.; Wanner, K.W. Efficacy of Thiamethoxam and Fipronil, Applied Alone and in Combination, to Control Limonius californicus and Hypnoidus bicolor (Coleoptera: Elateridae). Pest Manag. Sci. 2015, 71, 584–591. [Google Scholar] [CrossRef]
- Cycoń, M.; Markowicz, A.; Borymski, S.; Wójcik, M.; Piotrowska-Seget, Z. Imidacloprid Induces Changes in the Structure, Genetic Diversity and Catabolic Activity of Soil Microbial Communities. J. Environ. Manage. 2013, 131, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; An, J.; Dang, Z.; Lv, H.; Pan, W.; Gao, Z. Treating Wheat Seeds with Neonicotinoid Insecticides Does Not Harm the Rhizosphere Microbial Community. PLoS ONE 2018, 13, e0205200. [Google Scholar] [CrossRef]
- Parizadeh, M.; Mimee, B.; Kembel, S.W. Effects of Neonicotinoid Seed Treatments on Soil Microbial Gene Expression Vary with Time in an Agricultural Ecosystem. bioRxiv 2022, arXiv:2022.01.20.477174. [Google Scholar] [CrossRef]
- Broderick, N.A.; Raffa, K.F.; Handelsman, J. Midgut Bacteria Required for Bacillus thuringiensis Insecticidal Activity. Proc. Natl. Acad. Sci. USA 2006, 103, 15196–15199. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Hosokawa, T.; Fukatsu, T. Insect-Microbe Mutualism without Vertical Transmission: A Stinkbug Acquires a Beneficial Gut Symbiont from the Environment Every Generation. Appl. Environ. Microbiol. 2007, 73, 4308–4316. [Google Scholar] [CrossRef]
- Azhar, S.; Ahmad, K.S. Pedospheric Adsorption–Desorption of Anti-Moulting Agent Chlorfluazuron and Transfer in Agriculturally Significant Arcadian Soils. Sādhanā 2019, 44, 212. [Google Scholar] [CrossRef]
- Xie, G.; Li, B.; Tang, L.; Rao, L.; Dong, Z. Adsorption-Desorption and Leaching Behaviors of Broflanilide in Four Texturally Different Agricultural Soils from China. J. Soils Sediments 2021, 21, 724–735. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Rosi, E.J.; Gessner, M.O. Synthetic Chemicals as Agents of Global Change. Front. Ecol. Environ. 2017, 15, 84–90. [Google Scholar] [CrossRef]
- Peiffer, J.A.; Spor, A.; Koren, O.; Jin, Z.; Tringe, S.G.; Dangl, J.L.; Buckler, E.S.; Ley, R.E. Diversity and Heritability of the Maize Rhizosphere Microbiome under Field Conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553. [Google Scholar] [CrossRef]
- Donn, S.; Wheatley, R.E.; McKenzie, B.M.; Loades, K.W.; Hallett, P.D. Improved Soil Fertility from Compost Amendment Increases Root Growth and Reinforcement of Surface Soil on Slopes. Ecol. Eng. 2014, 71, 458–465. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, Variation, and Assembly of the Root-Associated Microbiomes of Rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, A.K.; Yin, C.; Hulbert, S.H. Community Structure, Species Variation, and Potential Functions of Rhizosphere-Associated Bacteria of Different Winter Wheat (Triticum aestivum) Cultivars. Front. Plant Sci. 2017, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Samoilova, E.S.; Kostina, N.V.; Striganova, B.R. Microbial Population of the Digestive Tract of Click Beetle Larvae (Elateridae, Coleoptera). Biol. Bull. 2016, 43, 457–467. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).